Abstract
Osteoarthritis is a chronic, painful condition that is now recognised as affecting a large proportion of cats. Non-steroidal anti-inflammatory drugs (NSAIDs) have proven efficacy in dogs and humans but there are limited published data on the use of NSAIDs in the long-term management of this condition in cats. This prospective study aimed to assess the long-term safety and palatability of oral meloxicam and its efficacy in treating osteoarthritic pain in cats when given at a dose of 0.01–0.03 mg/kg once daily. Forty cats diagnosed with osteoarthritis completed the trial with a mean treatment duration of 5.8 months. Gastrointestinal upset in 2/46 (4%) cats was the only adverse effect noted. No deleterious effect on renal function was detected in cats studied. Owners subjectively assessed treatment efficacy as good or excellent in 34/40 (85%) of cases. The results of this study showed oral meloxicam to be safe and palatable long-term treatment for osteoarthritis in cats when given with food at a dose of 0.01–0.03 mg/kg.
Osteoarthritis is a painful, degenerative condition that commonly affects one or more joints, typically involving the ‘elbow, hip and shoulder joints of older cats. In retrospective radiographic surveys up to 20% of cats have evidence of osteoarthritis (Godfrey 2005, Clarke et al 2005), although the exact percentage depends on the composition of the study cohort. Cats with osteoarthritis may present with a variety of clinical signs related to joint pain including difficulty in jumping, lameness, resentment of handling and stiff gait (Clarke and Bennett 2006), although many owners do not actually recognise the significance of these signs.
Diagnosis of osteoarthritis involves clinical examination and radiographic evidence of degenerative joint disease in the absence of signs referable to inflammation such as bone lysis, fever, anorexia or depression. Arthrocentesis and synovial fluid analysis may be required in some cases to rule out septic or immune-mediated arthritis (Schrader and Sherding 1994).
Despite the frequency with which this painful condition occurs, to the authors' knowledge, there is only one other published study investigating the long-term therapy of osteoarthritis in cats (Clarke and Bennett 2006). Current recommendations are based on strategies used in dogs, horses and people including weight management, exercise modification, non-steroidal anti-inflammatory drugs (NSAIDs) and disease modifying osteoarthritis agents (McLaughlin and Roush 2002).
Meloxicam (Metacam oral suspension; Boehringer Ingelheim Vetmedica) is an enolic acid derivative, belonging to the oxicam group of NSAIDs. It has been shown to be cyclooxygenase (COX)-2 selective in several species including dogs and horses (Kay-Mugford et al 2000, Brideau et al 2001). COX-2 selectivity has been postulated to minimise adverse effects by sparing essential constitutional COX-1 dependant prostaglandins. To the authors' knowledge data has not been published regarding meloxicam's COX-2 selectivity in cats. Studies in human and canine in vitro models have failed to show any adverse effects on cartilage integrity (Rainsford et al 1997, 1999). Long-term studies in young healthy cats at a daily maintenance dose of 0.05 mg/kg of meloxicam failed to produce gross or histological evidence of adverse gastrointestinal or renal effects (Dammgen 2007).
Meloxicam administered at 0.2 mg/kg subcutaneously (SC) is as effective as carprofen (4 mg/kg SC), ketoprofen (2 mg/kg SC) and tolfenamic acid (4 mg/kg SC) in providing postoperative analgesia after ovariohysterectomy in cats (Slingsby and Waterman-Pearson 2000). There was no significant difference between meloxicam and ketoprofen in providing short-term analgesia for acute and chronic locomotor disorders in cats, both agents provided effective analgesia (Lascelles et al 2001). Meloxicam also provided good control of osteoarthritic pain in dogs with minimal adverse effects with long-term use (Doig et al 2000). Clarke and Bennett (2006) used a loading dose of 0.1 mg/kg per os (PO) once daily followed by daily administration of 0.05 mg/kg PO in a prospective study of osteoarthritis in cats.
The aim of this study was to assess the long-term safety, palatability and efficacy of oral meloxicam administered at a dose of 0.01–0.03 mg/kg in controlling the clinical signs associated with osteoarthritis in cats.
