Abstract
Enteroaggregative Escherichia coli (EAEC) is an emerging cause of diarrheal illness. Clinical data suggest that diarrhea caused by EAEC is predominantly secretory in nature, but the responsible enterotoxin has not been described. Work from our laboratories has implicated a ca. 108-kDa protein as a heat-labile enterotoxin and cytotoxin, as evidenced by rises in short-circuit current and falls in tissue resistance in rat jejunal tissue mounted in an Ussing chamber. Here we report the genetic cloning, sequencing, and characterization of this high-molecular-weight heat-labile toxin. The toxin (designated the plasmid-encoded toxin [Pet]) is encoded on the 65-MDa adherence-related plasmid of EAEC strain 042. Nucleotide sequence analysis suggests that the toxin is a member of the autotransporter class of proteins, characterized by the presence of a conserved C-terminal domain which forms a β-barrel pore in the bacterial outer membrane and through which the mature protein is transported. The Pet toxin is highly homologous to the EspP protease of enterohemorrhagic E. coli and to EspC of enteropathogenic E. coli, an as yet cryptic protein. In addition to its potential role in EAEC infection, Pet represents the first enterotoxin within the autotransporter class of secreted proteins. We hypothesize that other closely related members of this class may also produce enterotoxic effects.
Enteroaggregative Escherichia coli (EAEC) is an emerging cause of pediatric diarrhea and has been associated with persistent enteric symptoms (8, 9, 14, 16, 29, 39, 44, 63). The pathogenesis of EAEC diarrhea is not completely defined; however, two prominent histopathologic features have been described: (i) formation of a thick mucus gel on the intestinal mucosa (60) and (ii) mucosal damage, apparently via the elaboration of mucosa-damaging toxin(s) (22, 28, 61). Clinical observations, including EAEC outbreaks (14, 30, 55), studies of endemic EAEC diarrhea, and adult volunteer studies, suggest that EAEC diarrhea is predominantly secretory in nature. Patient stools have been noted to contain mucus and often blood but generally not polymorphonuclear cells (16, 40). Such observations have led investigators to search for an EAEC enterotoxin(s).
Candidate EAEC enterotoxins have been reported. Savarino et al. (51, 52) described a heat-stable enterotoxin (EAST1), which is related to enterotoxigenic E. coli ST; EAST1 is present in ca. 40% of EAEC strains and is also found in strains of other diarrheagenic categories and in nonpathogenic E. coli (53). The role of EAST1 in diarrhea is questionable given the lack of diarrhea in volunteers challenged with EAST1-producing EAEC strains that colonized the intestine at high levels (40). Baldwin et al. (4) described a 120-kDa heat-labile EAEC protein which elicited rises in intracellular calcium in HEp-2 cells. No in vivo effect of this protein has been shown.
We have observed two severe outbreaks of EAEC diarrhea in Mexican hospitals (22) and have found that infants who died in these outbreaks manifested necrotic lesions of the ileal mucosa. We have also found that supernatants from the outbreak strains express two high-molecular-mass proteins (predicted molecular masses of 108 and 116 kDa) which, when injected into rat ileal loops, induce fluid accumulation and cytotoxic effects on the mucosa (22). These proteins were the predominant species in the supernatants of the outbreak strain and were recognized by the sera of the infected patients. It has been shown recently that the 108-kDa protein elicits rises in short-circuit current (Isc) in rat mucosal Ussing chambers (42), an effect which is accompanied by a fall in tissue resistance and damage to the tissue when examined under light microscopy (43).
In this work, we report the molecular cloning and nucleotide sequence analysis of the 108-kDa EAEC enterotoxin derived from a proven pathogenic strain. The toxin gene is located on the 65-MDa EAEC virulence plasmid (the AA plasmid) and is clustered within a locus of putative virulence-associated genes. The toxin is a member of a family of autotransporter proteins which feature serine protease motifs and are related to the immunoglobulin A proteases of Neisseria and Haemophilus species (33). Several proteins in this class have been described recently; however, this is the first instance of an autotransporter protein with enterotoxic activity and represents what may be a critical virulence factor of EAEC.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. Strain 042 was isolated from a child with diarrhea in the course of an epidemiological study in Lima, Peru, in 1983; this strain has been shown to cause diarrhea in adult volunteers in Baltimore (40). Strain 049766 was implicated in an outbreak of EAEC infection in Mexico City; JM221 was obtained from J. Mathewson. E. coli HB101 and DH5α were used as recipient strains for genetic manipulations. Strains were passed routinely on Luria-Bertani broth (L broth) or agar with the following antibiotics where appropriate: ampicillin (100 mg/ml), kanamycin (50 mg/ml), streptomycin (100 mg/ml), tetracycline (15 mg/ml), and chloramphenicol (20 mg/ml). All strains were stored at −70°C in Trypticase soy broth with 15% glycerol.
TABLE 1.
E. coli strains and plasmids used in this work
| Strain or plasmid | Descriptiona | Reference |
|---|---|---|
| Strains | ||
| 042 | Wild-type EAEC strain from Peru | 40 |
| 049766 | Wild-type EAEC strain from outbreak in Mexico | 22 |
| HB101 | K-12–B hybrid | 10 |
| DH5α | K-12 strain | 2 |
| UT5600 | proC leu6 trpE38 entA403 tsx ΔompP ΔompT | 34 |
| KS474 | F− ΔlacX74 galE galK thi rpsL (strA) ΔphoA (PvuII) degP41 (ΔPstI-Kanr) | 58 |
| JCB517 | dsbA::Kan-1 | 5 |
| Plasmid | ||
| pSPORT1 | High-copy-number cloning vector (Ap) | 18 |
| pBluescriptII SK | High-copy-number cloning vector (Ap) | 1 |
| pJRD215 | Low-copy-number cloning vector (Ap) | 19 |
| pRK415 | Low-copy-number cloning vector (Tc) | 35 |
| pJPN201 | 13-kb MluI fragment from pAA2 cloned into pJRD215 (Ap) | This work |
| pJPN204 | 11-kb PstI fragment from pAA2 cloned into pRK415 (Tc) | This work |
| pJPN205 | 2.8-kb MluI-PstI fragment from pAA2 cloned into pSPORT1 | This work |
| pJPN208 | 4.0-kb MluI-KpnI fragment from pJPN201 cloned into pSPORT1; expresses both Pet and EAST1 proteins (Ap) | This work |
| pCEFN1 | 3.9-kb PCR-derived fragment expressing Pet protein cloned into pSPORT1 (Ap) | This work |
Abbreviations: Ap, ampicillin resistance; Tc, tetracycline resistance; Cm, chloramphenicol resistance; Km, kanamycin resistance.
