Abstract
Eight cases of glomerular disease in young, related Abyssinian cats are described. Haematuria was the most consistent feature. Six cats developed the nephrotic syndrome. The short-term prognosis was good for cats with haematuria and fair for cats with the nephrotic syndrome as oedema resolved in three of the six cats. Light microscopic examination of renal biopsies from three cats was considered normal or revealed only mild abnormalities. In the three cases subjected to necropsy, histological abnormalities included mild mesangial hypercellularity and adhesions between the glomerular tuft and Bowman's capsule consistent with a focal proliferative glomerulopathy. Further investigation into this glomerulopathy will require ultrastructural and immunohistochemical studies to characterise the glomerular abnormality and genetic analyses to investigate its potential to be an inherited disease. Glomerular disease, potentially a familial one, should be considered in the investigation of persistent haematuria or proteinuria in Abyssinian and related cats.
Haematuria may be caused by lesions of the upper or lower urinary tract, or a systemic coagulopathy. In dogs and cats, haematuria is most often due to lower urinary tract disease (Forrester 2004). Renal causes of haematuria include glomerular diseases and non-glomerular conditions including neoplasia, nephroliths, telangiectasia, trauma, pyelonephritis and idiopathic renal haematuria (Forrester 2004). Haematuria has not been a prominent feature of glomerular disease in cats and dogs (Nash et al 1979, Cook and Cowgill 1996). In contrast, glomerular disease is a well-recognised cause of persistent haematuria in human patients and is most often due to immunoglobulin A (IgA) nephropathy, hereditary nephritis or inflammatory glomerulopathies (Falk et al 2004).
Acquired glomerular disease has been reported in both dogs and cats but there are some notable species differences. Glomerular pathology is not commonly diagnosed in cats with renal failure (DiBartola et al 1987, Minkus et al 1994). In contrast, necropsy surveys suggest that glomerular disease is the cause of 50–90% of canine renal diseases. Descriptions of canine glomerular disease include proliferative (mesangial, endocapillary and crescentic), membranous, membranoproliferative, sclerosing and minimal change diseases (Muller-Peddinghaus and Trautwein 1977, Macdougall et al 1986, Vilafranca et al 1994). Correlations between morphological changes, clinical features, prognosis, treatment and even aetiology have been made for many human glomerular diseases. In small animal medicine, such correlations have not yet been determined. Concurrent disease, for example, neoplasia and chronic inflammatory skin disease, is common in dogs with glomerulonephritis (57–88% of cases) and the granular immunofluorescence pattern most often described reflects injury due to immune complex deposition (Jaenke and Allen 1986, Center et al 1987, Cook and Cowgill 1996).
Although there are several reports of glomerulonephropathies in cats with feline leukaemia virus (FeLV) associated disease, feline infectious peritonitis and liver disease, the most commonly reported glomerular disease is membranous glomerulopathy (Glick et al 1978, Hayashi et al 1982, Johnson et al 1983). Primarily a disease of young cats, affecting male cats twice as often as females, this disease can manifest as either the nephrotic syndrome, renal failure or both (Nash et al 1979, Arthur et al 1986). Unlike glomerulopathies in dogs, significant concurrent disease has not been reported in cases of feline membranous glomerulopathy (Arthur et al 1986).
Familial renal diseases have been recognised in many different canine breeds. Specifically, familial forms of glomerular diseases include amyloidosis, hereditary nephritis and membranoproliferative glomerulonephritis (DiBartola 2005). Hereditary nephritis refers to a group of inherited disorders of the glomerular basement membrane. X-linked forms of hereditary nephritis have been diagnosed in Samoyeds (Jansen et al 1984) and a family of mixed breed dogs (Lees et al 1999), autosomal dominant forms exists in Bull Terriers (Hood et al 1990) and Dalmatian dogs (Hood et al 2002) and an autosomal recessive form has been described in English Cocker Spaniels (Lees et al 1998). The major forms of familial haematuria in people, thin basement membrane nephropathy and Alport's syndrome, are due to mutations in genes encoding for alpha 3, 4 or 5 chains of type IV collagen (Kashtan 2005). Specific mutations of the genes encoding collagen IV alpha 5 chains have been identified in Samoyeds and Novasota families (Kashtan 2002). While dogs with confirmed hereditary nephritis are frequently haematuric, proteinuria is the most prominent clinical feature (Lees et al 1999).
In contrast, the only recognised familial kidney diseases in cats are renal amyloidosis in Abyssinians and polycystic kidney disease in Persian and Persian-cross cats (Boyce et al 1984, Biller et al 1996). In Abyssinians, amyloid is deposited predominantly in the intersitium while glomerular deposits are variable, therefore, cats with renal amyloidosis typically develop chronic renal failure (CRF) (Chew et al 1982). In dogs with amyloidosis, amyloid is preferentially deposited in the glomerulus resulting in proteinuria (Greco 2001).
This paper describes glomerular disease in eight, closely related Abyssinian cats, characterised by haematuria and proteinuria.
