Abstract
A 6-month-old domestic shorthair female cat was presented with suspected diaphragmatic hernia (DH) that was later confirmed by thoracic radiography. The cat underwent exploratory celiotomy with a diaphragmatic rupture (DR) repair and recovered. Six days later, it was represented with vomiting and anorexia. Megaoesophagus (MO) and gastric dilatation were diagnosed by contrast radiography. A second celiotomy revealed no abnormalities and gastropexy was performed. Endoscopy demonstrated MO, oesophagitis and gastro-oesophageal reflux. MO persisted for several weeks and was an unexpected complication as no association between DR (or DH) and MO has never been described in the veterinary literature. The cat was treated medically with aggressive prokinetic and antacid therapy along with prolonged temporary oesophageal diversion (percutaneous endoscopic gastrostomy tube) with an excellent outcome.
Megaoesophagus (MO) is associated with generalised oesophageal dilatation due to neuromuscular disorders and is classified as congenital or acquired and as primary (idiopathic) or secondary (Twedt 1995, Mears and Jenkins 1997). The latter results from various conditions affecting the oesophageal neuromuscular function such as neuromuscular, endocrine or inflammatory disorders, toxic and infective agents, and oesophageal obstructive lesions (Jones et al 1989, Guilford 1990, Strombeck and Guilford 1996a,b). All forms of MO are uncommon in cats (Twedt 1995). Idiopathic congenital MO was previously suspected in cats (Hoenig et al 1990). A form of congenital MO, associated with gastric dilatation and outflow obstruction was described in Siamese kittens (Jones et al 1989).
Feline acquired idiopathic MO has also been described, but its pathogenesis remains unclear (Forbes and Leishman 1985). Secondary MO may result from several conditions that disrupt the neural reflex of swallowing or oesophageal muscle function (Guilford 1990). It has been speculated that differences in the amount of smooth muscle and presence of autonomic rather then somatic innervation account for the lower prevalence of secondary MO in cats compared to dogs (Twedt 1995). Oesophageal outflow obstruction has been shown to have major deleterious effects on oesophageal function in cats by decreasing its contraction amplitudes, duration of peristalsis and velocity (Mittal et al 1990). Experimentally, induced increased abdominal pressure in cats has been shown to correlate with slowing of oesophageal peristalsis and prolonged opening of the lower oesophageal sphincter (LES) (Ren et al 1991).
Approximately 85% of all feline and canine cases of diaphragmatic hernia (DH) or diaphragmatic rupture (DR) are caused by trauma (Al-Nakeeb 1971, Wilson et al 1971, Wilson and Hayes 1986), while congenital Peritoneopleural hernia has rarely been reported in cats (Keep 1950, Vages et al 1997).
Congenital DH has been associated with multiple anomalies in human patients, and transient MO was reported in a human neonate with congenital DH (Makhoul et al 2001). Gastro-oesophageal reflux (GOR) is a well-known complication in human congenital DH (Koot et al 1993, Nagaya et al 1994). In veterinary medicine, no direct association between DH (or DR), GOR and MO has ever been described, although vagal and recurrent laryngeal nerve anomalies were described in rats with experimentally induced congenital DH (Martinez et al 2004).
This report describes a case of transient MO and oesophagitis that has occurred unexpectedly following DR repair in a cat. The MO was treated medically and the case was followed to full recovery. The possible pathogenesis of the MO in this situation is discussed.
A 6-month-old domestic shorthair indoors/outdoors cat was referred to the Hebrew University Veterinary Teaching Hospital (HUVTH) with a history of sudden, acute dyspnoea and cyanosis that had developed upon a routine induction of anaesthesia for an elective spay. No signs were observed before this procedure. When the clinical signs occurred, the cat was intubated and positive pressure ventilation with 100% oxygen was applied, and it recovered. Auscultation revealed decreased respiratory and heart sounds on the right side of the thorax, and a DR (or DH) was suspected. The cat was immediately referred to the HUVTH.