Molecular cloning and nucleotide sequence analysis.
All genetic manipulations were performed by standard methods (2). Plasmid DNA was extracted by using a Plasmid Midi kit (Qiagen Inc., Chatsworth, Calif.). Purification of DNA fragments and extraction from agarose gel slices were performed with Geneclean (Bio 101, La Jolla, Calif.). Plasmid DNA was introduced into E. coli HB101 by transformation of competent cells (Gibco/BRL, Gaithersburg, Md.) according to the method of Hanahan (26). Colony blot hybridization was performed by standard methods (2), using as a probe the insert from clone pJPN205 (Fig. 1).
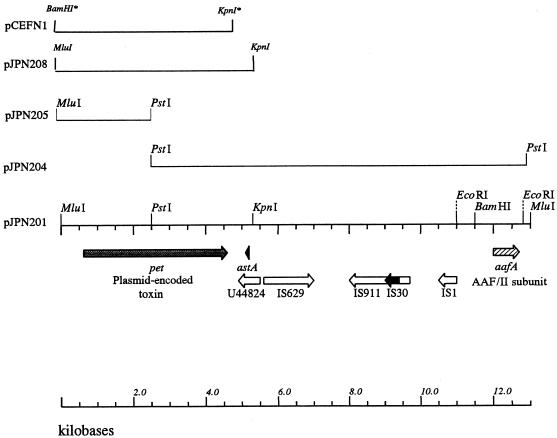
FIG. 1.
Map of the cloned insert of pJPN201. The fimbrial subunit of the AAF/II antigen is encoded on the far right end of the fragment (17). At the left end of the insert is the pet gene, followed in opposite orientation by the astA gene embedded within an IS-like element and then by four other IS-homologous sequences (see text). Subclones used for sequencing and expression of pet are indicated. Restriction sites introduced by PCR are indicated by asterisks.
The minimal clone of pet was constructed by PCR using oligonucleotide primers with the following sequences: 5′-ATGGATCCGGAAGACGGTTGTTGCGC-3′ (upstream) and 5′-GGGGTACCGGTTAGCTCTGAATTAAG-3′ (downstream).
DNA sequence determination was performed on an Applied Biosystems model 373A automated sequencer via dye terminator cycle sequencing with Taq polymerase (Perkin-Elmer Corp., Norwalk, Conn.) according to manufacturer’s instructions; sequencing was performed in the Biopolymer Laboratory, Department of Microbiology and Immunology, University of Maryland School of Medicine. Nucleotide sequence was analyzed with GENEPRO sequence analysis software (version 5.00; Riverside Scientific, Bainbridge Island, Wash.) and the Wisconsin GCG (Genetics Computer Group) sequence analysis package available through the Center of Marine Biotechnology, University of Maryland. The predicted amino acid sequence of each open reading frame (ORF) was compared with sequences of proteins listed in EMBL/GenBank by using the GCG TFASTA program and the BLAST algorithm (National Center for Biotechnology Information). Secondary-structure predictions were performed by Jähnig (32) or Emini et al. (21) algorithms, which are available in the HUSAR program package of the Deutsches Krebsforschungszentrum (Heidelberg, Germany).
Cosmid library construction.
Plasmid DNA was purified from strain 042 and digested partially with the restriction endonuclease Sau3a. The resulting fragments (15 to 30 kb in size) were ligated into the BamHI site of the cosmid vector pCVD301, and the ligation mix was packaged into phage by using the Gigapack packaging extract (Stratagene, Inc.). Recombinant phage were transfected into E. coli HB101. The library comprising 768 clones was maintained at −70°C in L broth containing 15% glycerol.
Protein methods.
Late-logarithmic-phase nutrient broth culture supernatant of strain 042 was subjected to 60 and 75% ammonium sulfate fractionation for 18 h at 4°C. Precipitates collected by centrifugation were dissolved and dialyzed in 0.07 M sodium phosphate buffer, pH 8.2. This suspension was treated with a 3.5 M solution of potassium phosphate, pH 6.8; the precipitate obtained was fractionated by chromatography in DEAE-cellulose and Sephadex columns (LKB Biotechnology, Uppsala, Sweden) and concentrated 10-fold by ultrafiltration through a Diaflo YM100 membrane (Amicon, Lexington, Mass.). The protein separated in polyvinylidene fluoride by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described by Laemmli (36) was transferred to Immobilon membranes (Millipore, Bedford, Mass.) prior to amino-terminal sequencing. Amino-terminal sequencing was performed by automated Edman degradation at the Protein and Nucleic Acid Facility, Stanford University, Palo Alto, Calif.
Outer membrane preparations were performed by concentrating overnight cultures of HB101(pCEFN1) and solubilizing the membranes in Triton X-100 as previously described (12). Cytoplasmic and periplasmic fractions were prepared as previously described (12). Preparations were separated by SDS-PAGE and were visualized by Coomassie blue staining.
Immunologic methods.
The purified Pet protein was injected subcutaneously in complete Freund’s adjuvant to New Zealand White rabbits weighing between 2.5 and 3.0 kg. Rabbits received subcutaneous boosters of 200 mg of total protein on days 0, 15, and 20. Rabbits were exsanguinated on day 25, and the serum collected was stored at −20°C until use.
Western immunoblots of the Pet protein were performed with the purified Pet protein (see above). Samples containing 100 mg of total protein were separated by SDS-PAGE, and the protein bands obtained were transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, N.H.) as described by Towbin et al. (59). The membranes were incubated overnight with rabbit antisera (anti-Pet) diluted 1:500. The Pet protein was visualized with goat anti-rabbit antibodies conjugated with alkaline phosphatase (Kirkegaard & Perry Laboratories, Gaithersburg, Md.).
Ussing chamber experiments.
Six pieces of rat jejunum removed from adult male Sprague-Dawley rats under sodium pentobarbital anesthesia were placed in ice-cold Ringer’s solution for mammals and gassed with an O2-CO2 (95%:5%) mixture. The excised segments were cut open along the mesenteric border, washed with cold Ringer’s solution, and mounted between the circular openings of six Ussing hemichambers. Each hemichamber was filled with 10 ml of gassed Ringer’s solution and kept at 37°C under constant O2-CO2 bubbling (41). Chambers were equilibrated for 30 min before experiments were initiated. After addition of the test sample, transepithelial electrical potential difference (PD) was measured at 10-min intervals under current-clamped conditions. Tissue conductance was determined at an applied current of 100 μA, and Isc was calculated by using Ohm’s law (25).