Methods
Four cases of renal disease in young, related Abyssinians were initially identified by one of the authors (RF) (cases 1, 2, 4 and 5). Information regarding history, clinical signs, pathological findings, treatment and outcomes was obtained from the clinical records. One cat (case 4) was still alive and available for further studies. Necropsy specimens from case 1 were retrieved from a commercial veterinary pathology laboratory for review.
Subsequently, four additional cats were managed by one of the authors (RF or JDW). For these cases, haematology, serum biochemistry, urinalysis, urine culture and urine protein:creatinine ratio (UPC) were performed through a single commercial laboratory. Where haematuria was identified in cystocentesed samples, further analyses of voided, midstream samples were performed to ensure that the haematuria was not iatrogenic. Indirect systolic blood pressure measurements were determined using a Parks Doppler device and appropriately sized paediatric cuff (cases 3, 7 and 8).
Kidney tissue was obtained at necropsy from two cats (cases 2 and 6). Renal biopsies were obtained during ovariohysterectomy from a further two cats (cases 3 and 8). One 20-gauge core biopsy was taken from each renal cortex using a spring-loaded automatic disposable device (Temno Evolution™ Cardinal Health, Ontario, Canada). Tissue for histological examination, obtained at either necropsy or biopsy, was sectioned at 5 and 2 μm, stained with haematoxylin and eosin (H&E), Congo red, Masson's trichrome and periodic acid–Schiff (PAS), respectively, and examined by three of the authors (JDW, KB, and CL).
Pedigree information was available for all cases. A diagram of genetic relationships was constructed using a commercial program (Wpdraw 141). To simplify the diagram, not all siblings have been shown in litters that do not contain affected cats. Shading was used to indicate the clinical status of each cat, where this was known. Cats were considered normal if the urine sediment contained ≤10 red or white cells/μl, urine specific gravity (USG) was >1.040, urine culture was negative and the UPC<0.4.
Results
Clinical signs were apparent in all cats by 3 years-of-age. Equal numbers of males and females were affected. Haematuria and proteinuria were the most consistent clinicopathological abnormalities. Haematuria was generally, but not invariably present, and could vary from gross to microscopic in the same cat throughout a day. Haematuria preceded the development of the nephrotic syndrome in two cases. The nephrotic syndrome developed in six cats and resolved in three. Haematuria and proteinuria persisted in three cats that remained otherwise well and resolved completely in one further case. Azotaemia, when present, was generally mild. Renal failure was present in one case at diagnosis. Four cats with the nephrotic syndrome died; two were euthanased, one died of unidentified causes and one cat whose subcutaneous oedema had resolved died from vehicular trauma 18 months later.
Four representative cases (cases 1, 3, 6 and 8) are described below. The remaining cases are described in Table 1. Available clinicopathological details of all cases are summarised in Table 2.
Table 1.
Clinical and pathological details of four additional cases of familial renal disease in Abyssinian cats
| Case number | Signalment | History | Abnormalities in physical examination and additional diagnostic tests | Treatment | Outcome |
|---|---|---|---|---|---|
| Case 2 | 15-month-old pregnant female | Episode of endometritis Cat mated 14 days earlier | Peripheral oedema, ascites and pleura effusion | Benazepril (2.5 mg orally once daily) Renal prescription diets | Euthanasia due to progressive dyspnoea 2 days later* |
| Case 4 | 9-month-old desexed male | One episode of acute diarrhoea 1 day before examination | Peripheral oedema | Single doses of frusemide, dexamethasone, antihistamine and amoxycillin | Peripheral oedema resolved over 1 week. No abnormalities on physical examination 15 months later. Serum biochemistry and haematology were normal, urinalysis revealed haematuria and pyuria of marginal significance |
| Case 5 | 18-month-old desexed male Polydipsia Swollen legs Lethargic Inappetent | Pyrexia Peripheral oedema Abdominal sonography: small amount of free abdominal fluid, renal cortices of slightly increased echogenicity | Amoxycillin–clavulanic acid (62.5 mg orally twice daily) Benazepril (2.5 mg orally once daily) | Pyrexia resolved within 24 h and peripheral oedema improved but haematuria and proteinuria persisted. Inappetence and weight loss developed 18 months later physical examination findings suggested anaemia and renomegaly but further diagnostics were not performed. The cat died of vehicular trauma soon after | |
| Case 7 | 18-month-old desexed male | Persistent haematuria | Abdominal ultrasound, coagulation profile and indirect blood pressure (160 mmHg) unremarkable | Benazapril (2.5 mg orally once daily) | Clinically normal but with persistent haematuria for 2 years after first examination. No azotaemia |
Necropsy confirmed six embryos in utero, pleural, pericardial and abdominal fluid. Light microscopy of renal samples revealed mild, diffuse mesangial hypercellularity, with many glomeruli demonstrating focal adhesions to Bowman's capsule. Focal hyaline granular changes in many proximal tubular epithelial cells with PAS staining. No significant interstitial inflammation or fibrosis. Congo red stains negative for amyloid.