Materials and Methods
The investigation was planned as a prospective case-control study. Forty-six cats diagnosed with osteoarthritis were enrolled over a 12-month period. The trial was concluded 3 months after the last cat was enrolled. Osteoarthritis was diagnosed based on history (clinical signs for over 2 months duration, lameness, difficulty in jumping and/or behavioural changes), physical findings (pain or decreased mobility of one or more joints) and radiography (narrowed joint space, periarticular osteophytes, subchondral bone sclerosis, ossified joint bodies). Where appropriate serum biochemistry, haematology and synovial fluid analysis were used to rule out inflammatory arthridites.
Cats were excluded if they had received corticosteroids or NSAIDs in the previous 2 weeks, long-acting corticosteroids in the previous 2 months, had current gastrointestinal signs, were pregnant or had systemic disease causing pyrexia. Pre-existing chronic renal insufficiency (IRIS stage 3) (n=3), diabetes mellitus (n=3) or hyperthyroidism (n=4) did not preclude enrolment in the study if the disease was stable and being appropriately managed. At enrolment, each osteoarthritic cat was assigned an age, breed, sex and pre-existing disease matched control cat without clinical evidence of osteoarthritis. Control cats were selected from the general clinic population.
The osteoarthritic group received a loading dose schedule of meloxicam (0.1 mg/kg PO once daily for 4 days) and thereafter at 0.1 mg/cat PO once daily irrespective of weight (giving a dose range of 0.01–0.03 mg/kg) with the medication given on the cat's normal diet. These doses were selected after consultation with the manufacturer at the time of the trial. The formulation of meloxicam used was 1.5 mg/ml and dispensed 0.1 mg meloxicam per drop. The control group received no treatment. Cats had full clinical examinations at recruitment, after 1 month of therapy and on completion of the trial. Simple discontinuous scales were used for veterinarian and owner assessments of general demeanor, lameness and overall efficacy (Doig et al 2000). In addition, owners were asked to score their cats for food intake and palatability of meloxicam (Doig et al 2000) (Table 1). Veterinary assessments were made at each examination and owner assessments were made at monthly intervals throughout the trial. At each examination owners were questioned about their cat's health and were asked to contact their veterinarian if any signs of illness occurred. They were asked to make records of any illness identified at home.
Table 1.
Discontinuous scales used to assess efficacy and palatability of long-term oral meloxicam administration for treatment of osteoarthritis in cats
| Score | Parameter | ||||
|---|---|---|---|---|---|
| General demeanor | Food intake | Lameness | Palatability | Overall efficacy | |
| 1 | Behaviour normal | Increased | None | Takes medication readily | Excellent, complete resolution of clinical signs |
| 2 | Slightly subdued | Unchanged | Mild lameness, slight weight shift observed | Sniffs at food a short while before taking medication | Good, obvious improvement |
| 3 | Very subdued | Slightlydecreased | Moderate lameness, marked weight shift observed | Shows reluctance in accepting medication, but will accept food | Fair, some improvement |
| 4 | Apathetic | Much reduced | Severe lameness, intermittent toe touching or non-weight bearing | Will not take medication at all | Poor, unchanged or worse |
The first 10 enrolled pairs of cats without concurrent disease had serum creatinine measurements at enrolment and after 1 month of therapy.
Statistical Analysis
Data from the assessment of lameness, general demeanor and food intake between enrolment and trial completion were analysed using paired samples t-tests. Creatinine levels in the meloxicam group at enrolment and after 1-month therapy were also compared using paired t-tests. Age, body weight and creatinine levels in treated versus control cats were compared at enrolment and after 1 month using the t-test. The number of illnesses and gastrointestinal side effects were compared using a χ2 and the Fisher exact test, respectively. The statistical analysis was performed by Decisions Research (Chippendale NSW) using Microsoft Excel 2000 and SPSS version 10.0. Statistical significance was accepted at the 5% level.
The trial was conducted in accordance with the NHMRC/CSIRO Code of Practice for the Care and Use of Animals in Research in Australia.