Upon admission (day 1) at the HUVTH, physical examination revealed mild tachycardia (heart rate 180/min), tachypnoea (respiratory rate 60/min) and presence of borborygmi with dull heart and respiratory sounds in the right hemithorax. No other abnormalities were detected. Thoracic radiography (Fig 1) demonstrated abdominal organs (liver lobes and gas-filled small intestinal loops) within the right thoracic cavity, and confirmed the diagnosis of peritoneopleural communication. Preoperative complete blood count (CBC) and serum biochemistry were unremarkable. The cat was anaesthetised (premedication with butorphanol [Torbugesic; Fort Dodge, 0.4 mg/kg IM], induction with propofol [Diprofol; Taro Pharmaceutical Industries, 1 mg/kg IV] and diazepam [Assival; Teva Pharmaceutical Industries, 0.5 mg/kg IV] and gas anaesthesia [Isoflurane; Nicholas Piramal, in 100% O2, 2 l/min via an endotracheal tube] with positive pressure ventilation when needed) and underwent an exploratory midline celiotomy that revealed a simple right ventrolateral radial tear of the muscular diaphragm. The margins of this tear appeared thick and fibrous, suggestive of a chronic, traumatic acquired DR. Three liver lobes and most of the small intestine were located in the right hemithorax. The pylorus and the duodenum were displaced cranially towards the diaphragmatic tear. The displaced viscera were reduced to the abdominal cavity and arranged in their correct anatomic position. A 12-FG chest tube was placed in the right thoracic wall through the eighth intercostal space and secured routinely. The diaphragmatic tear was sutured in simple continues pattern with polydeoxanon 3/0 (Serasynth; Serag-Wiessner) using no tension. The linea alba was closed with simple interrupted sutures (Nylon 3/0, monosof; Syneture) with some tension due to the reduction of the abdominal organs. No complications were observed during surgery, and recovery from anaesthesia was uneventful. Peri- and postoperative cefazolin (Kefazin; Vitamed, 25 mg/kg IV q8 h) was administered for 3 days. Routine intermittent chest tube drainage (q2–4 h) was performed over the next 24 h. After this period it was no longer productive and no respiratory abnormalities were observed so the chest tube was pulled out.
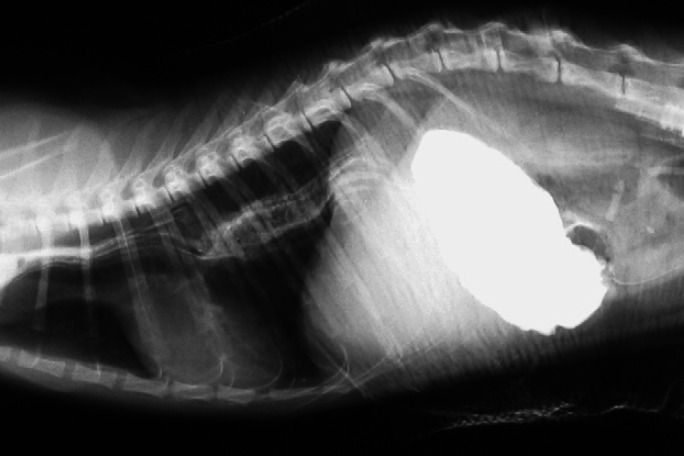
Fig 1.
A right lateral thoracic radiograph at day 1. Note the abdominal organs (liver lobes and gas-filled small intestines) within the thoracic cavity, compatible with the diagnosis of peritoneopleural hernia.
Over the next 2 days the cat remained bright and alert and ate well but vomited food on two occasions. This was thought to be due to increased abdominal pressure consequent to reduction and manipulation of the abdominal organs. These episodes were well controlled with metoclopramide (Pramin; Rafa Laboratories, 0.4 mg/kg SC q8 h). The cat was discharged 3 days postoperatively with cephalexin oral liquid suspension (Ceforal; Teva Pharmaceutical Industries, 25 mg/kg PO q8 h for 5 days), but this drug was given by the owners only twice over the first day after discharge.
The cat was represented 3 days later (day 6) with signs of anorexia and vomiting or regurgitation as well as mild (6–8%) dehydration. Thoracic and abdominal radiography demonstrated gastric dilatation, MO and right oesophageal displacement (Fig 2). The diaphragm appeared normal and there was no evidence to support recurrence of DR. The cat was hospitalised, and treated with intravenous lactated Ringer's solution, ranitidine (Zantac; GlaxoSmithKline, 2 mg/kg IV q12 h) and metoclopramide. An exploratory celiotomy was performed a day later (day 7) to exclude any organ malposition or iatrogenic lesions in the hiatus and LES region. The size, shape, colour and position of the abdominal organs were normal. The diaphragm appeared whole and was reopened ventromedially to the previous suture line to explore the thoracic cavity. No abnormalities were observed with exclusion of severe oesophageal dilatation. A left incisional gastropexy was performed. Postoperative treatment included cefazolin, metoclopramide (1 mg/kg/d IV in a constant infusion rate (CRI)), ranitidine, butorphanol (Turbogesic; Fort Dodge, 0.4 mg/kg SC q6 h) and sucralfate (Ulsanic; Teva Pharmaceutical Industries, 250 mg PO q8 h).