Table 2.
Summary of the clinicopathological abnormalities of Abyssinian cats with familial glomerulonephropathy
| Case number | Duration of follow-up | Urinalysis | Urine culture | UPC | Significant biochemistry and haematology results |
|---|---|---|---|---|---|
| Case 1 | 10 months |
|
Negative | 10.2 when clinical signs of the nephrotic syndrome developed |
|
| Case 2 | Euthanased 2 days after initial examination | 9.3 |
|
||
| Case 3 | 16 months |
|
Negative on four occasions | 0.3 to 12.4 |
|
| Case 4 | 5 months |
|
Negative at 5 months | 0.1 at 5 months |
|
| Case 5 | 2 weeks |
|
Negative | 90.4, 11.8 |
|
| Case 6 | 3 weeks |
|
Negative | 13.7 |
|
| Case 7 | 7 months |
|
Negative on three occasions | 0.4, 0.1 |
|
| Case 8 | 9 Months |
|
Negative on four occasions | 0.2 to 2.5 |
|
TPP =total plasma protein, reference range 56–80 g/l; creatinine reference range 70–160 μmol/l; albumin reference range 22–35 g/l; globulin reference range 28–48 g/l; cholesterol reference range 1.9–7.5 mmol/l; phosphorus reference range 1.29–2.26 mmol/l.
Case 1
A 5.5-month-old entire male Abyssinian was referred to the University of Melbourne Veterinary Hospital after 11 days of persistent haematuria, reduced appetite and weight loss. No other signs referable to the lower urinary tract were evident or reported. Haematology, serum biochemistry, a coagulation profile (one stage prothrombin time and activated partial thromboplastin time), abdominal sonography and systolic blood pressure were all within normal limits. Urinalysis revealed marked haematuria and proteinuria. Urine culture was negative. A renal biopsy was performed and considered normal on histological examination.
The cat developed peripheral and ventral subcutaneous abdominal oedema 10 months later. Hypoalbuminaemia, hypercholesterolaemia, haematuria, pyuria and proteinuria were present. Treatment with benazepril (Fortekor; Novartis, 2.5 mg once daily), aspirin (approximately 50 mg every 48 h) and renal prescription diets was started. Furosemide (10 mg twice daily) was added 6 weeks later. A further 2 months later the cat deteriorated developing pyrexia, tachycardia and pale mucous membranes. Spironolactone (6.25 mg bid) and amoxycillin–clavulanate (Clavulox; Pfizer, 62.5 mg bid) were trialled but oedema persisted and the cat died 1 day later.
Samples from left and right kidneys collected at necropsy were submitted for histological examination. Microscopically, a focal mesangioproliferative glomerulopathy was evident, with focal adhesions of the glomerular tuft to Bowman's capsule, small focal increases in mesangial cell numbers and matrix and a small number of parietal cell crescents (Fig 1). Bowman's capsule was frequently thickened. There were small foci of interstitial lymphocytes and occasional plasma cells, most obvious in the outer medulla. There was patchy fibrosis throughout the cortex. Congo red stains examined under polarised light were negative for amyloid.
Fig 1.
Renal specimen collected from case one at necropsy demonstrating focal mesangial hyperplasia and adhesions between the glomerular tuft and Bowman's capsule (2 μm section, PAS).
Case 3
A 2.5-year-old, entire female, clinically normal Abyssinian (litter sister of case 1) was investigated before a planned mating. Urinalysis showed haematuria and proteinuria. Serum biochemical analyses revealed a small increase in urea concentration. Repeat in-house urinalysis on a voided sample confirmed persistent haematuria (Table 2).
Two weeks later the cat experienced an episode of gastroenteritis that resolved with supportive treatment. A further week later, peripheral oedema developed. Clinicopathological abnormalities included hypoalbuminaemia, a small increase in serum urea and cholesterol concentrations, proteinuria, haematuria and pyuria (Table 2). Urine culture was negative. Treatment was started with benazepril (2.5 mg sid) and renal prescription diets. Two weeks later, hypoalbuminaemia persisted. A coagulation profile and bladder sonographic examination were unremarkable. Oedema resolved and serum albumin levels normalised over 2 months after which an ovariohysterectomy was performed and a core needle biopsy from each kidney was obtained. At this time indirect systolic blood pressure (140 mmHg) was within normal limits. Light microscopic examination of the biopsy was largely unremarkable but only a small number of glomeruli were present. Mild focal lymphocytic interstitial inflammation and tubular epithelial anisokaryosis were present (Fig 2). The cat has been closely monitored for 15 months. Despite reducing levels of proteinuria (UPC decreased from 12.4 to 0.3), haematuria has persisted. Pyuria was present on one subsequent occasion. Urine cultures were negative on three separate occasions. There have been no further episodes of oedema and azotaemia has not developed.
Fig 2.
Renal biopsy of case three demonstrating no significant glomerular changes and focal tubular anisokaryosis (5 μm section; H&E).
