Abstract
In this study we have investigated the prevalence of aelurostrongylosis, one of the most common feline pulmonary parasitic diseases, in cats from the north-west region of Portugal. For this purpose, 97 faecal samples were collected from cats at risk of being infected by Aelurostrongylus abstrusus in an animal shelter and in a municipal facility. Using the Baermann–Wetzel coprological technique, faecal shedding of first stage larvae (L1) was detected in 17.4% of the cats. Based on this result, it can be concluded that this lungworm infection seems to be common among feral cats in the north-west region of Portugal, in spite of the fact that clinical aelurostrongylosis is not frequently diagnosed by feline practitioners in the area. This parasitic disease should be included in the differential diagnosis of cats presenting with coughing or dyspnoea, and it also should be extended to asymptomatic animals with pulmonary nodules detected by image diagnosis.
Aelurostrongylus abstrusus is a metastrongyloid nematode with adults (less than 10 mm long) residing in the terminal bronchioles and alveolar ducts of cats. Adult females lay eggs which embryonate and from which first stage larvae (L1) emerge. The larvae are mobile and actively move from the terminal bronchioles towards the upper respiratory tract helped by the animal's mucocilliary clearance mechanism and cough. Larvae are then swallowed and then eliminated through the faeces.
In the external environment, these larvae infect a terrestrial gastropod (mainly snails and slugs) that acts as an intermediate host (Hobmaier and Hobmaier 1935, Miró and Gomez 1999, Lopez et al 2005). After two moults in the snails, L1 reaches the third larval stage (L3) which is infective for the definitive host. Although the cats may be infected by the ingestion of snails or slugs (Bowman and Lynn 1995) this is unlikely to be a common route of infection. Snails and slugs are usually eaten by amphibians, reptiles, birds and small mammals, which act as paratenic hosts (Gregory and Munday 1976, Bourdeau 1993). Cats usually become infected by hunting these paratenic hosts. The L3 larvae are released from the paratenic host after ingestion and migrate to the lungs where the life cycle is completed (Bowman and Lynn 1995).
Aelurostrongylus abstrusus is the most common metastrongylidae that uses cats as a definitive host (Sherding 1994, Kirk 1995, Sherding 2001) and is the most common feline pulmonary parasite (Bowman and Lynn 1995). It has a worldwide distribution (Aiello and Mays 1998, Soulsby 1988, Urquhart 1998) and has been reported all over Europe including Spain and Portugal (Cordero del Campillo et al 1994, Goicoa et al 1998, Miró et al 2004). The prevalence of infection with A abstrusus varies between studies. A prevalence of 2–5% has been reported in the USA (Williams 1984, Guerrero 2000) while in Europe the reported prevalence varies between 1% (Epe et al 1993) and 22% (Grabarevic et al 1999). However, these studies are not always comparable due to differences in the sampling areas or the number and characteristics of the sampled animals.
Clinical disease in infected animals is rarely reported (Hungerford 1990, Sherding 1994, Wills and Wolf 1995, Sherding 2001), but signs associated with aelurostrongylosis are similar to other forms of bronchitis (Ettinger and Feldman 2000) including feline allergic bronchitis (Knowlen 1993, Hawkins 1998, 2000).
Although several isolated cases have been reported in Portugal (Da Cruz and De Freitas 1948, Leitão 1963, Peleteiro et al 1990), to date there are no data available regarding the prevalence of this parasite. The main objective of this study was to determine the prevalence of A abstrusus infection in cats from the north-west region of Portugal.
Material and Methods
Sample Size and Study Design
From previous European studies, using an estimated prevalence of infection of 10%, we calculated that 97 cats would need to be sampled to provide an estimate with a 6% margin of error and a 95% confidence level (Win Episcope computer package, Thrusfield et al 2001).
