Abstract
This report describes an unusual case of uterine stump pyometra in a cat whose main clinical sign at presentation was abdominal straining. At the time of ovariohysterectomy, the surgeon reported that the uterine body had a purulent content. Nearly a month after the surgery the cat showed abdominal straining. The enlarged uterine stump, filled with purulent fluid, had caused a compression of the rectum and secondary intestinal sub-occlusion. Surgical revision consisted of draining the purulent content of the remnant of the uterine body and ablating as much of it as possible; checking of the ovarian pedicles revealed the presence of a small fragment of whitish tissue on the right side, which was shown to contain, by means of histological observation and immunohistochemical staining, ovarian tissue. Four months after surgical revision the queen did not show any pathological signs and 1 year later she is still in good health.
At the end of July 2009, an 8-month-old spayed mixed breed queen, living indoors with free access to the outdoors, was taken to the Veterinary Teaching Hospital of The Faculty of Veterinary Medicine of Turin University because of worsening of abdominal straining, a condition that had appeared about 3 weeks after ovariohysterectomy. The cat had also shown reduced appetite and activity. Ovariohysterectomy had been performed about 6 weeks earlier and the practitioner had reported that the uterus had a purulent content. Two weeks after surgery the queen had shown enlarged mammary glands and milk secretion, and cabergoline (5 μg/kg) had been administered for 5 days.
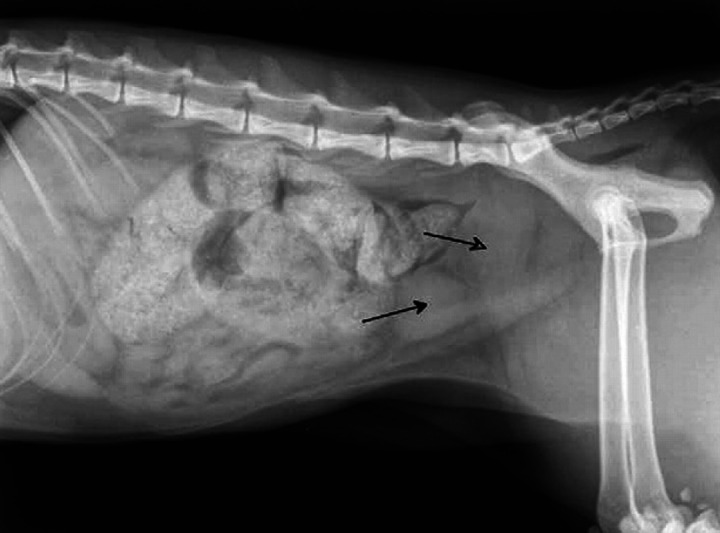
The cat appeared in quite good condition, alert and not dehydrated. Abdominal palpation was not painful but revealed the presence of a highly distended colon with soft content. The hypothesis of an intestinal sub-occlusion, which was the cause of the straining reported by the owner was confirmed by a radiological examination (Fig 1). Abdominal ultrasound revealed the presence of a fluid-filled structure referable to a uterine stump, measuring 3.7×2.6 cm, compressing the rectum dorsally. Cell blood count, biochemical parameters (aspartate aminotransferase, alanine aminotransferase, creatine kinase, γ-glutamyltransferase, creatinine, urea, glucose, total bilirubin, triglycerides, total cholesterol, alkaline phosphatase, amylase and lipase) and total serum protein were within normal limits. Feline leukaemia virus and feline immunodeficiency virus tests were negative. A surgical revision was suggested in order to eliminate the cause of the obstruction. After sedation, anaesthesia was induced with propofol and maintained with isoflurane in oxygen.
Fig 1.
Right lateral radiographic projection showing distended colon and rectum and enlarged uterine stump (open arrow) dorsal to the bladder (closed arrow).