Case 6
A 2-year-old desexed female Abyssinian presented with a 12 h history of peripheral and ventral abdominal oedema. Blood and urine tests revealed azotaemia, hypoalbuminaemia, haematuria and proteinuria (Table 2). Treatment was started with benazepril (2.5 mg once daily) and renal prescription diets. The owners reported improvements in oedema, appetite and demeanour. Two weeks later the cat developed upper respiratory signs and lost weight. The dose of benazepril was halved and antibiotics (amoxycillin–clavulanate 50 mg twice daily and marbofloxacin [Zeniquin, Pfizer] 12.5 mg once daily) were started but hypoalbuminaemia persisted and azotaemia increased. The cat deteriorated further over several days, with increasing oedema and persistent anorexia. The cat was euthanased and a necropsy performed. Samples from the spleen, pancreas, liver, heart and lungs were grossly and microscopically unremarkable. Mild multifocal lymphocytic enteritis was present. Renal abnormalities included proximal tubular proteinosis and hyaline casts. Moderate focal proximal tubular dilation with flattened epithelium and a multifocal to diffuse mononuclear interstitial inflammatory infiltrate were present, most prominent in the outer medulla. Hyaline intracytoplasmic granules, consistent with protein reabsorption droplets, were present in many proximal tubular cells (Fig 3). Glomerular changes included a focal increase in mesangial matrix and cells, and frequent adhesions between Bowman's capsule and the glomerular tuft, consistent with a mild focal mesangioproliferative glomerulopathy. Congo red stains were negative for amyloid.
Fig 3.
Renal specimen collected from case six at necropsy demonstrating intra-cytoplasmic protein reabsorption granules, tubular proteinosis and hyaline casts (2 μm section, PAS).
Case 8
A 5-month-old entire female Abyssinian developed gross haematuria without additional signs of lower urinary tract disease. Serum biochemical tests revealed no significant abnormalities. Urinalysis demonstrated marked haematuria and mild proteinuria. Urine tests repeated on three occasions over 9 months demonstrated persistent haematuria and mild proteinuria. Urine culture was negative on each occasion (Table 2). A coagulation profile and sonographic examination of the bladder were normal. Core needle biopsies from each kidney were obtained at the time of ovariohysterectomy. Light microscopic examination of the biopsy was unremarkable. Protein reabsorption droplets were present in proximal tubular epithelial cells. There was no significant glomerular or interstitial pathology. Benazapril (1.25 mg once daily) was started. Over 10 months of monitoring, haematuria has persisted. The cat subsequently experienced a single episode of gastrointestinal disease that responded to supportive treatment. Blood pressure was normal (indirect systolic blood pressure 140 mmHg) while the cat was on benazapril. Renal prescription diets were started at 9 months-of-age.
Familial Relationships
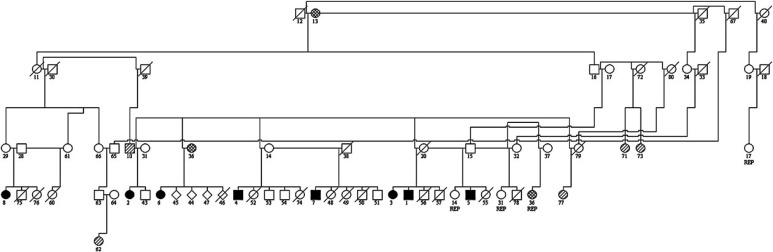
To date, equal numbers of male and female cats have been diagnosed. Cats 3 and 55 and cats 4 and 57 comprised two separate litters from the same dam and sire. Affected cats were, therefore, from eight separate litters (Fig 4).
Fig 4.
Genetic relationship between Abyssinians with glomerular disease. Number=assigned number for pedigree analysis, □=male, ○=female, ⋄=unknown. Filled symbols represent known cases of glomerular disease. Open symbols represent cats where the health of the cat is unknown. Cross-striped symbols represent cats with normal urinalysis results. Hatched symbols represent cats with CRF. REP=cat has been replicated on the diagram.
All affected cases have a common mating among their ancestors (cats 12 and 13). In the litters that are known to contain an affected cat, 8/28 of littermates were affected. If this disease is proven to be a heritable one, this prevalence would suggest an autosomal recessive trait.
Cat 36, the dam of case 6, developed CRF. Tests for proteinuria and haematuria were negative. Cat 13 is reported to have died of CRF but no details are available.
Cats 20, 55, 60, 74 and 78 have had urinalyses performed to screen for evidence of glomerular disease. These cats were clinically normal and gross haematuria has not been observed. However, each cat had at least one abnormal urine result with haematuria (ranging from 10 to >1000 red blood cells [RBCs]/μl) in all five cats and mild pyuria (10–20 white blood cells [WBCs]/μl) in three. UPCs were less than 0.5 and urine cultures were negative in all cases.
Discussion
The presence of a glomerulopathy is supported by key clinicopathological features, such as the nephrotic syndrome, proteinuria, renal haematuria and histopathological evidence of renal disease. The presence of similar clinicopathological signs in eight, young, closely related cats, living in different environments is consistent with a familial disease (DiBartola 2005).