Samples used in Ussing chamber experiments consisted of 100 ml of L-broth cultures grown overnight at 37°C without shaking. After centrifugation at 12,000 × g for 10 min, supernatants were concentrated and size fractionated (>50 kDa) by passage through Biomax-50 Ultrafree filters (Millipore). Samples were adjusted to a concentration of 25 μg of protein/ml, and 100 μl of each sample was added to the mucosal hemichamber of rat jejunum preparations.
Nucleotide sequence accession number.
The sequence of the pet gene has been submitted to GenBank under accession no. AF056581.
RESULTS
Cloning and sequencing of the 108-kDa toxin gene.
We had previously raised polyclonal antiserum to the 108-kDa EAEC protein derived from strain 049766 (implicated in an EAEC outbreak in Mexico). We used this antiserum to localize the toxin gene in strain 042. Use of the anti-108-kDa protein antiserum in Western immunoblotting of concentrated culture supernatants from E. coli HB101 containing the 65-MDa plasmid pAA2 (from strain 042) revealed the presence of the 108-kDa protein, whereas culture supernatants from HB101 lacking pAA2 were negative for the toxin. To clone the plasmid-encoded toxin gene, a cosmid library of plasmid pAA2 was constructed in vector pCVD301. A portion of the cosmid bank was subjected to restriction analysis in order to identify a small subset of clones which were representative of the entire parent plasmid. This series of experiments resulted in the selection of 11 overlapping cosmid clones which encompassed the large majority of the plasmid. Subsequent Western immunoblot analysis of this cosmid subset revealed that two of the 11 cosmid clones expressed high-molecular-weight bands that reacted with anti-108-kDa protein antiserum (not shown). Restriction mapping of these two cosmid clones demonstrated an overlapping region of approximately 20 kb.
The two toxin-encoding cosmids shared a common 13-kb MluI fragment. A series of subclones was constructed from this region (Table 1 and Fig. 1). Western immunoblot analysis suggested that pJPN201, carrying the full 13-kb MluI fragment, expressed the 108-kDa protein, whereas the nested clone pJPN204 did not.
Sequence analysis of the toxin gene.
Single-stranded nucleotide sequencing was performed on the insert of pJPN201. Due to the absence of the 108-kDa protein in supernatants of HB101(pJPN204), we expected to find an ORF near the left terminus of the cloned insert. Indeed, analysis of the nucleotide sequence of pJPN201 revealed a large ORF (3,885 bp in length) starting 617 bp from the MluI site at the left end of the pJPN201 insert (Fig. 1). The G+C content of this ORF was 43.6%, significantly lower than average for the E. coli genome. Sequence analysis of the cloned insert did not reveal any other ORFs which could potentially encode a protein greater than 80 kDa in size. In recognition of the fact that the large ORF apparently encodes the high-molecular-weight toxin described by Eslava et al. (22) and by Navarro-Garcia et al. (43), this gene has been designated pet (EAEC plasmid-encoded toxin). Figure 1 illustrates the map of pJPN201 including the position of pet and the subclones that were used for sequencing and phenotypic analysis.
We identified a potential pet promoter which had a −10 region (TTTAAT) and a −35 region (GTAACA) positioned 48 and 70 bp, respectively, upstream from the ATG start codon. A possible rho-independent stem-loop transcriptional termination signal was also identified 6 bp downstream of the TGA termination codon of the pet gene. The presence of the promoter is consistent with the ability of clone HB101(pJPN201) to express the Pet product.
Downstream from the pet gene are five insertion sequence (IS)-homologous ORFs (Fig. 1). Immediately downstream from pet is a potential ORF of 581 bp (in the antisense direction), the predicted product of which exhibits 49% identity with a transposase of Burkholderia cepacia (accession no. U44828). Within this ORF, in the same orientation as the transposase and 647 bp downstream of the 3′ end of the pet gene, lies a gene homologous to the astA gene, which encodes the 38-amino-acid EAST1 heat-stable enterotoxin (51). Interestingly, the astA gene of strain 042 is 100% identical at the amino acid level with the predicted sequence of the astA gene from enterotoxigenic E. coli strain H10407 (64; accession no. S81691); this EAST1 differs in only one residue from the EAST1 protein of EAEC strain 17-2. Immediately upstream of the B. cepacia IS-like element, and in the opposite orientation, is a sequence of 1,310 bp which is 97% identical to an IS629 element of Shigella sonnei (37). IS629 is 95% identical to the IS1203 element found recently in pathogenic E. coli O111:H− (45). Further downstream from pet lies an element identical to Shigella dysenteriae IS911 (47), the sequence of which is interrupted by a complete IS30 element (13). Upstream of this element lies a complete IS1 element (50).
To facilitate further analyses, a minimal clone of the pet gene (pCEFN1) was constructed by PCR and cloned into pSPORT1 (Fig. 1). The insert was flanked by the native MluI site (upstream) and an engineered KpnI site (downstream) and spanned from 610 bp upstream of pet to 50 bp downstream of the termination codon. This fragment included the predicted pet promoter but not the astA gene. Of note, all known promoters of pSPORT1 are aligned in opposite orientation to the cloned pet gene in pCEFN1.
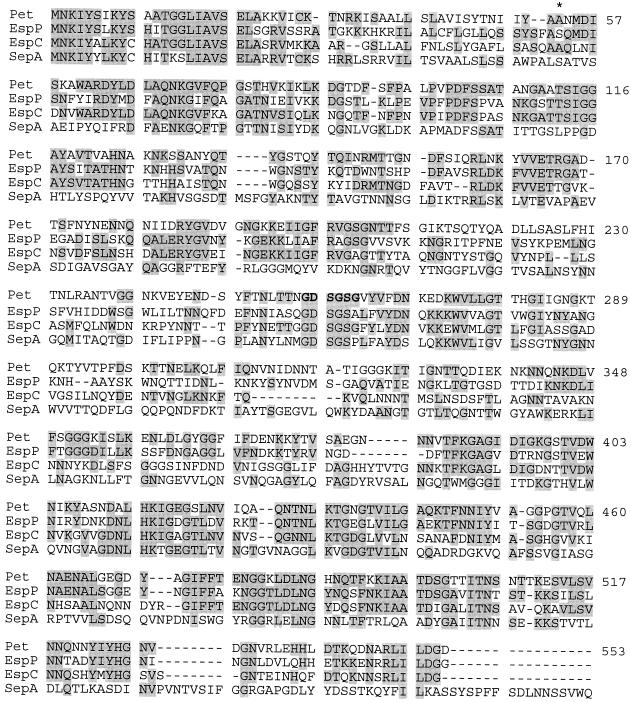
Assuming that the first ATG codon of the ORF corresponds to the translational start codon, the pet gene would encode a 1,295-aminio-acid protein with a predicted molecular mass of 140.0 kDa and a calculated isoelectric point of 6.71. Comparison of the deduced amino acid sequence with those listed in GenBank databases revealed 58% overall identity (83% similarity) with the recently described EspP protein of enterohemorrhagic E. coli (11) (Fig. 2). In addition, pet displayed 55% identity (70% similarity) and 44% identity (60% similarity) with the espC gene product of enteropathogenic E. coli and with SepA, the major secreted protein of Shigella flexneri, respectively. Significant homology was also seen with other members of the so-called autotransporter family of bacterial virulence factors. Notably, the homologies displayed are not uniformly distributed over the sequences; the N-terminal passenger domain (encoding the mature protein) of pet displays 49, 45, and 31% identity to the EspP, EspC, and SepA passenger domains, respectively, whereas the C-terminal β domains (the C-terminal β barrel) exhibited 90, 80, and 78% identity, respectively.