Our study population were stray cats housed in an animal shelter and a municipal service responsible for the capture of stray cats from the city of Porto. Both facilities receive wild, lost or abandoned animals from the north region of Portugal, mainly from an area of 80 km around the city of Porto representing rural and urban cat populations.
All the sampled cats were stray cats greater than 2 months of age. Once inside the shelter or in the municipal service facilities there was no contact with paratenic hosts and, therefore, no opportunity for new infections to develop.
Faecal Samples
Faecal samples were obtained directly from the cages where the animals were housed and collected in individual sterile plastic containers. Samples were kept at 4°C and analysed within 4 h of collection.
Detection and Identification of A Abstrusus
Using the Baermann–Wetzel method (Bowman and Lynn 1995), approximately 30–35 ml of each faecal sample was analysed. Briefly, faecal samples were deposited over a plastic reticulated support (1 mm pores) covered with a double gauze (0.8 mm pores) and preheated water at 30°C was added, approximately 20 ml. After 24 h incubation at room temperature, 3–5 ml of sediment was collected and centrifuged for 3 min at 250 g. The pellet was analysed under the light microscope. Two microscopic preparations were evaluated from each faecal sample. A sample was considered positive when at least one first stage larvae (L1) was identified.
Results
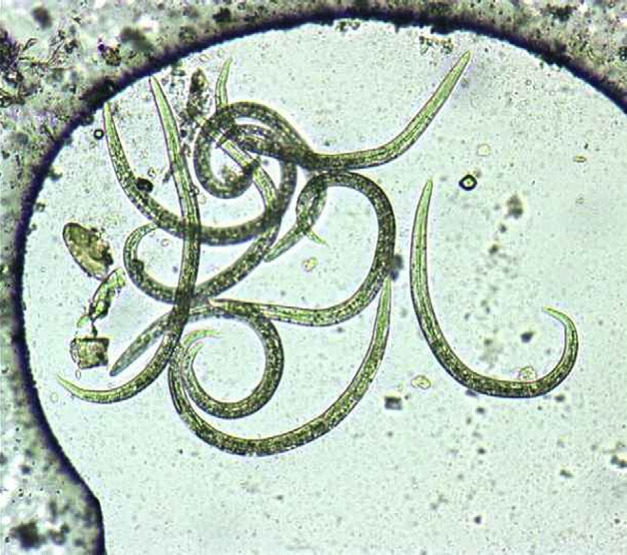
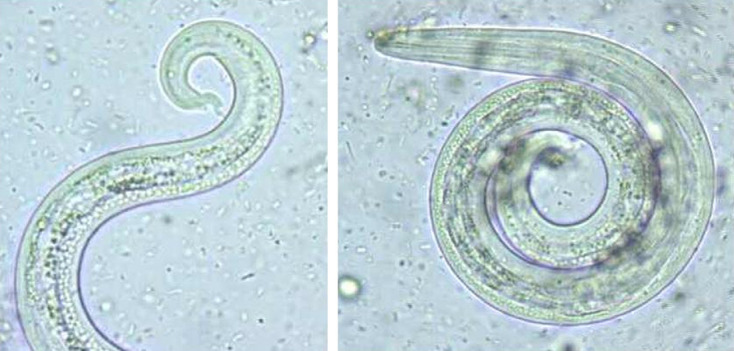
In the period comprised between September of 2003 and May of 2005, faecal samples were collected from 97 stray cats. Using the Baermann–Wetzel method, A abstrusus L1 were detected from 17 cats (17.4%). A high number of L1 was detected in the majority of the positive samples and, therefore, the diagnosis was readily achieved (Fig 1). However, in a few cases only two to three L1 were observed. The Baermann–Wetzel method allowed good preservation of morphological characteristics, so the conical shape of the L1 head and the presence of a subterminal spine in the tail with an S shape were clearly identified (Fig 2).
Fig 1.
Appearance of Baermann–Wetzel sediment in a positive animal eliminating high number of A abstrusus first stage larvae (L1) (100×).