Gross haematuria was the first detectable sign of glomerular disease, appearing as early as 5 months-of-age in two cats. Tissue was not available from all cases, and histological changes detected by light microscopy in renal biopsy specimens were non-specific or unremarkable. Consequently, haematuria of glomerular origin was confirmed by eliminating other causes of haematuria and/or by the development of the nephrotic syndrome. Notably, haematuria was present in all cats in this series without other signs of lower urinary tract disease.
Haematuria of glomerular origin in people is frequently associated with misshapen RBCs (Falk et al 2004), a feature that was not been identified in these cats. Theoretically, haematuria of glomerular origin results from the passage of RBCs across the normally continuous glomerular basement membranes (GBMs) (Lin et al 1983). In people, anatomic gaps in the GBM have been demonstrated in several inflammatory glomerular diseases associated with capillary wall necrosis, the presence of leukocytes and intramembranous electron-dense deposits. Gaps are potentially due to proteases from leukocytes, intramembranous electron-dense deposits causing mechanical fragility and anoxic damage from capillary thrombosis (Stejskal et al 1973). The elasticity of the GBM, deformability of RBCs and high intraglomerular capillary pressure are all proposed to contribute to the ability of RBCs to ‘squeeze’ through gaps in the GBM (Lin et al 1983). The mechanism for glomerular haematuria in non-inflammatory glomerular diseases is less well understood. Recent electron microscopy studies of thin basement membrane disease have shown RBCs passing into the urinary ultrafiltrate, across a transendothelial defect and a localised gap in the GBM (Collar et al 2001).
Five cats, closely related to the affected cats, had microscopic haematuria on at least one occasion. The significance of this finding in the absence of proteinuria in clinically normal cats is unknown. Repeated urinalysis will be required to determine if haematuria persists. In people with gross haematuria, further investigations are routine, but the need for routine investigation of microscopic haematuria is less clear, especially as the prevalence of asymptomatic microscopic haematuria in people ranges from 0.19 to 21% (Woolhandler et al 1989, Yun et al 2004).
Light microscopic findings in kidneys were similar in all four cases subjected to necropsy. The morphological diagnosis was a focal proliferative glomerulonephritis, characterised by a mild increase in mesangial cells and matrix and focal adhesions of the glomerular tuft to Bowman's capsule. The degree of interstitial scarring varied between cases. Although consistent, these glomerular changes are not pathognomonic for a single disease.
Biopsies from two cats were examined by light microscope by three of the authors (JDW, CL and KB). Another biopsy was obtained and examined at the University of Melbourne. Biopsies were considered histologically normal, or to have minimal changes. Case 8 was characterised by haematuria with only mild proteinuria and the presence of glomerular disease was presumed by the absence of clinical or ultrasonographic signs of lower urinary disease. The other cats however, developed the nephrotic syndrome either before or after biopsies were obtained, and glomerular pathology was evident at necropsy in one case. The lack of light microscopic glomerular abnormalities does not preclude the existence of disease. For example, renal abnormalities on light microscopic examination may be subtle or even absent in IgA and hereditary nephropathies, both of which are common causes of haematuria in people (D'Agati et al 2005a,b). Although light microscopic changes, including mesangial expansion, focal thickening and splitting of the capillary basement membrane, have been described in dogs with hereditary nephritis, these changes vary with the stage of disease and definitive diagnosis requires immunostaining and electron microscopy examination (Lees et al 1998, 1999, Rha et al 2000). The morphological diagnosis in these Abyssinians must be considered preliminary until sufficient case material becomes available to perform immunohistochemistry and ultrastructural studies.
There is clinical evidence to suggest that this disease has a waxing and waning course, with fluctuating levels of haematuria, episodic pyuria and resolution of the nephrotic syndrome in some cases. If the disease is truly episodic, further work is required to determine if urinalysis is an adequate screening tool. It is also clear that the expression of this disease is highly variable between individuals.
It is possible that glomerular disease in these cats was triggered or exacerbated by an episode of inflammatory gastrointestinal disease. Clinical signs of gastrointestinal disease, hypoglobulinaemia and pyrexia were present in some cats immediately before or at the same time as the development of the nephrotic syndrome. Only one cat (case 2) had necropsy samples of small intestine available; mild lymphocytic enteritis was present. Exposure to foreign antigens can trigger the development of nephritogenic autoantibodies in people, although this has not been demonstrated in dogs or cats (Brady and Brenner 2001). The role of an inflammatory or infectious disease inciting or exacerbating renal disease in these cats remains speculative.