A median ventral laparotomy revealed the presence of an enlarged uterine stump, with granulomatous tissue and adhesions to the bladder. Grossly, the uterine wall appeared thinned and the uterine lumen showed a remarkable amount of purulent exudate, which was drained. A sample was taken for culture and sensitivity testing. The remnant of uterine tissue was removed as completely as possible. Bilateral exploration of the abdomen, from the caudal pole of each kidney to the uterine stump was performed and an increased vascularisation of the right ovarian pedicle was detected, together with the presence of a small fragment of whitish tissue which was removed for histological examination. At the end of the surgical procedure an enema consisting of warm water and mineral oil was administered. Two more enemas were administered during the following days, and the intestinal functionality was re-established. The cat was treated with amoxicillin–clavulanic acid (12.5 mg/kg q 12 h), a therapy that was confirmed after culture results and antimicrobials susceptibility tests (β-haemolytic Streptococcus species). Recovery was uneventful.
Histopathological examination of the fragment of tissue removed from the right ovarian pedicle showed a mild inflammatory process with presence of haemorrhagic areas and scar tissue with fibroblasts. Other areas were characterised by the presence of both small cells with uniform round or oval hyperchromatic nuclei with finely granular chromatin, resembling granulosa cells, and larger cells with round or oval nuclei and cytoplasmic vacuolisation resembling luteal cells (Fig 2a).
Fig 2.
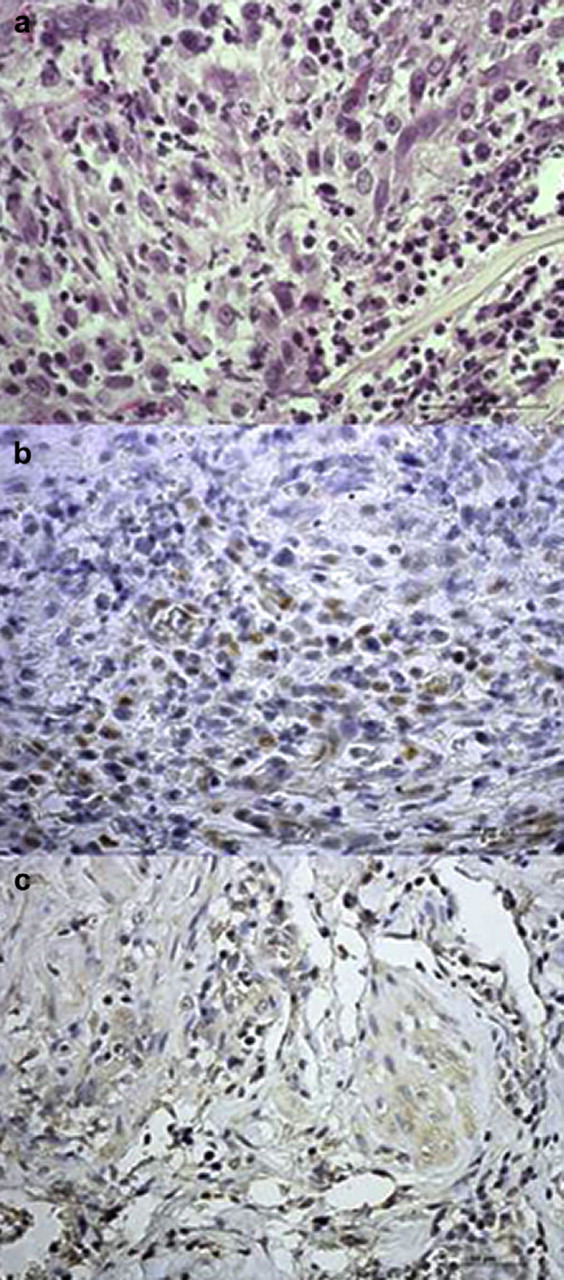
(a) Microphotograph of the fragment of tissue removed from the right ovarian pedicle showing the presence of both small cells with uniform round or oval hyperchromatic nuclei with finely granular chromatin, resembling granulosa cells, and larger cells with round or oval nuclei and cytoplasmic vacuolisation resembling luteal cells (haematoxylin and eosin 60×). (b) PR immunostaining in the tissue fragment, showing strongly positive nuclei of putative granulosa cells (60×). (c) Aromatase immunostaining in the tissue fragment, showing a diffuse cytoplasmic immunoreactivity in both granulosa and luteal putative cells (40×).