FIG. 2.
Alignment of the predicted Pet protein with its closest homologs, EspP (accession no. X97542), EspC (U69128), and SepA (Z48219). Shaded residues represent identity. Coordinates are shown for Pet only. The asterisk at residue 53 indicates the first amino acid of the mature Pet protein. The arrowhead at residue 1018 indicates the position of cleavage of the β domain. The serine protease motif is in boldface.
Several features of the autotransporter family were evident within the predicted pet gene product. First, analysis of the predicted Pet amino acid sequence revealed the presence of a putative Walker A box (62) nucleotide binding motif (G281IIGNGK) which has been described for a number of other members of this class, though a function for these motifs has not yet been shown (11, 48, 54, 56). Second, a serine protease motif (GDSGSP) has been found in several of the closest homologs of Pet. Although this site has been shown to act in proteolysis in Haemophilus and Neisseria autotransporters, (3, 27), a function has not been determined for this motif in the autotransporters of members of the family Enterobacteriaceae. At the corresponding site in Pet, the sequence was determined to be G256DSGSGV. Computer-aided analysis of the deduced amino acid sequence of Pet indicated that the protein possessed the characteristics of a signal sequence (31), with positively charged amino acids followed by a hydrophobic region and a signal peptidase cleavage site at residue 52. The length of this signal sequence would be unusually long for E. coli but similar to those predicted for other autotransporters. To confirm the site of cleavage of the mature protein, Pet was isolated from culture supernatants of E. coli 049766 and the N-terminal amino acid sequence was determined. The derived amino acid sequence (ANMDISKAWARD......) indeed localized the site of cleavage between residues A52 and A53.
Processing and export of Pet.
Since the ca. 108-kDa protein present in culture supernatant fluids is smaller than predicted from the N-terminal processed ORF (1,243 amino acids; molecular mass of 134.4 kDa), the Pet protein apparently undergoes a posttranslational processing step, namely, cleavage of the passenger domain from the β domain. Members of the autotransporter family of proteins are exported through the outer membrane of the bacterium via the presence of a characteristic C-terminal amphipathic region (β domain) comprising an even number of antiparallel β sheets; this region of the protein forms a β-barrel structure in the outer membrane through which the passenger domain of the protein passes. The high homology between the β domains of Pet and the EspP, EspC, and SepA proteins suggests that the β domain of Pet functions as an outer membrane translocator and that cleavage of the passenger domain occurs during this step. Based on the sequence homology with other autotransporters the cleavage site was predicted to be between N1018 and N1019.
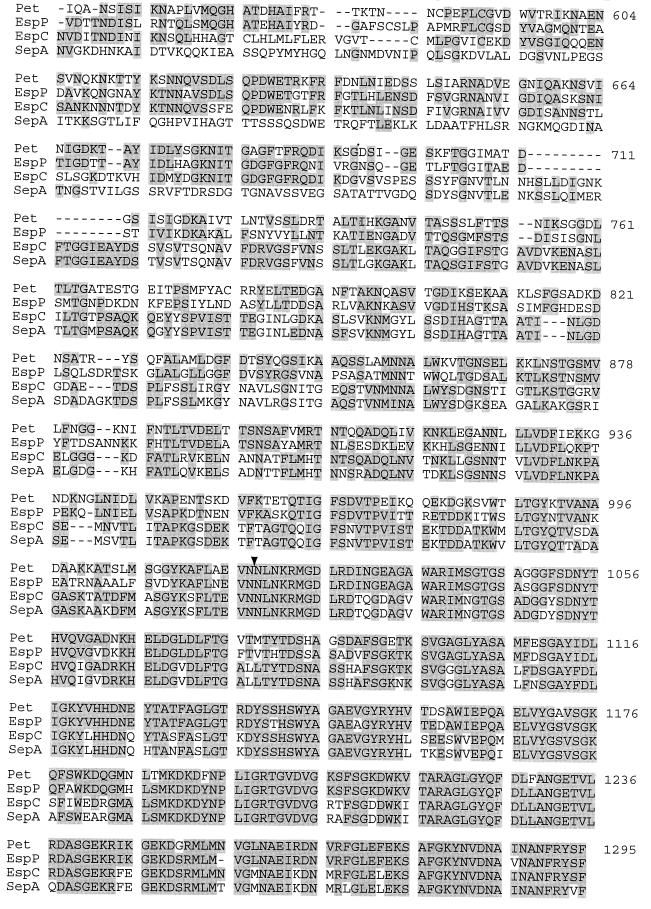
To localize accurately the site of C-terminal processing, the outer membrane β domain was visualized by SDS-PAGE analysis of envelopes from HB101(pCEFN1) extracted with Triton X-100 and compared with similar extracts of HB101(pSPORT1). These analyses revealed the presence of a 30-kDa species in the fractions obtained from HB101(pCEFN1) that was absent from the similar fractions of the control strain (Fig. 3a). As expected, the N-terminal amino acid sequence of this protein (NLNKRMGDLR...) placed the site of cleavage between N1018 and N1019. Cleavage at this point, and at the site of cleavage of the signal sequence (A52-A53), would result in a secreted Pet product of 104.2 kDa, a mass which agrees well with the mass of 108 kDa predicted for Pet on SDS-PAGE analysis.
FIG. 3.