The short-term prognosis was good for cats with haematuria alone, fair for cats with the nephrotic syndrome as oedema resolved in three of six cats but poor for cats in renal failure. Two cats with persistent haematuria have been monitored for at least 10 months without developing significant proteinuria or renal failure. Although proteinuria, as assessed by the UPC has been variable, there has been a trend to reduced levels of proteinuria over time in these cats. High levels of proteinuria are a poor prognostic indicator in both cats and people (Hunsicker et al 1997, Syme et al 2006). Two of the cats with the nephrotic syndrome died or were euthanased, probably because of complications of the nephrotic syndrome (eg, dyspnoea, thromboembolic disease) rather than renal failure as there was minimal renal scarring present histopathologically. In one cat (case 2) it is possible that the oedema was exacerbated by increased circulating blood volume and peripheral vasodilation, changes typically associated with pregnancy in women (Paller and Connaire 2004). Further monitoring is required to determine if progression to renal failure is a feature of this disease.
Three cases are currently being treated with angiotensin converting enzyme (ACE) inhibitors and renal prescription diets. The efficacy of treatment is unknown as peripheral oedema and proteinuria resolved in one cat without on-going treatment. People, dogs and cats with proteinuric renal disease benefit from the use of ACE inhibitors (Anonymous 1997, Grauer et al 2000, King et al 2006). The most compelling rationale for this benefit is the ability of ACE inhibitors to improve GBM size-selectivity, reduce transglomerular protein movement and inflammation associated with tubular epithelial protein uptake and processing (Ruggenenti and Remuzzi 2000). Additional benefits from reduction of systolic blood pressure are less likely in cats, as hypertension in this species appears reasonably refractory to ACE inhibition (Steele et al 2002), but cats may benefit from a reduction in glomerular hypertension if present (Brown et al 2001).
This study describes a new, potentially familial glomerular disease of Abyssinian cats that should be considered in the investigation of persistent haematuria or proteinuria of Abyssinians and related breeds. However, further characterisation of the pathogenesis and appearance of this glomerulopathy will require ultrastructural and immunohistochemical studies, continued monitoring of the current clinical cases and the early detection of future cases.
Acknowledgements
The following veterinarians and veterinary hospitals treated individual cases: M Lethlean at Dromana Veterinary Hospital and C Goode at The University of Melbourne Veterinary Hospital treated case 1. V Gavens at Ringwood East Personal Petcare continues to treat case 4. S Gibson and R Denny at North Randwick Veterinary Clinic and D Foster at Veterinary Specialist Centre treated case 5. E. Maclean from Blaxland Veterinary Clinic continues to treat case 8. Richard Malik is supported by the Valentine Charlton Bequest. Many thanks to Dr Lorna Rasmussen and Gribbles Veterinary Pathology for assistance with histopathology and clinical pathology testing. The authors greatly appreciate Professor Paul Canfield reviewing the manuscript.
Addendum
Cat 8 has now been followed for over 2 years, during which time haematuria had persisted without evidence of progressive renal disease. There was no haematuria present on the most recent urinalysis.
Three additional cases of renal disease among young Abyssinian cats have come to the authors' attention.
A 3.5-year-old, desexed male developed chronic renal disease characterised by haematuria and mild proteinuria. A 3-year-old desexed female was euthanased with a severe non-regenerative anaemia. Azotaemia was present, however, urinalysis was not performed. Light microscopic examination of renal tissue obtained at necropsy confirmed glomerular disease was present in both cats. A 9-year-old desexed male developed gross haematuria at 1 year-of-age. Repeated urinalysis revealed haematuria and proteinuria. No other abnormalities were detected on urine culture, abdominal radiographs and ultrasound and a coagulation profile. Gross haematuria resolved spontaneously. Recent urinalysis revealed persistent haematuria without proteinuria. There is at least one cat in common in the ancestry of the three cases and the initial eight cases.