Results
Forty-six pairs of cats were enrolled in the trial. One treated and one control cat with pre-existing chronic renal insufficiency died of histologically confirmed end stage renal failure during the trial. An additional cat in the meloxicam group died of pulmonary oedema referable to hypertrophic cardiomyopathy. Two other cats were euthanased in the control group, one with sublingual squamous cell carcinoma and one with small intestinal adenocarcinoma. Meloxicam was withdrawn from two cats that (in the opinion of the attending veterinarian) required corticosteroids for diseases that developed during the trial period: one cat suffered spinal trauma and another developed plasmacytic/lymphocytic stomatitis. This left complete data for 40 pairs of cats with duration of therapy of 5.8±3.4 months (mean±SEM).
At enrolment the mean age and body weight of the meloxicam group (12.9±4.2 years, 5.0±1.4 kg) did not differ significantly from the control group (12.4±3.5 years, 4.9±1.2 kg; P=0.53 and P=0.97, respectively). There was no significant change in weight during the trial, at completion the meloxicam group averaged 5.0±1.2 kg (P=0.75) and the control group 4.8±1.3 kg (P=0.58). The daily dosage per kilogram for the maintenance of meloxicam therapy ranged from 0.01 mg/kg to 0.03 mg/kg. Concurrent disease was present in 10 pairs of cats, four with hyperthyroidism, three with chronic renal insufficiency (IRIS stage 3) and three with diabetes mellitus.
Illness
A range of illnesses developed in both the meloxicam and control groups (Table 2). There was no significant difference in the number of illness events occurring (meloxicam=11, control group=12; P=0.851). Four cases of gastrointestinal upset were reported in the meloxicam group compared with two in the control group (P=0.438) (Table 2). Two cats in the meloxicam group had vomiting associated with trichobezoars in the vomitus and the cause of vomiting was believed to be unrelated to the treatment. However, two other cats consistently vomited after administration of meloxicam necessitating withdrawal of the drug. The vomiting ceased immediately after withdrawal of meloxicam and no other evidence of clinical disease was associated with the vomiting. One cat was subsequently rechallenged with meloxicam and vomited again after drug administration. The owner of the other cat was unwilling to administer meloxicam again. Both of these cats were receiving a dose of 0.02 mg/kg daily.
Table 2.
Illnesses occurring in control and treatment groups during the investigation of long-term oral meloxicam administration in cats
| Meloxicam group (n=11) | Control group (n=12) |
|---|---|
Fatal.
Necessitated withdrawal from trial.
Palatability
Initially 43/46 cats (94%) readily accepted food containing meloxicam, 2/46 (4%) were reluctant and 1/46 (2%) would only eat the food after many hours. After 2 months all (44/44) cats readily accepted food containing meloxicam and this continued throughout the trial period.
Creatinine
There was no statistically significant difference in serum creatinine between treated and control cats (n=10) at enrolment (169±70 μmol/l and 156±52 μmol/l, respectively; P=0.65). There was no difference in serum creatinine after therapy with meloxicam at enrolment and after 1 month of therapy (169±70 μmol/l and 184±82 μmol/l, respectively; P=0.66). The progression of renal disease in the cats with pre-existing chronic renal insufficiency is presented in Table 3.
Table 3.
Changes in serum creatinine levels after long-term oral meloxicam administration in three cats with pre-existing chronic renal failure
| Duration of therapy (months) | Creatinine level (reference range 71–212 μmol/l) | |||
|---|---|---|---|---|
| Meloxicam | Control | |||
| Enrolment | Completion | Enrolment | Completion | |
| 4 | 327 | 388 | 345 | 420 |
| 6 | 403 | 654 | 340 | 880 |
| 9 | 285 | 410 | 253 | 305 |
Efficacy
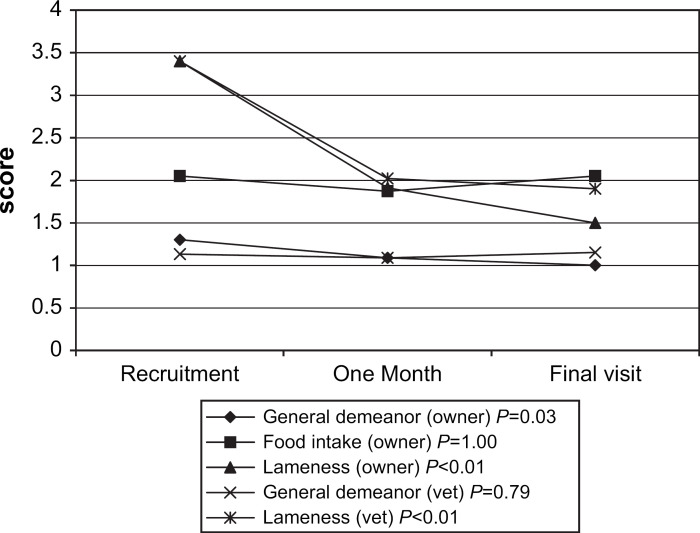
Owner and veterinarian assessments of the effectiveness of meloxicam in controlling the clinical signs of osteoarthritis are summarised in Fig 1. Statistically significant improvements occurred in owner assessed general demeanor (P=0.03) and in both owner and veterinarian assessed lameness (P<0.01 for both pairwise comparisons). No change was observed in food intake or veterinarian assessed general demeanor.