(a) SDS-PAGE analysis of clone pCEFN1, encoding the complete pet gene. Lanes: A, Triton X-100-insoluble fraction of HB101(pCEFN1); B, Triton X-100-insoluble fraction of HB101 harboring the cloning vector pSPORT1; C, supernatant of HB101(pCEFN1); D, supernatant of HB101(pSPORT1). The arrow at 104 kDa represents the Pet passenger domain (the mature form of the protein); the arrow at ca. 30 kDa represents the β domain inserted into the bacterial outer membrane. (b) Western immunoblot of bacterial supernatants reacted with anti-Pet antiserum. Lanes: A, periplasmic fraction of HB101(pCEFN1); B, periplasmic fraction of HB101(pJPN205) harboring the C-terminal deletion mutant of the pet gene; C, cytoplasmic fraction of HB101(pCEFN1); D, cytoplasmic fraction of HB101(pJPN205); E, supernatant of HB101(pCEFN1); F, supernatant of HB101(pJPN205). Arrows denote expected sizes of mature Pet and the expected truncated species produced by pJPN205. Smaller species reacting with antibodies in lanes B and E most likely represent breakdown products of the mature toxin.
Structural predictions of the β domain of Pet, from the N1019 cleavage site to the terminal phenylalanine residue, were performed by using the algorithm {Hb(i) = [h(i ± 4) + h(i ± 2) + h(i)]/5} described by Jähnig (32). According to these predictions, the β domain of Pet consists of at least 14 membrane-spanning amphipathic β strands interrupted by large external loops and generally short periplasmic loops, spanning amino acid positions 1032 to 1295 of the Pet precursor. These results were confirmed by calculating the regions of high surface probability as described by Emini et al. (21), using the GCG program from the Wisconsin sequence analysis package, since such regions are always located between the β strands. An alpha helix was not predicted upstream of the amphipathic strands.
To test the hypothesis that the β domain is involved in translocation of the Pet passenger domain to the external milieu, the deletion mutant pJPN205 (truncated at residue 770) was analyzed for expression of the mature Pet protein. By Western immunoblotting, HB101(pJPN205) supernatants did not reveal a protein consistent with this truncated passenger domain (Fig. 3b, lane F), although an appropriate-size protein species was detected in the bacterial periplasm (Fig. 3b, lane B) and, to a much lesser extent, in the cytoplasm (Fig. 3b, lane D). These data confirm that the C-terminal β domain is required for Pet translocation to the external milieu.
The possible role of several endogenous membrane-associated enzymes in the processing and export of Pet was investigated. The pCEFN1 clone was transformed into E. coli UT5600 (ompP ompT), KS474 (degP), and JCB517 (dsbA). The resulting constructions were screened for processing of Pet by SDS-PAGE analysis of concentrated culture supernatants. Each strain yielded a 104-kDa species, suggesting that normal processing of the Pet precursor occurs in the absence of the DegP, OmpP, and OmpT proteases and in the absence of the DsbA isomerase.
Phenotypic analysis of the Pet protein.
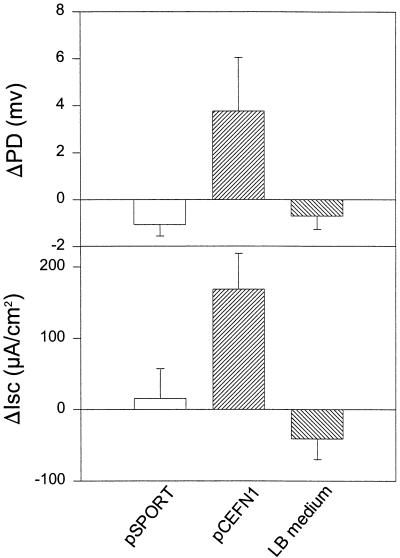
We have shown that concentrated supernatant from strain 049766 produces an increase in jejunal PD and Isc (43). The >50-kDa fraction of supernatants from HB101(pJPN201) also induced rises in jejunal PD and Isc which were not induced by concentrated supernatants from HB101(pJRD215) or HB101(pJPN204). Supernatants derived from the minimal clone of pet were also found to induce rises in Isc (Fig. 4), which were significantly higher than those induced by the cloning vector, suggesting that Pet is the enterotoxic moiety. Rises in Isc and PD induced by the pet clone were similar in timing and degree to those induced by the parent strain 042.
FIG. 4.
Enterotoxic activity of the Pet protein derived from pCEFN1. Supernatants from overnight cultures were size fractionated (>50 kDa), and 5 μg of protein was added per Ussing chamber into which was mounted full-thickness rat jejunal tissue. The supernatants of HB101(pCEFN1) and HB101(pSPORT1) are illustrated in lanes C and D, respectively of Fig. 3a. Data points represent the means of at least three experiments; error bars represent standard errors of the means. The insert of pCEFN1 generates significant rises in PD and Isc compared with negative controls (P < 0.05 by Student’s t test).
The ability of the Pet protein to act as a protease was tested by separating concentrated supernatants through casein or gelatin zymogram gels (NOVEX). Strains 042, HB101(pCEFN1), and HB101(pJPN201) all yielded zones of clearing at 108 kDa, whereas HB101 and HB101(pSPORT) supernatants did not reveal proteolytic activity at this molecular mass (data not shown).
Regulation of Pet expression.
The EspP protein has been shown to be regulated by temperature (11). To obtain information on the regulation of pet expression, we analyzed the expression of Pet from EAEC strain 042 grown at different temperatures (20, 37, and 42°C). The bacteria were grown to an optical density at 600 nm of 1.0, and concentrated culture supernatants were analyzed by SDS-PAGE for the presence of Pet. The mature Pet protein was observed in similar amounts from supernatants of 042 grown at all three temperatures (not shown), suggesting that the pet gene is not strictly temperature regulated.
Prevalence of the pet gene among EAEC.
To determine the prevalence of the pet gene among clinical isolates, colony blot hybridization studies were performed with a restriction fragment internal to the pet gene, corresponding to the region encoding residues 62 to 781 of the Pet protein. Against a collection of EAEC strains from various epidemiological studies around the world, 5 of 34 strains (15%) yielded a positive hybridization signal with the probe (Table 2).
TABLE 2.