Fig 2.
Morphological characteristics of A abstrusus first stage larvae (L1) (400×): conical shape of the head and subterminal spine in the tail with an S shape.
Discussion
In this study the prevalence of A abstrusus infection in stray cats from the north-west region of Portugal was investigated. This area is characterised by an Atlantic wet climate with relatively high humidity and moderate temperatures. Previous studies have demonstrated that A abstrusus L1 may survive in such humid environments for a few months (Sherding 1994, 2001) or more than 5 months in the presence of organic matter (Pennisi et al 1994). Moreover, both intermediate and paratenic hosts (Castells and Magro 1993, Lopez et al 2005) exist in this region and the relatively high population of cats with access to paratenic hosts means the life cycle can be easily completed.
Our results show that 17.4% [95% confidence interval (CI): 9.9–24.9%] of the stray cats sampled (ie, cats at high risk of infection) were shedding L1 in the faeces. Although this shows quite a high prevalence, we believe this may be an underestimate the true prevalence of the parasite. Firstly, the Baermann–Wetzel technique may only have a 90% sensitivity in detecting the parasite in faeces (Birchard and Sherding 1994). Furthermore, faecal shedding of L1 is not a constant phenomenon (Barsanti and Prestwook 1983, Hawkins 1998, Miró and Gomez 1999, Hawkins 2000) and infected animals also cannot be detected during the prepatent period. It has also been demonstrated that after 5 months of infection, cats cease to eliminate L1 (Pennisi et al 1994) and several studies have shown that the adult parasite may be present for several years in cats' lungs without producing L1 (Bourdeau 1993). Ribeiro et al (2001) also reported eliminating and non-eliminating cycles in the same chronically parasitised cats and, therefore, these cats can be of importance in the maintenance of the A abstrusus life cycle.
As far as we know there are no previous data concerning the prevalence of aelurostrongylosis in Portugal. Our results are similar to those of Grabarevic et al (1999) in Croatia but are higher than those reported in other European countries such as Germany where Epe et al (1993) found only 1% of positive cats, or Spain where Miró et al (2004) described less than 2% of positive cats.
Part of these differences can be explained by climate variations between the areas, but differences in the cat populations studied and in the technique used for the coprological analyses of the samples may help to explain the different results. The Baermann–Wetzel method is considered the most reliable for the diagnosis of this helminth infection (Barsanti and Hubbell 1980, Knowlen 1993, Sherding 1994, Birchard and Sherding 1994, Bowman and Lynn 1995, Wills and Wolf 1995, Aiello and Mays 1998, Hawkins 1998), so the use of other techniques such as the modified Telemann method (as used by Miró et al 2004), or variations in the Baermann–Wetzel technique itself (eg, faecal quantity and incubation time), may lead to underestimations of the true prevalence. In our study we used a high quantity of faeces and a long incubation time (24 h) in order to increase the sensitivity of the method.
The fact that aelurostrongylosis is relatively common in cats from the north-west of Portugal contrasts with a very limited number of clinical reports of this parasite as a cause of disease. This may partly be explained by the fact that the Baermann–Wetzel coprological technique is uncommonly used in Portuguese clinical practice (Speare and Tinsley 1987). Although this parasite commonly results in an asymptomatic infection, where clinical signs develop they may mimic those of other diseases such as feline allergic bronchitis (Knowlen 1993, Hawkins 1998, 2000), and may even respond positively to treatment with corticoids and bronchodilators (Center and Randolf 1993, Sherding 1994, 2001) further complicating the diagnosis.
Use of X-ray, necropsy, tracheal swab (Birchard and Sherding 1994, Aiello and Mays 1998, Owens and Biery 1999) and haematological results (Barsanti and Hubbell 1980) may help in establishing the diagnosis. However, they show less sensitivity than the Baermann technique (Barsanti and Prestwook 1983, Knowlen 1993, Hawkins 2000).