References
- Anonymous Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia), Lancet 349, 1997, 1857–1863. [PubMed] [Google Scholar]
- Arthur J.E., Lucke V.M., Newby T.J., Bourne F.J. The long-term prognosis of feline idiopathic membranous glomerulonephropathy, Journal of the American Animal Hospital Association 22, 1986, 731–737. [Google Scholar]
- Biller D.S., DiBartola S.P., Eaton K.A., Pflueger S., Wellman M.L., Radin M.J. Inheritance of polycystic kidney disease in Persian cats, Journal of Heredity 87, 1996, 1–5. [DOI] [PubMed] [Google Scholar]
- Boyce J.T., DiBartola S.P., Chew D.J., Gasper P.W. Familial renal amyloidosis in Abyssinian cats, Veterinary Pathology 21, 1984, 33–38. [DOI] [PubMed] [Google Scholar]
- Brady H.R., Brenner B.M. Pathogenesis of glomerular injury. Braunwald E., Fauci A.S., Kasper D.L., Hauser S.L., Longo D.L., Jameson J.L. Harrison's Principles of Internal Medicine, 15th edn, 2001, McGraw-Hill: New York, 1572–1580. [Google Scholar]
- Brown S.A., Brown C.A., Jacobs G., Stiles J., Hendi R.S., Wilson S. Effects of the angiotensin converting enzyme inhibitor benazepril in cats with induced renal insufficiency, American Journal of Veterinary Research 62, 2001, 375–383. [DOI] [PubMed] [Google Scholar]
- Center S.A., Smith C.A., Wilkinson E., Erb H.N., Lewis R.M. Clinicopathologic, renal immunofluorescent, and light microscopic features of glomerulonephritis in the dog: 41 cases (1975–1985), Journal of the American Veterinary Medical Association 190, 1987, 81–90. [PubMed] [Google Scholar]
- Chew D.J., DiBartola S.P., Boyce J.T., Gasper P.W. Renal amyloidosis in related Abyssinian cats, Journal of the American Veterinary Medical Association 81, 1982, 139–142. [PubMed] [Google Scholar]
- Collar J.E., Ladva S., Cairns T.D., Cattell V. Red cell traverse through thin glomerular basement membranes, Kidney International 59, 2001, 2069–2072. [DOI] [PubMed] [Google Scholar]
- Cook A., Cowgill L. Clinical and pathological features of protein-losing glomerular disease in the dog: a review of 137 cases (1985–1992), Journal of the American Animal Hospital Association 32, 1996, 313–322. [DOI] [PubMed] [Google Scholar]
- D'Agati V.D., Jennette J.C., Silva F.G. Hereditary nephropathies. King D.W. Non-Neoplastic Kidney Diseases, 4th edn, 2005a, ARP Press: Maryland, 75–90. [Google Scholar]
- D'Agati V.D., Jennette J.C., Silva F.G. IgA nephropathy and Henoch–Schonlein purpura. King D.W. Non-Neoplastic Kidney Diseases, 4th edn, 2005b, ARP Press: Maryland, 297–306. [Google Scholar]
- DiBartola S.P. Familial renal disease in dogs and cats. Ettinger S.J., Feldman E.C. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat, 5th edn, 2005, WB Saunders: Philadelphia, 1698–1703. [Google Scholar]
- DiBartola S.P., Rutgers H.C., Zack P.M., Tarr M.J. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984), Journal of the American Veterinary Medical Association 190, 1987, 1196–1202. [PubMed] [Google Scholar]
- Falk R., Jennette J.C., Nachman P. Primary glomerular disease (Online). Brenner B. Brenner & Rector's the Kidney, 2004, Saunders: Philadelphia, 1293–1356. [Google Scholar]
- Forrester S. Diagnostic approach to hematuria in dogs and cats, Veterinary Clinics of North America, Small Animal Practice 34, 2004, 849–867. [DOI] [PubMed] [Google Scholar]
- Glick A.D., Horn R.G., Holscher M. Characterization of feline glomerulonephritis associated with viral-induced hematopoietic neoplasms, American Journal of Pathology 92, 1978, 321–322. [PMC free article] [PubMed] [Google Scholar]
- Grauer G.F., Greco D.S., Getzy D.M., Cowgill L.D., Vaden S.L., Chew D.L., Polzin D.J., Barsanti J.A. Effects of enalapril versus placebo as a treatment for canine idiopathic glomerulonephritis, Journal of Veterinary Internal Medicine 14, 2000, 526–533. [DOI] [PubMed] [Google Scholar]
- Greco D.S. Congenital and inherited renal disease of small animals, Veterinary Clinics of North America, Small Animal Practice 31, 2001, 393–399. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Ishida T., Fujiwara K. Glomerulonephritis associated with feline infectious peritonitis, Japanese Journal of Veterinary Science 44, 1982, 909–916. [DOI] [PubMed] [Google Scholar]
- Hood J.C., Huxtable C., Nait I., Smith C., Sinclair R., Savige J. A novel model of autosomal dominant Alport syndrome in Dalmatian dogs, Nephrology Dialysis Transplantation 17, 2002, 2094–2098. [DOI] [PubMed] [Google Scholar]
- Hood J.C., Robinson W.F., Huxtable C.R., Bradley J.S., Sutherland R.J., Thomas M.A.B. Hereditary nephritis in the bull terrier: evidence for inheritance by an autosomal dominant gene, Veterinary Record 126, 1990, 456–459. [PubMed] [Google Scholar]
- Hunsicker L.G., Adler S., Caggiula A., England B.K., Greene T., Kusek J.W., Rogers N.L., Teschan P.E. Predictors of the progression of renal disease in the modification of diet in renal disease Study, Kidney International 51, 1997, 1908–1919. [DOI] [PubMed] [Google Scholar]