Prevalence of the pet gene among EAEC strains
| Site | pet probe result | Strain(s) |
|---|---|---|
| Chile | Negative | 17-2 |
| Mexico | Negative | 60A, 93A, JM221 |
| India | Negative | 34b |
| Peru | Negative | H46-2, H92-1, H32-1, H223-1, H11-1, H232-1, H145-1, H191-1, H38-1, Peru-10 |
| Positive | Peru-49 | |
| Philippines | Negative | DS61R2, DS65R3, DS67R2 |
| Positive | DS244R3 | |
| Thailand | Negative | 44-1-1, 6-1-1, 103-1-1, 144-1-1, 501-1-1, 309-1-1, 253-1-1, 239-1-1, 278-1-1, 495-1-1, 232-1-1 |
| Positive | 216-1-1, 199-1-4, 435-1-1 |
DISCUSSION
The pathogenesis of EAEC diarrhea is poorly understood. Clinical descriptions suggest that EAEC diarrhea is secretory in nature and therefore perhaps due to the presence of an as yet unidentified enterotoxin. The low-molecular-weight putative enterotoxin EAST1 has been found in some EAEC strains, but its role in diarrhea has yet to be proven. Eslava et al. (22) have described a ca. 108-kDa EAEC protein that is able to elicit fluid accumulation and mucosal destruction in rat ileal loops. Navarro-Garcia et al. (43) suggested that this protein elicits rises in Isc and decreases of electrical resistance in rat jejunal tissue mounted in an Ussing chamber, accompanied by damage to the epithelial cells (43). Using molecular methods, we have characterized this high-molecular-weight protein and have demonstrated that it is a plasmid-encoded autotransporter enterotoxin of EAEC.
Our analysis of the gene encoding the Pet enterotoxin shows homology with members of the autotransporter family of bacterial proteins. This family comprises a rapidly growing number of virulence determinants of gram-negative bacteria (33). The class takes its name from its so-called type IV secretion mechanism (24), in which an N-terminal amino acid leader sequence directs secretion through the general secretory pathway into the bacterial cytoplasm; once in the periplasm, a C-terminal amphipathic region forms a β barrel in the outer membrane, allowing the processed N-terminal protein to pass through into the extracellular milieu. In some cases, the protein remains anchored in the outer membrane (7), whereas in other cases, the protein is released into the supernatant. Secondary structure analyses of the predicted Pet product by the method of Jähnig (32) suggested the presence of 14 amphipathic β strands, each strand consisting of 10 to 14 amino acids. An even number of antiparallel transmembrane segments would place the first and last strands in opposite orientation and would allow closing of the β barrel, a feature observed for the trimeric porins such as OmpF (15). Thus, our analyses suggest that the β domain forms a pore through which the passenger domain is translocated to the surface, as is typical of the type IV mechanism.
The release of the Pet passenger domain apparently occurred by proteolytic cleavage between residues N1018 and N1019. In the case of the immunoglobulin A1 proteases of Neisseria gonorrhoeae, this processing step is a result of autoproteolysis involving the serine protease site of the molecule (46). The presence in Pet of a putative serine protease active site suggests that a similar step could also occur in the case of Pet. To characterize further the processing step involved in the maturation of Pet, export of the passenger domain was investigated in E. coli strains lacking either the periplasmic protease DegP, the OmpT and OmpP proteases of the outer membrane, or DsbA, the disulfide bond isomerase. The results indicated that formation of the passenger and β domains was independent of these four enzymes and implied that either another unidentified protease is involved or autoprocessing may occur.
A number of autotransporter proteins from Enterobacteriaceae have been reported recently. Among these are EspP from enterohemorrhagic E. coli (11), EspC from enteropathogenic E. coli (57), She (49), and SepA (6) from S. flexneri and Tsh (48). Each of these proteins is >100 kDa, is processed and exported by the type IV mechanism, and features a serine protease active site motif. The precise roles of these proteins have not been determined, however; only Pet has been tested rigorously for enterotoxic activity.
Analysis of nucleotide sequence data identified a number of IS-like elements flanking the genes encoding members of the autotransporters of Enterobacteriaceae. Of note is the presence of an IS629 (IS1203) element downstream of the pet gene. Other workers have shown that IS629 elements are linked to the presence of putative virulence loci, but of specific interest is the association of this IS-like element with the espP, sepA, and she genes. Furthermore, espP of E. coli O157:H7 is flanked by both an IS629 element and an IS1 element (although in the opposite orientation to the pet gene), while the identical gene from O26:H− is flanked by IS629 and remnants of an IS911 element (20). These data coupled to the fact that pet has a G+C content significantly lower than the average for E. coli (51%) suggests that the gene may have been acquired by strain 042 via horizontal transfer. Certainly, the association of most autotransporters in E. coli and Shigella with the IS629-like elements suggests a role for this element in the evolution and spread of these homologs among the Enterobacteriaceae.
The enterotoxic activity induced by Pet is consistent with the secretory diarrhea seen in most patients with EAEC enteritis. However, the rises in Isc induced by the Pet protein are accompanied by a fall in tissue resistance, and light microscopic examination of the tissue after exposure to the toxin in an Ussing chamber reveals damage to the tissue (43). In light of observations suggesting that EAEC causes cytotoxic effects in in vitro intestinal culture and T84 cells, it is tempting to speculate that the Pet toxin may have cytopathic effects as well (28, 38). This possibility is currently under investigation. As well, we have observed that while only a small fraction of EAEC strains express the pet gene, it is quite possible that only these strains are in fact diarrheagenic. Indeed, human volunteer studies suggest that strain 042 induced diarrhea in healthy adults whereas three other strains did not induce enteric symptoms (40). Our DNA probe analysis for the pet gene suggests that of the four strains fed, only strain 042 expresses Pet. Moreover, Pet was initially isolated from EAEC strain 049766, which was implicated in a highly virulent outbreak of diarrhea in Mexico. The hypothesis that only Pet-producing EAEC strains are capable of inducing diarrhea is currently being tested in epidemiological studies.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI33096 and TW00499 (to J.P.N.) and by DGAPA IN-208493 (to C.E.).