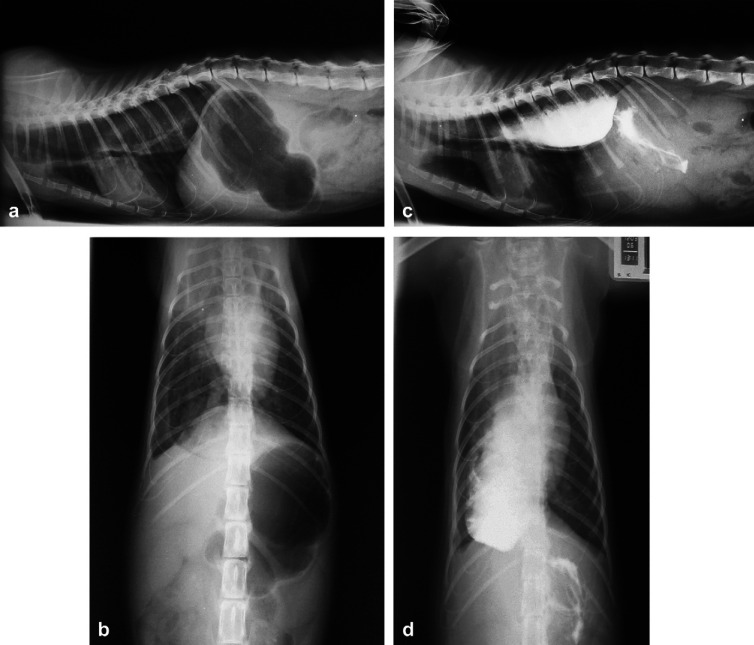
Fig 2.
Thoracic and abdominal radiographs at day 6. (a) Right lateral survey, (b) ventrodorsal survey, (c) right lateral contrast, (d) ventrodorsal contrast, demonstrating gastric dilatation, and MO. No sedation or anaesthesia was used while obtaining these radiographs. Note that the diaphragm appears normal.
The cat continued to present signs of anorexia, regurgitation, vomiting and hypersalivation over the next 24 h. On day 8, endoscopy was performed under general anaesthesia. This procedure revealed severe oesophageal dilatation with its displacement to the right, severe oesophageal mucosal erosions and oesophagitis (Fig 3). During the endoscopy, the LES was open and GOR was observed. The gastric mucosa appeared normal. A percutaneous endoscopic gastrostomy (PEG) tube was placed.
Fig 3.
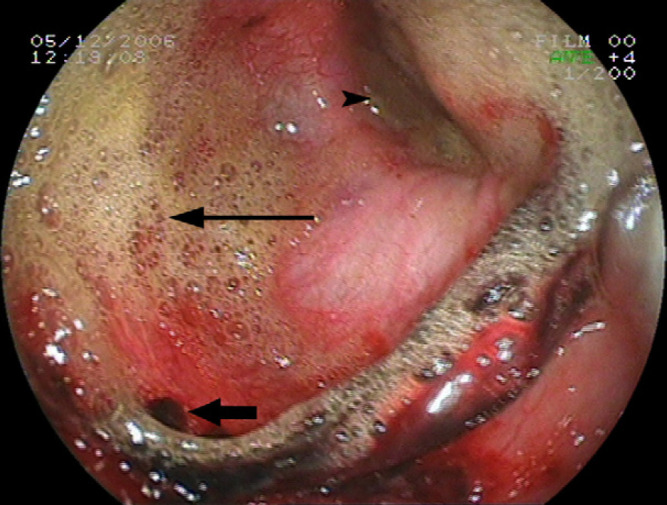
An endoscopic view of the evaluation performed at day 8 showing oesophageal dilatation, severe mucosal erosions and oesophagitis (thick arrow). Note the semi opened LES (arrow head) and marked GOR (thin arrow).