Fig 1.
Mean owner and veterinarian assessed discontinuous scales (0–4) for general demeanor, food intake and lameness in 40 cats administered oral meloxicam for the treatment of osteoarthritic pain. (P values are for comparison between recruitment and final visit.)
At completion of the trial, overall efficacy was rated as excellent or good by 34/40 (85%) of owners and in 32/40 (80%) by veterinarians (Table 4).
Table 4.
Owner and veterinarian assessed overall efficacy of oral metacam administration in the treatment of osteoarthritis in 40 cats
| Owner assessment (%) | Veterinarian assessment (%) | |
|---|---|---|
| Excellent | 40 | 23 |
| Good | 45 | 58 |
| Fair | 3 | 8 |
| Poor | 12 | 11 |
Discussion
The most important finding in this study was that long-term oral administration of meloxicam at a maintenance dose of 0.01–0.03 mg/kg was considered safe and was not associated with an increased incidence of disease. It is essential to note that meloxicam has recently been registered for chronic use in cats at a maintenance dose of 0.05 mg/kg which is higher than the dose used in this study. There were numerous episodes of illness reported to the investigators in both the treated and control groups, which was not surprising considering the advanced age of the cohort and the duration of the trial. Other than the two cats that vomited in temporal association with oral meloxicam administration there was no other evidence of disease attributable to meloxicam treatment.
NSAIDs have a long history of use in human and canine patients for the treatment of osteoarthritic pain and while they are considered a first line treatment, their use has been associated with numerous adverse effects. Adverse effects reported have related to a range of body systems including gastrointestinal, renal, hepatic, coagulation and articular cartilage (Sweetman 2002). The most common side effects in both humans and dogs relate to the gastrointestinal tract including vomiting, diarrhoea, dyspepsia, gastric erosion and perforating ulcers. Gastrointestinal signs are caused by two distinct mechanisms, a direct irritant effect of the medication on the gastrointestinal mucosa or via COX inhibition (Booth 2001, Sweetman 2002).
Four cats treated with meloxicam had episodes of vomiting compared to one in the control group. Two of these had trichobezoars in their vomitus and meloxicam was not considered the cause of vomiting. Vomiting in the remaining two cats was attributed to meloxicam administration and this was confirmed by rechallenge in one cat. Neither cat showed a decrease in food intake or other clinical signs of illness. Endoscopic examination of these cats was not considered clinically appropriate and so it was undetermined if gastric erosion or ulceration was present. In these two cats, drug withdrawal was effective in resolving the vomiting, but dose reduction was not attempted. Minor gastrointestinal side effects, such as dyspepsia and vomiting occur in 5–50% of people on long-term NSAID therapy (MacDonald 2000), and NSAID withdrawal is required in 5% of dogs (Nell et al 2002), which is comparable to the 4% of cats reported here.