REFERENCES
- 1.Alting-Mees M A, Sorge J A, Short J M. pBluescriptII: multifunctional cloning and mapping vectors. Methods Enzymol. 1992;216:483–495. doi: 10.1016/0076-6879(92)16044-k. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1989. [Google Scholar]
- 3.Bachovchin W W, Plaut A G, Flentke G R, Lynch M, Kettner C A. Inhibition of IgA1 proteinases from Neisseria gonorrhoeae and Haemophilus influenzae by peptide prolyl boronic acids. J Biol Chem. 1990;265:3738–3743. [PubMed] [Google Scholar]
- 4.Baldwin T J, Knutton S, Sellers L, Hernandez H A M, Aitken A, Williams P H. Enteroaggregative Escherichia coli strains secrete a heat-labile toxin antigenically related to E. coli hemolysin. Infect Immun. 1992;60:2092–2095. doi: 10.1128/iai.60.5.2092-2095.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardwell J C, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;67:581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 6.Benjelloun-Touimi Z, Sansonetti P J, Parsot C. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol Microbiol. 1995;17:123–135. doi: 10.1111/j.1365-2958.1995.mmi_17010123.x. [DOI] [PubMed] [Google Scholar]
- 7.Benz I, Schmidt M A. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27), is synthesized via a precursor molecule. Mol Microbiol. 1992;6:1539–1546. doi: 10.1111/j.1365-2958.1992.tb00875.x. [DOI] [PubMed] [Google Scholar]
- 8.Bhan M K, Khoshoo V, Sommerfelt H, Raj P, Sazawal S, Srivastava R. Enteroaggregative Escherichia coli and Salmonella associated with nondysenteric persistent diarrhea. Pediatr Infect Dis J. 1989;8:499–502. doi: 10.1097/00006454-198908000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Bhan M K, Raj P, Levine M M, Kaper J B, Bhandari N, Srivastava R, Kumar R, Sazawal S. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J Infect Dis. 1989;159:1061–1064. doi: 10.1093/infdis/159.6.1061. [DOI] [PubMed] [Google Scholar]
- 10.Boyer H W, Roulland-Dussoix R. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–468. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 11.Brunder W, Schmidt H, Karch H. EspP, a novel extracellular serine protease of enterohemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol Microbiol. 1997;24:767–778. doi: 10.1046/j.1365-2958.1997.3871751.x. [DOI] [PubMed] [Google Scholar]
- 12.Caffrey P, Owen P. Purification and N-terminal sequence of the a subunit of antigen 43, a unique protein complex associated with the outer membrane of Escherichia coli. J Bacteriol. 1989;171:3634–3640. doi: 10.1128/jb.171.7.3634-3640.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caspers P, Dalrymple B, Iida S, Arber W. IS30, a new insertion sequence of Escherichia coli K12. Mol Gen Genet. 1984;196:68–73. doi: 10.1007/BF00334094. [DOI] [PubMed] [Google Scholar]
- 14.Cobeljic M, Miljkovic-Selimovic B, Paunovic-Todosijevic D, Velickovic Z, Lepsanovic Z, Zec N, Savic D, Ilic R, Konstantinovic S, Jovanovic B, Kostic V. Enteroaggregative Escherichia coli associated with an outbreak of diarrhoea in a neonatal nursery ward. Epidemiol Infect. 1996;117:11–16. doi: 10.1017/s0950268800001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowan S W, Schirmer T, Rummel G, Steiert M, Ghosh R, Pauptit R A, Jansonius J N, Rosenbusch J P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992;358:727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- 16.Cravioto A, Tello A, Navarro A, Ruiz J, Villafan H, Uribe F, Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet. 1991;337:262–264. doi: 10.1016/0140-6736(91)90868-p. [DOI] [PubMed] [Google Scholar]
- 17.Czeczulin J, Balepur S, Hicks S, Phillips A, Navarro-Garcia F, Nataro J P. Aggregative adherence fimbriae II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect Immun. 1997;65:4135–4145. doi: 10.1128/iai.65.10.4135-4145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Alessio J M, Gruber C E, Cain C, Noon M C. Construction of directional cDNA libraries using the superscript plasmid system. Focus. 1990;12:47–50. [Google Scholar]
- 19.Davison J, Heusterspreute M, Chevalier N, Ha-Thi V, Brunel F. Vectors with restriction site banks. V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene. 1987;51:275–280. doi: 10.1016/0378-1119(87)90316-7. [DOI] [PubMed] [Google Scholar]
- 20.Djafari S, Ebel F, Deibel C, Kramer S, Hudel M, Chakraborty T. Characterization of an exported protease from Shiga toxin-producing Escherichia coli. Mol Microbiol. 1997;25:771–784. doi: 10.1046/j.1365-2958.1997.5141874.x. [DOI] [PubMed] [Google Scholar]
- 21.Emini E A, Hughes J V, Perlow D S, Boger J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol. 1985;55:836–839. doi: 10.1128/jvi.55.3.836-839.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eslava C, Villaseca J, Morales R, Navarro A, Cravioto A. Abstracts of the 93rd General Meeting of the American Society for Microbiology. 1993. Washington, D.C: American Society for Microbiology; 1993. Identification of a protein with toxigenic activity produced by enteroaggregative Escherichia coli, abstr. B-105; p. 44. [Google Scholar]
- 24.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guandalini S, Fasano A, Migliavacca M, Marchesano G, Ferola A, Rubino A. Effects of Berberine on basal and secretagogue-modified ion transport in the rabbit ileum in vitro. J Pediatr Gastroenterol Nutr. 1987;6:953–960. doi: 10.1097/00005176-198711000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 27.Hendrixson D R, de la Morena M L, Stathopoulos C, St. Geme J W. Structural determinants of processing and secretion of the Haemophilus influenzae Hap protein. Mol Microbiol. 1997;26:505–518. doi: 10.1046/j.1365-2958.1997.5921965.x. [DOI] [PubMed] [Google Scholar]
- 28.Hicks S, Candy D C, Phillips A D. Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun. 1996;64:4751–4760. doi: 10.1128/iai.64.11.4751-4760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huppertz H I, Rutkowski S, Aleksic S, Karch H. Acute and chronic diarrhoea and abdominal colic associated with enteroaggregative Escherichia coli in young children living in western Europe. Lancet. 1997;349:1660–1662. doi: 10.1016/S0140-6736(96)12485-5. [DOI] [PubMed] [Google Scholar]
- 30.Itoh Y, Nagano I, Kunishima M, Ezaki T. Laboratory investigation of enteroaggregative Escherichia coli O untypeable:H10 associated with a massive outbreak of gastrointestinal illness. J Clin Microbiol. 1997;35:2546–2550. doi: 10.1128/jcm.35.10.2546-2550.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izard J W, Kendall D A. Signal peptides: exquisitely designed transport promoters. Mol Microbiol. 1994;13:765–773. doi: 10.1111/j.1365-2958.1994.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 32.Jähnig F. Structure predictions of membrane proteins are not that bad. Trends Biochem Sci. 1990;15:93–95. doi: 10.1016/0968-0004(90)90188-h. [DOI] [PubMed] [Google Scholar]