Over the next 10 days, the signs of ileus, regurgitation and hypersalivation resolved slowly. Tube feeding of small volumes of several liquid diet formulae (High Calorie [Eukanuba; Iams] mixed with Nutran [Nestle]) q2 h failed due to delayed gastric emptying and ileus. Large residual gastric fluid volumes prevented the scheduled feedings. Medical treatment included cisapride (Prepulsid; Janssen–Cilag, 2.5 mg, via the PEG tube, q8 h), lactulose (Laevac; Kabi Fresenius, 2 ml via the PEG tube q12 h), erythromycin (Erythro-Teva; Teva Pharmaceutical Industries, 0.5 mg/kg via the PEG tube, q8 h), metoclopramide, omeprazole (Losec; Teva Pharmaceutical Industries, 0.7 mg/kg via the PEG tube, q24 h), sucralfate and butorphanol. On day 18, tube feedings with liquid diets were eventually successful, providing 25% of the cat's basal energy requirements (BER). Thoracic radiography, performed on that day, demonstrated an improvement of the MO. Over the next few days, tube feeding volumes were gradually increased, reaching twice the cat's BER, divided three times daily.
On day 35, the cat was discharged with the PEG tube still in place and was fed (same liquid diets as above) and medicated (sucralfate, omeprazole and cisapride) by the owners through the tube. One week later (day 43), the cat continued to improve and contrast thoracic radiography revealed no abnormalities (Fig 4). Oral feeding began in combination with PEG tube feeding. Three weeks later, the cat was able to eat normally, had gained weight and no longer required PEG tube feeding (day 64). At this time the PEG tube was pulled out and all treatments were discontinued. Six months later, the owners reported that the cat was clinically improved and appeared normal.
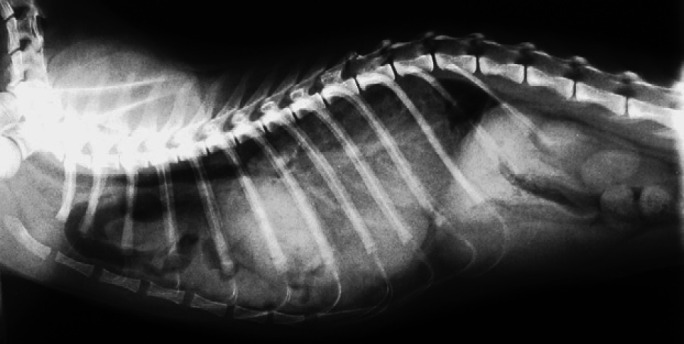
Fig 4.
A right lateral contrast thoracic radiograph performed on day 43 demonstrating significant improvement of the oesophagus.
Congenital diaphragmatic defects in dogs and cats are usually located in the dorsolateral region when intermediate parts of the left lumbar muscles or both crura and tendon are absent, and such cases rarely survive the neonatal period (Feldman et al 1968, Valentine et al 1988, Hunt and Johnson 2003), thus it seems unlikely that such a condition existed in the present cat. Feline traumatic, DRs have been reported to be circumferential, radial or a combination of these, with a prevalence of 59%, 18% and 23% of all traumatic ruptures, respectively (Garson et al 1980). Although clinical signs were absent in this cat prior to the first induction of anaesthesia at the age of 6 months, the presence of a thickened fibrous tissue at the diaphragmatic tear as well as its location suggest that this diaphragmatic defect was a chronic, probably acquired and of a traumatic aetiology.
An asymptomatic oesophageal dilatation during the neonatal period has been reported rarely in human medicine but not in cats (Feng and Kong 1999). Although presence of an asymptomatic MO before the surgery cannot be excluded, it was most probably a post surgical sequel, as the cat had no clinical or radiographic signs of MO (eg, regurgitation, vomiting and hypersalivation) prior to the procedure, and the initial clinical signs of MO appeared only 2 days postoperatively. The latter might have been caused by immediate surgery-related complications such as increased abdominal pressure, postoperative pancreatic irritation, post anaesthetic oesophagitis, or drug intolerance.
Irritant oesophagitis in cats had been reported due to several oral medications (German et al 2005, Beatty et al 2006) and formulations (Westfall et al 2001). However, in this case, during hospitalisation all drugs were given intravenously or into the PEG tube. The only oral medication used after the first surgery was a liquid cephalexin suspension, and no tablets or capsules were medicated. This formulation, to the best of our knowledge, has never been reported to induce oesophagitis. In addition, the exposure to this drug was minimal, thus we believe it was not responsible for the severe oesophagitis in this cat.