- Jaenke R.S., Allen T.A. Membranous nephropathy in the dog, Veterinary Pathology 23, 1986, 718–733. [DOI] [PubMed] [Google Scholar]
- Jansen B., Thorner P.S., Singh A., Patterson J.M., Lumsden J.H., Valli V.E., Baumai R., Basrur P.K. Animal model of human disease: hereditary nephritis in Samoyed dogs, American Journal of Pathology 116, 1984, 175–178. [PMC free article] [PubMed] [Google Scholar]
- Johnson M.E., DiBartola S.P., Gelberg H.B. Nephrotic syndrome and pericholangiohepatitis in a cat, Journal of the American Animal Hospital Association 19, 1983, 191–196. [Google Scholar]
- Kashtan C. Animal models of Alport syndrome, Nephrology Dialysis Transplantation 17, 2002, 1359–1361. [DOI] [PubMed] [Google Scholar]
- Kashtan C.E. Familial hematurias: what we know and what we don't, Pediatric Nephrology 20, 2005, 1027–1035. [DOI] [PubMed] [Google Scholar]
- King J.N., Gunn-Moore D.A., Tasker S., Gleadhill A., Strehlau G. Tolerability and efficacy of benazapril in cats with chronic kidney disease, Journal of Veterinary Internal Medicine 20, 2006, 1054–1064. [DOI] [PubMed] [Google Scholar]
- Lees G.E., Helman R.G., Kashtan C.E., Michael A.F., Homco L.D., Millichamp N.J., Camacho Z.T., Templeton J.W., Ninomiya Y., Sado Y., Naito I., Kim Y. New form of X-linked dominant hereditary nephritis in dogs, American Journal of Veterinary Research 60, 1999, 373–383. [PubMed] [Google Scholar]
- Lees G.E., Helman C.E., Kashtan R.G., Michael A.F., Homco L.D., Millichamp N.J., Ninomiya Y., Sado Y., Naito I., Kim Y. A model of autosomal recessive Alport syndrome in English cocker spaniel dogs, Kidney International 54, 1998, 373–383. [DOI] [PubMed] [Google Scholar]
- Lin J.T., Wada H., Maeda H., Hattori M., Tanaka H., Uenoyama F., Suehiro A., Noguchi K., Nagai K. Mechanism of hematuria in glomerular disease. An electron microscopic study in a case of diffuse membranous glomerulonephritis, Nephron 35, 1983, 68–72. [DOI] [PubMed] [Google Scholar]
- Macdougall D.F., Cook T., Steward A.P., Cattell V. Canine chronic renal disease: prevalence and types of glomerulonephritis in the dog, Kidney International 29, 1986, 1144–1151. [DOI] [PubMed] [Google Scholar]
- Minkus G., Reusch C., Horauf A., Breuer W., Darbes J., Krafy W., Hermanns W. Evaluation of renal biopsies in cats and dogs – histopathology in comparison with clinical data, Journal of Small Animal Practice 35, 1994, 465–472. [Google Scholar]
- Muller-Peddinghaus R., Trautwein G. Spontaneous glomerulonephritis in dogs. I. Classification and immunopathology, Veterinary Pathology 14, 1977, 1–13. [DOI] [PubMed] [Google Scholar]
- Nash A., Wright N., Spencer A., Thompson H., Fisher E. Membranous nephropathy in the cat: a clinical and pathological study, Veterinary Record 105, 1979, 71–77. [DOI] [PubMed] [Google Scholar]
- Paller M.S., Connaire J.J. Kidney and hypertension in pregnancy (Online). Brenner B. Brenner & Rector's the Kidney, 2004, Saunders: Philadelphia, 1659–1686. [Google Scholar]
- Rha J., Labato M.A., Ross L.A., Breitschwerdt E., Alroy J. Familial glomerulonephropathy in a litter of Beagles, Journal of the American Veterinary Medical Association 216, 2000, 46–50. [DOI] [PubMed] [Google Scholar]
- Ruggenenti P., Remuzzi G. The role of protein traffic in the progression of renal diseases, Annual Review of Medicine 51, 2000, 315–327. [DOI] [PubMed] [Google Scholar]
- Steele J.L., Henik R.A., Stepein R.L. Effects of angiotensin-converting enzyme inhibition on plasma aldosterone concentration, plasma renin activity, and blood pressure in spontaneously hypertensive cats with chronic renal disease, Veterinary Therapeutics 3, 2002, 157–166. [PubMed] [Google Scholar]
- Stejskal J., Pirani C.L., Okada M., Mandelanakis N., Pollak V.E. Discontinuities (gaps) of the glomerular capillary wall and basement membrane in renal diseases, Laboratory Investigation 28, 1973, 149–169. [PubMed] [Google Scholar]
- Syme H.M., Markwell P.J., Pfeiffer D., Elliot J. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria, Journal of Veterinary Internal Medicine 20, 2006, 528–535. [DOI] [PubMed] [Google Scholar]
- Vilafranca M., Wohlsein P., Trautwein G., Leopold-Temmler B., Nolte I. Histological and immunohistological classification of canine glomerular disease, Journal of Veterinary Medicine. Series A 41, 1994, 599–610. [DOI] [PubMed] [Google Scholar]
- Woolhandler S., Pels R.J., Bor D.H., Himmelstein D.U., Lawrence R.S. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders. I. Hematuria and proteinuria, Journal of the American Medical Association 262, 1989, 1214–1219. [PubMed] [Google Scholar]
- Yun E.J., Meng M.V., Carroll P.R. Evaluation of the patient with hematuria, Medical Clinics of North America 88, 2004, 329–343. [DOI] [PubMed] [Google Scholar]