Based on the present study, we can conclude that A abstrusus seems to be highly prevalent among cats of the north-west region of Portugal and, therefore, aelurostrongylosis should be considered in the differential diagnosis of cats presenting with coughing and dyspnoea.
As infected animals cease to eliminate L1 5 months after infection, aelurostrongylosis should be considered in all animals with respiratory problems and animals with pulmonary nodules detected by imaging diagnosis, such as X-ray. In those cats with pulmonary nodules, there will be no sign of a cough as the cat's cough receptors are located exclusively in the trachea.
Given the fact that infection is generally asymptomatic (Center and Randolf 1993, Kirk 1995; Corcoraan 2000) clinicians don't have any reason to ask for coprology analysis, using Baermann technique, in apparent healthy animals. Furthermore, a cough can be a slight signal but when the owners bring the animals to the veterinary practise, they are no longer actively eliminating the L1 that are the main cause of a cough (Williams 1984). A cough is the most commonly communicated sign, but only appears in the first stages of parasitosis, as it is caused by the mechanical irritation of L1 larvae, when they are ascending over the trachea. This is due to the cat's cough receptor anatomy. Receptors are located mainly in large airways, as laryngeal, trachea and tracheal bifurcation areas (Korpas and Tomozi 1979, Corcoraan 2000). The cat doesn't have cough receptors in the alveoli or in alveolar sacs. Consequently, the cough reflex is not elicited by processes within the pulmonary parenchyma (Sherding 1994) where the adult parasite inhabits. Theoretically, cats that eliminate L1 and are seriously affected at the pulmonary level by the parasite or those that cease to eliminate L1 won't present with an effusive cough. Thus, the veterinarian will not perform the Baermann technique.
It is important to detect non-eliminating L1 parasitised cats. Some chronically infected animals may have eliminating and non-eliminating cycles (Hamilton 1968, Ribeiro and Lima 2001): the eliminating ones will perpetuate the parasite life cycle. In addition, if the infected cats don't eliminate L1 in faeces, it will be difficult to distinguish aelurostrongylosis from other respiratory diseases based on clinical signs or radiological findings (Bourdeau 1993, Hawkins 1998).
This study acknowledges the prevalence of feline lungworm parasitosis. Further work is needed to improve the diagnosis of non-eliminating L1 larvae animals (with and without clinical signs) and to recognise the clinical significance of this parasitosis.
Acknowledgements
The authors wish to thank Jorge Ribeiro DMV and Dr. Augusto José Ferreira de Matos DMV (ICBAS, Universidade do Porto) for critical review of the manuscript. Alexandra Guerreiro, DMV from animal shelter of Porto and Adélia Fernandes, DMV from municipal shelter of Porto.
References
- Aiello E., Mays A. The Merck's Veterinary Manual, 8th edn, 1998, Merck and Co.: Philadelphia, pp. 1061–1064, 1117–1121. [Google Scholar]
- Barsanti J.A., Hubbell J. Serum proteins in normal cats and cats infected with Aelurostrongylus abstrusus, American Journal of Veterinary Research 41, 1980, 775–778. [PubMed] [Google Scholar]