The exact mechanism leading to formation of MO in this cat is unclear. Possibly, the long-standing preoperative malposition and cranial displacement of the stomach and the duodenum led to kinking, oedema and LES malfunction resulting in partial obstruction and oesophageal dilatation. However, preoperative GOR, if present, was subclinical making this mechanism unlikely. Moderately increased intragastric pressure produced by abdominal compression leads to increased LES pressure that remains above the intragastric pressure and thus protects against GOR. In contrast, markedly increased intragastric pressure results in LES relaxation, allowing development of GOR (Strombeck and Guilford 1996a,b). In our cat, reduction of the chronically displaced abdominal organs and with repair of the DR probably led to a significant increase in the abdominal pressure within a short time. Concurrent correction of the left displacement and stretching of the oesophagus might have decreased the mechanical resistance of gastro-oesophageal junction to GOR and subsequently to oesophageal erosions and inflammation.
Previous studies in brachycephalic dogs suggested that chronic respiratory depression promotes GOR and that in over 80% of such cases, gastrointestinal tract signs had resolved upon relief of the upper respiratory tract obstruction. It has been speculated that the combination of increased abdominal pressure generated by recurrent vomiting as well as the negative intrathoracic pressure generated by increased inspiratory efforts promotes GOR (Poncet et al 2005, 2006). Possibly, in this cat, the combination of the chronically displaced abdominal organs within the thoracic cavity with subsequent lung compression and the postoperative chest drainage resulted in a relatively low intrathoracic pressure. This might have induced a greater propensity to GOR. As the chest tube was pulled out after 24 h from surgeries and no respiratory efforts were observed, the role of this mechanism in the pathogenesis of GOR in this case remains unclear.
Bilateral peripheral vagal lesions due to inflammation, trauma or surgery are necessary to induce oesophageal hypomotility and MO (Twedt 1995). Such damage might have occurred either congenitally, at the time of trauma leading to the DR or during surgery. Congenial oesophageal disease seems unlikely in this cat as it fully recovered eventually and full oesophageal function was restored. Moreover, an association between DH and MO secondary to vagal or recurrent laryngeal nerve lesions has been reported only in rats with congenital rather than traumatic ruptures (Martinez et al 2004). In our patient, the tear and surgical intervention were nowhere near the vagus nerve position and thus could not account for the post surgical complications leading to MO. To the best of our knowledge, transient postoperative neurapraxia due to manipulations or reduction of abdominal organs has never been documented in the literature.
There is a dependent control of the LES by the caudal oesophagus, and this control is lost when the caudal part of the oesophagus is diseased, such as occurs with oesophagitis. This has been demonstrated experimentally in cats by disruption of the reception of sensory information from the oesophagus that maintains LES closure (Eastwood et al 1975, Strombeck and Guilford 1996a,b). Caudal oesophageal distension due to GOR might have aggravated LES relaxation. Such distension has been shown to stimulate vagus-mediated LES relaxation (Price et al 1979).
The association of delayed gastric empting and feline MO has been documented, but the exact mechanism remains unclear (Pearson et al 1974). Experimental gastric dilatation in cats resulted in decreased oesophageal motility (Mittal et al 1990, Ren et al 1991), and transient MO has been reported after occurrence of canine gastric dilatation and volvulus (Oakes and Pechman 1992). In one report, pyloric hypertrophy with gastric outflow obstruction was described in a cat along with MO that resolved after pyloroplasty (Jones et al 1989). The possibility that the DR repair induced gastric outflow abnormalities, gastric dilatation and subsequent GOR, oesophagitis and MO cannot be excluded in this case.
In the present cat, the appearance of clinical signs after surgery along with the radiographic and endoscopic findings suggests that the DR repair led to gastro-oesophageal and LES malfunction. These were followed by gastric dilatation, GOR, oesophagitis and MO. Whatever the mechanism leading to GOR in this cat was, we believe that when GOR had developed it induced severe oesophagitis and the latter probably had a major role in the formation of MO. Oesophagitis secondary to GOR was rarely reported in cats and little is known of its typical clinical presentation (Wilkinson 1970, Lobetti and Leisewitz 1996, Han et al 2003) making this an unexpected complication in the present case. Gastropexy and prolonged temporary oesophageal diversion using the PEG tube, with aggressive prokinetic and antacid therapy allowed oesophageal healing and recovery of its function.