Histopathological examination of the uterine stump revealed a mild cystic endometrial hyperplasia, together with a diffuse chronic inflammatory infiltrate which was present in all layers of the uterine wall.
In order to confirm the hypothesis of ovarian remnant, 4 μ thick sections from the tissue fragment were subjected to immunohistochemistry with anti-progesterone receptor (PR) and anti-aromatase antibodies. PR expression is selectively induced in granulosa cells of preovulatory follicles by luteinising hormone (LH) 1–3 and persists in the corpus luteum of the ovary. 4,5 Aromatase is a key enzyme in ovarian steroidogenesis and plays an important role in sexual differentiation, oestrogen biosynthesis, fertility and carcinogenesis. It is highly conserved among mammals and it is expressed in granulosa cells and participates in conversion of androgens to oestrogens. 6
The fragment of tissue we removed from the right ovarian pedicle showed a positive staining for both PR and aromatase. In particular, nuclei of putative granulosa cells stained strongly positive for PR, while putative luteal cells showed a mild cytoplasmic staining (Fig 2b). Immunohistochemistry for aromatase showed a diffuse cytoplasmic immunoreactivity in several areas where both granulosa and luteal cells were detected (Fig 2c).
The reported findings suggested the presence of ovarian tissue in the fragment.
Four months after surgical revision, the cat had gained weight and the owner reported that no pathological signs had occurred since the operation. At ultrasound the uterine stump was appreciable as a small (1×0.5 cm) hyperechoic area dorsal to the bladder which was interpreted as fibrosis. To this day (ie, about 1 year later) the cat has been healthy.
In cats, uterine stump pyometra is reported to occur in neutered queens either because of ovarian remnants or following administration of progestagens. 7,8 Ovarian remnant syndrome is usually diagnosed when a previously spayed queen shows recurrent behavioural signs of oestrous. 9,10 In our case, the queen had been in heat for the first time at about 6 months of age, but neither signs of oestrus were present after ovariohysterectomy, nor had she ever received progestagens. The interval between ovariohysterectomy and uterine stump pyometra diagnosis was around 40 days; our hypothesis is that uterine stump pyometra was sustained by the presence of functional ovarian luteal tissue. The duration of luteal function is about 40–50 days in the non-pregnant queen, 11 and, in our case, the luteal tissue localised in the examined fragment could represent the remnant of old corpora lutea. We believe that ovarian luteal tissue maintained the conditions of endometrial hyperplasia and increase of the secretory activity of the endometrial glands, 7,12 which allowed the persistence/recurrence of the uterine infection. We did not measure serum progesterone concentration at the moment of surgical revision, but, according to our hypothesis, it could have been low or around basal. From the history of the queen, we know that she had been treated with cabergoline because of mammary secretion following ovariohysterectomy: the prolactin increase causing milk secretion is likely to have been induced by a sharp decline in progesterone concentration, and this means that the queen had ovulated and had corpora lutea on ovaries when she had undergone ovariohysterectomy. In cats, cabergoline has an antiprolactinic action and induces abortion 13 by means of a slow (3–4 days) reduction in plasma progesterone concentration. 14 After the end of cabergoline effect, residual luteal tissue may have regained functionality. However, we cannot exclude that the observed condition might have developed also in the absence of functional luteal tissue: in fact, although a correlation between pyometra and corpora lutea presence has been observed in cats, 8 cats with pyometra may also have low serum progesterone concentration because the effects of progesterone on uterine mucosa and glands may persist beyond the end of the luteal phase. 7
Acknowledgements
The authors are grateful to the Centro di Referenza di Patologia Comparata ’Bruno Maria Zaini’ for the skilful assistance.