- Barsanti J.A., Prestwook A.K. Parasitic diseases of the respiratory tract. Kirk R.W. Current Veterinary Therapy VIII, 1983, WB Saunders: Philadelphia, 241. [Google Scholar]
- Birchard S., Sherding R. Saunders Manual of Small Animal Practice, 1st edn, 1994, WB Saunders: Pennsylvania, p. 579. [Google Scholar]
- Bourdeau P. L'aelurostrongylose féline, Recueil de Médecine Vétérinaire 169, 1993, 409–414. [Google Scholar]
- Bowman D.D., Lynn R.C. Georgis' Parasitology for Veterinarians, 6th edn, 1995, WB Saunders: Philadelphia, pp. 196, 295,–296, 310,, 326,–327, 394–396. [Google Scholar]
- Castells A., Magro M. Guía de mamíferos en libertad en España y Portugal, 1993, Pirámide: Madrid, pp. 179–182 [Google Scholar]
- Center S.A., Randolf Eosinofilia. August J.R. Consultas en Medicina Interna Felina, 1993, Interamericana: Buenos Aires, 377–378. [Google Scholar]
- Corcoraan B. Diagnóstico diferencial da tos, Manual de medicina e cirugía cardiorrespiratória en pequenos animais, 2000, BSAVA: Gloucestershire. [Google Scholar]
- del Campillo M. Cordero, Castañón L., Reguera A. Indice-Catálogo de zooparasitos ibéricos, 2nd edn, 1994, Universidad de León Secretariado de Publicaciones: León, pp. 203, 301,, 309. [Google Scholar]
- Da Cruz A., De Freitas L. Rev Med Vet Lisbon 43, 1948, 222. [Google Scholar]
- Epe C., Ising-Volmer S., Stoye M. Ergebnisse parasitologischer Kotuntersuchungen von Eqiden, Hunden, Katzen und Iageln derf Jahre 1984–1991, Deutsche tierärztliche Wochenschrift 100, 1993, 426–428. [PubMed] [Google Scholar]
- Ettinger S.J., Feldman E.C. Textbook of Veterinary Internal Medicine, 2000, Saunders Publishers: New York. [Google Scholar]
- Goicoa A., Barreiro A., Díez P., Morrondo P. Estudio de la bronconeumonia (Aelurostrongylus abstrusus) del gato, Consulta de Difusión Veterinaria 6, 1998, 64–68. [Google Scholar]
- Grabarevic Z., Curic S., Tustonja A., Artukovic B., Šimec Z., Ramadan K., Zivicnjak T. Incidence and regional distribution of the lungworm Aelurostrongylus abstrusus in cats in Croatia, Veterinary Archives 69, 1999, 279–287. [Google Scholar]
- Gregory G.G., Munday B.L. Internal parasites of feral cats from the Tasmanian Midlands and King Island, Australian Veterinary Journal 52, 1976, 317–320. [DOI] [PubMed] [Google Scholar]
- Guerrero J. The Lungworms, 2000, University of Pennsylvania, School of Veterinary Medicine, Available from: <http://cal.vet.upenn.edu/parasit/ppthtml/Lect7/sld001.htm>. [Google Scholar]
- Hamilton J.M. Studies on reinfestation of the cat with Aelurostrongylus abstrusus, Journal of Comparative Pathology 78, 1968, 69–72. [DOI] [PubMed] [Google Scholar]
- Hawkins E.C. Disorders of the pulmonary parenchyma. Nelson R.W., Couto C.G. Small Animal Internal Medicine, 2nd edn, 1998, Mosby: St Louis, pp. 301–302, 263,, 1242. [Google Scholar]
- Hawkins E.C. Disorders of the lower respiratory tract. Section IX—The respiratory system. Ettinger S.J., Feldman E.C. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat, 2000, WB Saunders: Philadelphia, 1070–1091. [Google Scholar]
- Hobmaier M., Hobmaier A. Intermediate host of Aelurostrongylus abstrusus of the cat, Proceedings of the Society for Experimental Biology and Medicine 32, 1935, 1641–1647. [Google Scholar]
- Hungerford T.G. Gastrointestinal and respiratory tract parasites of cats. Hungerford T.G. Diseases of Livestock, 9th edn, 1990, McGraw-Hill: Sydney, pp. 1488–1495, 941–943. [Google Scholar]