References
- Al-Nakeeb S.M. Canine and feline traumatic diaphragmatic hernias, Journal of the American Veterinary Medical Association 159, 1971, 1422–1427. [PubMed] [Google Scholar]
- Beatty J.A., Swift N., Foster D.J., Barrs V.R. Suspected clindamycin-associated oesophageal injury in cats: five cases, Journal of Feline Medicine and Surgery 8, 2006, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood G.L., Castell D.O., Higgs R.H. Experimental oesophagitis in cats impairs lower esophageal sphincter pressure, Gastroenterology 69, 1975, 146–153. [PubMed] [Google Scholar]
- Feldman D.B., Bree M.M., Cohen B.J. Congenital diaphragmatic hernia in neonatal dogs, Journal of the American Veterinary Medical Association 153, 1968, 942–944. [PubMed] [Google Scholar]
- Feng F.H., Kong M.S. Congenital esophageal stenosis treated with endoscopic balloon dilation: report of one case, Acta Paediatrica Taiwanica 40, 1999, 351–353. [PubMed] [Google Scholar]
- Forbes D.C., Leishman D.E. Megaesophagus in the cat, Canadian Veterinary Journal 26, 1985, 354–356. [PMC free article] [PubMed] [Google Scholar]
- Garson H.L., Dodman N.H., Baker G.J. Diaphragmatic hernia. Analysis of fifty-six cases in dogs and cats, Journal of Small Animal Practice 21, 1980, 469–481. [DOI] [PubMed] [Google Scholar]
- German A.J., Cannon M.J., Dye C., Booth M.J., Pearson G.R., Reay C.A., Gruffydd-Jones T.J. Oesophageal strictures in cats associated with doxycycline therapy, Journal of Feline Medicine and Surgery 7, 2005, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford W.G. Megaesophagus in the dog and cat, Seminars in Veterinary Medicine and Surgery (Small Animal) 5, 1990, 37–45. [PubMed] [Google Scholar]
- Han E., Broussard J., Baer K.E. Feline esophagitis secondary to gastroesophageal reflux disease: clinical signs and radiographic, endoscopic, and histopathological findings, Journal of the American Animal Hospital Association 39, 2003, 161–167. [DOI] [PubMed] [Google Scholar]
- Hoenig M., Mahaffey M.B., Parnell P.G., styles M.E. Megaesophagus in two cats, Journal of the American Veterinary Medical Association 196, 1990, 763–765. [PubMed] [Google Scholar]
- Hunt G.B., Johnson K.A. Diaphragmatic, pericardial and hiatal hernia. Slatter D.H. Textbook of Small Animal Surgery, 3rd edn, 2003, WB Saunders: Philadelphia, 471–487. [Google Scholar]
- Jones B.D., Jergens A.E., Guilford W.J. Diseases of the esophagus. Ettinger S.J. Textbook of Veterinary Internal Medicine, 3rd edn, 1989, WB Saunders: Philadelphia, 1255–1277. [Google Scholar]
- Keep J.M. Congenital diaphragmatic hernia in a cat, Australian Veterinary Journal 26, 1950, 193–196. [DOI] [PubMed] [Google Scholar]
- Koot V.C., Bergmeijer J.H., Bos A.P., Molenaar J.C. Incidence and management of gastro-oesophageal reflux after repair of congenital diaphragmatic hernia, Journal of Pediatric Surgery 28, 1993, 48–52. [DOI] [PubMed] [Google Scholar]
- Lobetti R., Leisewitz A. Gastroesophageal reflux in two cats, Feline Practice 24, 1996, 5–9. [Google Scholar]
- Makhoul I.R., Shoshany G., Smolkin T., Epelman M., Sujov P. Transient mega-esophagus in a neonate with congenital diaphragmatic hernia, European Radiology 11, 2001, 867–869. [DOI] [PubMed] [Google Scholar]
- Martinez L., Gonzales-Reyes, Burgos E., Tovar J.A. The vagus and recurrent laryngeal nerves in experimental congenital diaphragmatic hernia, Pediatric Surgery International 20, 2004, 253–257. [DOI] [PubMed] [Google Scholar]
- Mears E.A., Jenkins C.C. Canine and feline megaesophagus, Compendium on Continuing Education for the Practicing Veterinarian 19, 1997, 313–326. [Google Scholar]