Renal complications from chronic NSAID administration occur in humans and it is likely that this also occurs in cats. However, reports of long-term clinical use of NSAIDs in cats are lacking. In humans adverse effects on the kidney occur due to inhibition of renal prostaglandins, initiation of interstitial nephritis or chronic nephropathy (Sweetman 2002). Inhibition of prostaglandin production reduces the kidney's ability to auto-regulate perfusion in the face of reduced systemic blood pressure or flow. Hence, the potential for nephrotoxicity is most prevalent during times of dehydration, hypovolaemia or hypotension, and NSAID use is contraindicated in these states. For this reason the author always advises clients to discontinue meloxicam administration and seek veterinary advice if their cat is showing any signs of illness or has reduced food intake. A previous study of peri-operative meloxicam use in dogs failed to demonstrate nephrotoxicity using biochemical and histopathological methods (Matthews et al 2001). The current trial studied the clinical effects on renal disease (as assessed by examination, weight change and history) of long-term meloxicam usage. During the course of the current trial, one cat in the meloxicam group and two cats in the control group were diagnosed with renal failure. There was no increase in the prevalence of renal disease associated with meloxicam usage during the course of the trial, or significant alteration in creatinine in the initial subset of cats studied (n=10). The cats that had biochemically detectable pre-existing renal disease did not appear to deteriorate faster than their controls, although there was no statistical evaluation performed as there were only three pairs of cat with pre-existing renal disease in the trial. Further trials are needed to evaluate the effects of meloxicam in cats with pre-existing renal disease to determine safety in this population of cats, both at the doses used in this study and at the higher daily dose (0.05 mg/kg) now recommended by the manufacturer. It is worthy of note that in humans meloxicam does not require a dose reduction in mild to moderate renal failure (Sweetman 2002). However, it is prudent to use NSAIDs in the presence of chronic renal failure only when no other effective alternative exists and even then with great caution and frequent monitoring. Other therapeutic options that can be considered include nutraceuticals such as glucosamine and chondroprotective agents such as pentosan polysulfate (Beale 2004).
Several NSAIDs have been shown to have deleterious effects on canine articular cartilage (Rainsford et al 1999) and this raises concerns about the choice of NSAID for the management of osteoarthritis. The effects of meloxicam on both canine and human articular cartilage have been investigated and the results of this work showed meloxicam to have no deleterious effects (Rainsford et al 1999).
To date there have been no reports of hepatotoxicity associated with the use of meloxicam in cats or dogs and no evidence of clinically apparent hepatic dysfunction was observed during the course of this study.
Ease of administration and palatability of drug formulations are major concerns in feline practice, especially when medications are required for prolonged periods of time. Meloxicam is significantly more palatable than ketoprofen when given as oral therapy to cats (Lascelles et al 2001). Meloxicam is commercially available as a honey scented elixir in a dropper bottle, containing either 0.05 mg or 0.1 mg/drop meloxicam and is registered for use in dogs. Recently meloxicam has been registered for use in cats in Europe.
The majority of cats treated with meloxicam in this trial had good to excellent control of their osteoarthritic signs as rated by both owners and veterinarians. A limitation of this study is the lack of a control group for the efficacy data. Both owners and veterinarians were asked to assess lameness, general demeanor and overall efficacy independently and there was agreement in the degree of improvement of lameness and overall efficacy, however, significant potential for bias remains. No placebo group was used as it was considered unethical to withhold analgesic treatment from patients that were clinically considered to require it. Additionally, there is no recognised standard therapy for osteoarthritis with which to compare chronic meloxicam treatment. This study shows that the majority of owners were satisfied with meloxicam therapy, though this may have been in part a ‘placebo’ effect.
This study showed that oral meloxicam was highly palatable and safe for long-term treatment of osteoarthritis in cats, including those of advanced age. The only adverse effect associated with its use in this trial was vomiting in a small percentage of cats. The commercially available elixir was highly palatable to cats even over many months. Further investigations are required to assess the safety of this drug in cats with mild to moderate chronic renal disease.
Acknowledgements
Funding for this trial was kindly provided by Boehringer Ingelheim Vetmedica Australia.
References
- Beale B.S. Use of nutraceuticals and chondroptectants in osteoarthritic dogs and cats, Veterinary Clinics of North America Small Animal Practice 34, 2004, 271–289. [DOI] [PubMed] [Google Scholar]
- Booth D.M. The analgesic, antipyretic, anti-inflammatory drugs. Adams H.R. Veterinary Pharmacology and Therapeutics, 8th edn, 2001, Iowa State University Press: Iowa, 433–434. [Google Scholar]
- Brideau C., Van Staden C., Chan C.C. In vitro effects of cyclooxygenase inhibitors in whole blood of horses, dogs and cats, American Journal of Veterinary Research 62, 2001, 1755–1760. [DOI] [PubMed] [Google Scholar]
- Clarke S.P., Bennett D. Feline osteoarthritis: a prospective study of 28 cases, Journal of Small Animal Practice 47, 2006, 439–445. [DOI] [PubMed] [Google Scholar]
- Clarke S.P., Mellor D., Clements D.N., Gemmill T., Farrell M., Carmichael S., Bennett D. Prevalence of radiographic signs of degenerative joint disease in a hospital population of cats, Veterinary Record 157, 2005, 793–799. [DOI] [PubMed] [Google Scholar]
- Dammgen J. (2007) The use of Metacam 0.5mg/ml oral suspension in cats with osteoarthritis. In: Metacam Symposium on Arthritic Disease in Cats, pp. 19–20.