- 33.Jose J, Jähnig F, Meyer T F. Common structural features of IgA1 protease-like outer membrane protein autotransporters. Mol Microbiol. 1995;18:377–382. doi: 10.1111/j.1365-2958.1995.mmi_18020378.x. [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann A, Stierhof Y-D, Henning U. New outer membrane-associated protease of Escherichia coli K-12. J Bacteriol. 1994;176:359–367. doi: 10.1128/jb.176.2.359-367.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 36.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 37.Matsutani S, Ohtsubo E. Complete sequence of IS629. Nucleic Acids Res. 1990;18:1899. doi: 10.1093/nar/18.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nataro J P, Hicks S, Phillips A D, Vial P A, Sears C L. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect Immun. 1996;64:4761–4768. doi: 10.1128/iai.64.11.4761-4768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nataro J P, Kaper J B, Robins-Browne R, Prado V, Levine M M. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987;6:829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Nataro J P, Yikang D, Cookson S, Cravioto A, Savarino S J, Guers L D, Levine M M, Tacket C O. Heterogeneity of enteroaggregative E. coli virulence demonstrated in volunteers. J Infect Dis. 1995;171:465–468. doi: 10.1093/infdis/171.2.465. [DOI] [PubMed] [Google Scholar]
- 41.Navarro-García F, López-Revilla R, Tsutsumi V, Reyes J L. Entamoeba histolytica: electrophysiologic and morphologic effects of trophozoite lysates on rabbit colon. Exp Parasitol. 1993;77:162–169. doi: 10.1006/expr.1993.1073. [DOI] [PubMed] [Google Scholar]
- 42.Navarro-Garcia F, Villaseca J M, Eslava C, López-Revilla R, Cravioto A. Abstracts of the 95th General Meeting of the American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1995. Toxigenic activity in Ussing chambers of a heat-labile protein secreted by enteroaggregative Escherichia coli, abstr. B-105. [Google Scholar]
- 43.Navarro-García F, Eslava C, Villaseca J M, López-Revilla R, Czeczulin J R, Srinivas S, Nataro J P, Cravioto A. In vitro effects of a high-molecular-weight heat-labile enterotoxin from enteroaggregative Escherichia coli. Infect Immun. 1998;66:3149–3154. doi: 10.1128/iai.66.7.3149-3154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pai M, Kang G, Ramakrishna B S, Venkataraman A, Muliyil J. An epidemic of diarrhoea in south India caused by enteroaggregative Escherichia coli. Indian J Med Res. 1997;106:7–12. [PubMed] [Google Scholar]
- 45.Paton A W, Paton J C. Characterization of IS1203, an insertion sequence in Escherichia coli O111:H−. Gene. 1994;150:67–70. doi: 10.1016/0378-1119(94)90859-1. [DOI] [PubMed] [Google Scholar]
- 46.Pohlner J, Halter R, Beyreuther K, Meyer T F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature. 1987;325:458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- 47.Prere M F, Chandler M, Fayet O. Transposition in Shigella dysenteriae: isolation and analysis of IS911, a new member of the IS3 group of insertion sequences. J Bacteriol. 1990;172:4090–4099. doi: 10.1128/jb.172.7.4090-4099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Provence D L, Curtiss R., III Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect Immun. 1994;62:1369–1380. doi: 10.1128/iai.62.4.1369-1380.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajakumar K, Sasakawa C, Adler B. Use of a novel approach, termed island probing, identifies the Shigella flexneri she pathogenicity island which encodes a homolog of the immunoglobulin A protease-like family of proteins. Infect Immun. 1997;65:4606–4614. doi: 10.1128/iai.65.11.4606-4614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saedler H, Heiss B. Multiple copies of the insertion-DNA sequences IS1 and IS2 in the chromosome of E. coli K-12. Mol Gen Genet. 1973;122:267–277. doi: 10.1007/BF00278602. [DOI] [PubMed] [Google Scholar]
- 51.Savarino S J, Fasano A, Robertson D C, Levine M M. Enteroaggregative Escherichia coli elaborate a heat-stable enterotoxin demonstrable in an in vitro rabbit intestinal model. J Clin Invest. 1991;87:1450–1455. doi: 10.1172/JCI115151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Savarino S J, Fasano A, Watson J, Martin B M, Levine M M, Guandalini S, Guerry P. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 represents another subfamily of E. coli heat-stable toxin. Proc Natl Acad Sci USA. 1993;90:3093–3097. doi: 10.1073/pnas.90.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Savarino S J, McVeigh A, Watson J, Cravioto A, Molina J, Echeverria P, Bhan M K, Levine M M, Fasano A. Enteroaggregative Escherichia coli heat-stable enterotoxin is not restricted to enteroaggregative E. coli. J Infect Dis. 1996;173:1019–1022. doi: 10.1093/infdis/173.4.1019. [DOI] [PubMed] [Google Scholar]
- 54.Schmitt W, Haas R. Genetic analyses of the Helicobacter pylori vacuolating cytotoxin: structural similarities with the IgA protease type of exported protein. Mol Microbiol. 1994;12:307–319. doi: 10.1111/j.1365-2958.1994.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 55.Smith H R, Cheasty T, Rowe B. Enteroaggregative Escherichia coli and outbreaks of gastroenteritis in UK. Lancet. 1997;350:814–815. doi: 10.1016/s0140-6736(05)62611-6. [DOI] [PubMed] [Google Scholar]
- 56.St Geme J W, III, Cutter D, Barenkamp S J. Characterization of the genetic locus encoding Haemophilus influenzae type b surface fibrils. J Bacteriol. 1996;178:6281–6287. doi: 10.1128/jb.178.21.6281-6287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stein M, Kenny B, Stein M A, Finlay B B. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J Bacteriol. 1996;178:6546–6554. doi: 10.1128/jb.178.22.6546-6554.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strauch K L, Johnson K, Beckwith J. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol. 1989;171:2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tzipori S, Montanaro J, Robins-Browne R M, Vial P, Gibson R, Levine M M. Studies with enteroaggregative Escherichia coli in the gnotobiotic piglet gastroenteritis model. Infect Immun. 1992;60:5302–5306. doi: 10.1128/iai.60.12.5302-5306.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vial P A, Robins-Browne R, Lior H, Prado V, Kaper J B, Nataro J P, Maneval D, Elsayed A, Levine M M. Characterization of enteroadherent-aggregative Escherichia coli, a putative agent of diarrheal disease. J Infect Dis. 1988;158:70–79. doi: 10.1093/infdis/158.1.70. [DOI] [PubMed] [Google Scholar]
- 62.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wanke C A, Schorling J B, Barrett L J, de Souza M A, Guerrant R L. Potential role of adherence traits of Escherichia coli in persistent diarrhea in an urban Brazilian slum. Pediatr Infect Dis J. 1991;10:746–751. doi: 10.1097/00006454-199110000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto T, Echeverria P. Detection of the enteroaggregative Escherichia coli heat-stable enterotoxin 1 gene sequences in enterotoxigenic E. coli strains pathogenic for humans. Infect Immun. 1996;64:1441–1445. doi: 10.1128/iai.64.4.1441-1445.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]