- Mittal R.K., Ren J., McCallum R.W., Shaffer H.A., Sluss J. Modulation of feline esophageal contractions by bolus volume and outflow obstruction, American Journal of Physiology (Gastrointestinal and Liver Physiology) 258, 1990, 208–215. [DOI] [PubMed] [Google Scholar]
- Nagaya M., Akatsuka H., Kato J. Gastroesophageal reflux occurring after repair of congenital diaphragmatic hernia, Journal of Pediatric Surgery 29, 1994, 1447–1451. [DOI] [PubMed] [Google Scholar]
- Oakes M.G., Pechman R.D. What is your diagnosis? Gastric volvulus, megaesophagus and aspiration pneumonia, Journal of the American Veterinary Medical Association 200, 1992, 835–836. [PubMed] [Google Scholar]
- Pearson H., Gaskell C.J., Gibbs C., Waterman A. Pyloric and oesophageal dysfunction in the cat, Journal of Small Animal Practice 15, 1974, 487–501. [DOI] [PubMed] [Google Scholar]
- Poncet C., Dupre G., Freiche V., Estrada M., Poubanne Y., Bouvy B. Prevalence of gastrointestinal tract lesions in brachycephalic dogs with upper respiratory syndrome: clinical study in 73 cases (2000–2003), Journal of Small Animal Practice 46, 2005, 273–279. [DOI] [PubMed] [Google Scholar]
- Poncet C., Dupre G., Freiche V., Bouvy B. Long-term results of upper respiratory syndrome surgery and gastrointestinal tract medical treatment in 51 brachycephalic dogs, Journal of Small Animal Practice 47, 2006, 137–142. [DOI] [PubMed] [Google Scholar]
- Price L.M., El-Sharkawy T.Y., Mui H.Y., Diamant N.E. Effect of bilateral cervical vagotomy on balloon-induced lower esophageal sphincter relaxation in the dog, Gastroenterology 77, 1979, 324–329. [PubMed] [Google Scholar]
- Ren J.L., Dodds W.J., Martin C.J., Dantas R.O., Mittal R.K., Harrington S.S., Kern M.K., Brasseur J.G. Effect of increased intra-abdominal pressure on peristalsis in feline esophagus, American Journal of Physiology (Gastrointestinal and Liver Physiology) 261, 1991, 417–425. [DOI] [PubMed] [Google Scholar]
- Strombeck D.R., Guilford W.G. Disease of swallowing. Guilford W.G., Cener S.A., Strombeck D.R., Williams D.A., Meyer D.J. Strombeck's Small Animal Gastroenterology, 3rd edn, 1996a, WB Saunders: Philadelphia, 216–268. [Google Scholar]
- Strombeck D.R., Guilford W.G. Pharynx and esophagus: normal structure and function. Guilford W.G., Cener S.A., Strombeck D.R., Williams D.A., Meyer D.J. Strombeck's Small Animal Gastroenterology, 3rd edn, 1996b, WB Saunders: Philadelphia, 202–210. [Google Scholar]
- Twedt D.C. Disease of the esophagus. Ettinger S.J. Textbook of Veterinary Internal Medicine, 4th edn, 1995, WB Saunders: Philadelphia, 1124–1142. [Google Scholar]
- Vages A.K., Bertrand S., Hill R.C., Neuwirth L., Schaer M. True diaphragmatic hernia in a cat, Veterinary Radiology and Ultrasound 38, 1997, 116–119. [DOI] [PubMed] [Google Scholar]
- Valentine B.A., Cooper B.J., Dietze A.E., Noden D.M. Canine congenital diaphragmatic hernia, Journal of Veterinary Internal Medicine 2, 1988, 109–112. [DOI] [PubMed] [Google Scholar]
- Westfall D.S., Twedt D.C., Steyn P.F., Oberhauser E.B., VanCleave J.W. Evaluation of esophageal transit of tablets and capsules in 30 cats, Journal of Veterinary Internal Medicine 15, 2001, 467–470. [DOI] [PubMed] [Google Scholar]
- Wilkinson G.T. Chronic papillomatous esophagitis in a young cat, Veterinary Record 87, 1970, 355–356. [DOI] [PubMed] [Google Scholar]
- Wilson G.P., Hayes H.M. Diaphragmatic hernia in the dog and cat: a 25-year overview, Seminars in Veterinary Medicine and Surgery (Small Animal) 1, 1986, 318–326. [PubMed] [Google Scholar]
- Wilson G.P., Newton C.D., Burt J.K. A review of 116 diaphragmatic hernias in dogs and cats, Journal of the American Veterinary Medical Association 159, 1971, 1142–1145. [PubMed] [Google Scholar]