References
- 1. Park O.-K., Mayo K. Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge, Mol Endocrinol 5, 1991, 967–978. [DOI] [PubMed] [Google Scholar]
- 2. Natraj U., Richards J.S. Hormonal regulation, localization and functional activity of the progesterone receptor in granulosa cells of rat preovulatory follicles, Endocrinology 133, 1993, 761–769. [DOI] [PubMed] [Google Scholar]
- 3. Park-Sarge O.-K., Parmer T.G., Gu Y., Gibori G. Does the rat corpus luteum express the progesterone receptor gene?, Endocrinology 136, 1995, 1537–1543. [DOI] [PubMed] [Google Scholar]
- 4. Shao R., Markström E., Friberg P., Johansson M., Billig H. Expression of progesterone receptor (PR) A and B isoforms in mouse granulosa cells: stage-dependent PR-mediated regulation of apoptosis and cell proliferation, Biol Reprod 68, 2003, 914–921. [DOI] [PubMed] [Google Scholar]
- 5. Press M.F., Greene G.L. Localization of progesterone receptor with monoclonal antibodies to the human progestin receptor, Endocrinology 122, 1988, 1165–1175. [DOI] [PubMed] [Google Scholar]
- 6. Tsonis C.G., Carson R.S., Findlay J.K. Relationships between aromatase activity, follicular fluid oestradiol 17 beta and testosterone concentrations, and diameter and atresia of individual ovine follicles, J Reprod Fertil 72, 1984, 152–163. [DOI] [PubMed] [Google Scholar]
- 7. Johnston S.D., Kustritz M.V. Root, Olson P.N.S. Disorders of the feline uterus and uterine tubes. Johnston S.D., Kustritz M.V. Root, Olson P.N.S. Canine and feline theriogenology, 1st edn, 2001, Saunders: Philadelphia, 463–471. [Google Scholar]
- 8. Agudelo C.F. Cystic endometrial hyperplasia–pyometra complex in cats, Vet Q 27, 2005, 173–182. [PubMed] [Google Scholar]
- 9. Wallace M.S. The ovarian remnant syndrome in the bitch and queen, Vet Clin North Am Small Anim Pract 21, 1991, 501–507. [DOI] [PubMed] [Google Scholar]
- 10. Miller D.M. Ovarian remnant syndrome in dogs and cats: 46 cases (1988–1992), J Vet Diagn Invest 7, 1995, 572–574. [DOI] [PubMed] [Google Scholar]
- 11. Paape S.R., Shille V.M., Seto H., Stabenfeldt G.H. Luteal activity in the pseudopregnant cat, Biol Reprod 13, 1975, 470–474. [DOI] [PubMed] [Google Scholar]
- 12. Lawler D.F., Evans R.H., Reimers T.J., Colby E.D., Monti K.L. Histopathologic features, environmental factors, and serum estrogen, progesterone, and prolactin values associated with ovarian phase and inflammatory uterine disease in cats, Am J Vet Res 52, 1991, 1747–1753. [PubMed] [Google Scholar]
- 13. Jöchle W., Arbeiter K., Post K., Ballabio R., D’Ver A.S. Effects on pseudopregnancy, pregnancy and interoestrous intervals of pharmacological suppression of prolactin secretion in female dogs and cats, J Reprod Fertil Suppl 39, 1989, 199–207. [PubMed] [Google Scholar]
- 14. Verstegen J.P., Onclin K., Silva L.D., Donnay I. Abortion induction in the cat using prostaglandin F2 alpha and a new anti-prolactinic agent, cabergoline, J Reprod Fertil Suppl 51, 1997, 259–263. [PubMed] [Google Scholar]