- Doig P.A., Purbrick K.A., Hare J.E., McKeown D.B. Clinical efficacy and tolerance of meloxicam in dogs with chronic osteoarthritis, Canadian Veterinary Journal 41, 2000, 296–300. [PMC free article] [PubMed] [Google Scholar]
- Godfrey D.R. Osteoarthritis in cats: a retrospective radiological study, Journal of Small Animal Practice 46, 2005, 425–429. [DOI] [PubMed] [Google Scholar]
- Kay-Mugford P., Benn S.J., LaMarre J., Conlon P. In vitro effects of non-steroidal anti-inflammatory drugs on cyclooxygenase activity in dogs, American Journal of Veterinary Research 61, 2000, 802–810. [DOI] [PubMed] [Google Scholar]
- Lascelles B.D.X., Henderson A.J., Hackett I.J. Evaluation of the clinical efficacy of meloxicam in cats with painful locomotor disorders, Journal of Small Animal Practice 42, 2001, 587–593. [DOI] [PubMed] [Google Scholar]
- MacDonald T.M. Epidemiology and pharmacoeconomic implications of non-steroidal anti-inflammatory drug-associated gastrointestinal toxicity, Rheumatology 39 (suppl 2), 2000, 13–20. [DOI] [PubMed] [Google Scholar]
- McLaughlin R.M., Roush J.K. Medical therapy for patients with osteoarthritis, Veterinary Medicine 97, 2002, 135–144. [Google Scholar]
- Matthews K.A., Pettifer G., Foster R., McDonell W. Safety and efficacy of preoperative administration of meloxicam, compared with that of ketoprofen and butorphanol in dogs undergoing abdominal surgery, American Journal of Veterinary Research 62, 2001, 882–888. [DOI] [PubMed] [Google Scholar]
- Nell T., Bergman J., Hoeijmakers M., Van Laar P., Horspool L.J.I. Comparison of vedaprofen and meloxicam in dogs with musculoskeletal pain and inflammation, Journal of Small Animal Practice 43, 2002, 208–212. [DOI] [PubMed] [Google Scholar]
- Rainsford K.D., Ying C., Smith F.C. Effects of meloxicam, compared with other NSAIDs, on cartilage proteoglycan metabolism, synovial prostaglandin E2 production, and production of interleukins 1, 6, and 8, in human and porcine explants in organ culture, Journal of Pharmacy and Pharmacology 48, 1997, 991–998. [DOI] [PubMed] [Google Scholar]
- Rainsford K.D., Skerry T.M., Chindemi P., Delaney K. Effects of the NSAIDs meloxicam and indomethacin on cartilage proteogylcan synthesis and joint responses to calcium pyrophosphate crystals in dogs, Veterinary Research Communications 23, 1999, 101–113. [DOI] [PubMed] [Google Scholar]
- Schrader S.C., Sherding R.G. Disorders of the skeletal system. Sherding R.G. The Cat: Diseases and Management, 2nd edn, 1994, Churchill Livingstone: New York, 1599–1648. [Google Scholar]
- Slingsby L.S., Waterman-Pearson A.E. Postoperative analgesia in the cat after ovariohysterectomy by use of carprofen, ketoprofen, meloxicam or tolfenamic acid, Journal of Small Animal Practice 41, 2000, 447–450. [DOI] [PubMed] [Google Scholar]
- Sweetman S.C. Nonsteroidal anti-inflammatory drugs. Sweetman S.C. Martindale: The complete Drug Reference, 33rd edn, 2002, Pharmaceutical Press: London, 63–64. [Google Scholar]