- Kirk R.W. Cardiopulmonary diseases, Current Veterinary Therapy XII. Small Animal Practice, 1995, WB Saunders: Philadelphia, 896–897. [Google Scholar]
- Knowlen G.G. El gato con tos. August J.R. Consultas en Medicina Interna Felina, 1993, Interamericana: Buenos Aires, 198–201. [Google Scholar]
- Korpas J., Tomozi Z. Cough and other respiratory reflexes. Herzoy Progress in Respiratory Research vol. 12, 1979, Karger: Basel, 15. [Google Scholar]
- Leitão J.L. Parasitas dos animais domésticos em Portugal metropolitano, 1963, Fundación Caloustre Gulbenkian: Lisboa. [Google Scholar]
- Lopez C., Panadero R., et al. Larval development of Aelurostrongylus abstrusus (Nematoda, Angiostrongylidae) in experimental infected Cernuella (Cernuella) virgata (Mollusca, Helicidae), Parasitology Research 95, 2005, 13–16. [DOI] [PubMed] [Google Scholar]
- Miró G., Gomez M. Parasitosis respiratorias y cardiopulmonares. Cordero M., et al. Parasitologia Veterinaria, 1a edición, 1999, Mc Graw-Hill-Interamericana: Madrid, 696–699. [Google Scholar]
- Miró G., Montoya A., Jiménez S., et al. Prevalence of antibodies to Toxoplasma gondii and intestinal parasites in stray, farm and housed cats in Spain, Veterinary Parasitology 126, 2004, 249–255. [DOI] [PubMed] [Google Scholar]
- Owens J., Biery D. Radiographic Interpretation for the Small Animal Clinician, 2nd edn, 1999, Williams and Wilkins: Baltimore, p. 168. [Google Scholar]
- Peleteiro M.C., Meireles J.F.S., Bento J.L. Um caso pneumonia parasitaria em gato por Aelurostrongylus abstrusus. (1988–1989), 1990, Anais da Faculdade de Medicina-Veterinaria: Lisboa, pp. 25–26, 73–82. [Google Scholar]
- Pennisi M.G., Niutta P.P., Giannetto S. Parásitos pulmonares en el gato (Aelurostrongylus abstrusus), Revue de Médecine Vétérinaire 11, 1994, 568–572. [Google Scholar]
- Ribeiro V.M., Lima W.S. Larval production of cats infected and re-infected with Aelurostrongylus abstrusus (Nematoda: Protostrongylidae), Revue de Médecine Vétérinaire 152, 2001, 815–829. [Google Scholar]
- Sherding R.G. The Cat Diseases and Clinical Management, 2nd edn, 1994, Churchill Livingstone: New York, pp. 615–617, 592–621. [Google Scholar]
- Sherding R. (2001) Broncho-pulmonary parasite infections. In: Proceedings of the World Small Animal Veterinary Association (WSAVA) Congress, September, Vancouver, Canadá.
- Soulsby E.J. Parasitología y Enfermedades Parasitarias en Animales Domésticos, 7a edición, 1988, Interamericana: México, p. 280. [Google Scholar]
- Speare R., Tinsley D.J. Survey of cats for Strongyloides felis, Australian Veterinary Journal 64, 1987, 191–192. [DOI] [PubMed] [Google Scholar]
- Thrusfield M., Ortega C., de Blas I., Noordhuizen J.P., Frankena K. Win Episcope 2.0: improved epidemiological software for veterinary medicine, Veterinary Record 148, 2001, 567–572. [DOI] [PubMed] [Google Scholar]
- Urquhart G.M. Helmintologia Veterinaria. Urquhart G.M., Armour J., Duncan J.L., Dunn A.M., Jennings F.W. Parasitología Veterinaria, 2nd edn, 1998, Guanabara Koogan: Rio de Janeiro, 54–57. [Google Scholar]
- Williams J.F. Continental parasitarias del tracto respiratorio. Kirk R.W. Current Veterinary Therapy VII, 1984, Compañía Continent Continental: México. [Google Scholar]
- Wills J., Wolf A. Manual de Medicina Felina, 1995, Acribia: Zaragoza, pp. 142–143 [Google Scholar]