Abstract
The effect of probiotic therapy in chronic kidney disease (CKD) in cats is poorly defined, but gaining in popularity. However, cat owners often prefer to administer probiotics by combining them with food, rather than administering capsules intact, as is prescribed by the manufacturer. The efficacy of such non-recommended administration is unknown. In this double-blinded, controlled clinical trial, 10 cats with naturally-occurring CKD were randomized to receive either a probiotic–prebiotic combination (synbiotic) or psyllium husk (prebiotic only) for 2 months. Medications were sprinkled and mixed into food or given as a slurry. Blood urea nitrogen (BUN) and creatinine were measured twice prior to administration of medication, and then monthly for 2 months during the medication administration. Owners and clinicians were masked as to treatment. The maximal percentage change in BUN and creatinine was calculated for each cat. No differences in percentage change were detected between groups (P=0.8 for both BUN and creatinine). The synbiotic supplement used in this study, when applied to food or administered as a slurry fails to reduce azotemia in cats with CKD. Therefore, owners should not administer this synbiotic in this manner.
Chronic kidney disease (CKD) is a common geriatric feline disorder and presents with high morbidity and mortality. 1,2 Therapy for CKD is varied and generally directed at preserving renal function, minimizing uremic toxin production, promoting solute excretion via fluid diuresis, countering anemia, protecting the gastrointestinal tract from secondary uremic injury and providing calcium and phosphorus homeostasis, either via specific drugs, 3 or via nutritional management. 4,5
Recently, investigators have examined the ability of probiotic–prebiotic combination (known as ‘synbiotic’) therapy to aid in reducing azotemia – a process called ‘enteric dialysis’. 6–10 Specific bacteria capable of metabolizing urea, creatinine, indoles, phenol and nitrosamine into non-toxic metabolites, have been selected for this purpose. One such synbiotic (Azodyl; Vetoquinol, USA) contains strains of three naturally occurring bacteria (Streptococcus (enterococcus) thermophilus, Lactobacillus acidophilus, and Bifidobacterium longum) combined with a prebiotic (psyllium husk) in an enteric-coated capsule that releases the contents within the ileo-colic region. Studies in nephrectomized rats and pigs demonstrated reductions in azotemia after administration of this synbiotic. 9,10 Studies in humans have also suggested that synbiotic therapy can alter azotemia in patients with CKD. 6–8 One case series suggested that this synbiotic, administered by sprinkling onto food, reduced azotemia by up to 36% in cats with CKD. 11 This method of administration is contrary to that recommended by the manufacturer for two reasons: (1) S thermophilus and B longum are subject to acid degradation in the stomach and (2) B longum is an obligate anaerobe, so activation prior to delivery in the distal intestine likely inactivates the organism. Owners of cats with CKD are increasingly interested in ‘natural’ supplemental therapy, such as synbiotic therapy, which comes encapsulated. However, regular, frequent, administration of intact capsules is problematic for many owners. Consequently, owners often elect to administer the capsule contents by sprinkling them onto food or in a slurry, against the manufacturers’ recommendations. Whether this method of administration is effective in reducing azotemia is unknown.
Therefore, we sought to examine the ability of this synbiotic to reduce azotemia in cats with CKD when it was applied in a non-recommended manner, by either sprinkling capsule contents onto food, or mixing contents into a slurry and administering per os. We hypothesized that this synbiotic would fail to reduce azotemia in these patients when administered in this manner.
Materials and methods
Subject recruitment and enrollment
Cats with stable CKD were recruited prospectively via a trial website (http://www.vin.com/ART) from August 2007 to July 2010. Cats of any age and sex were eligible for study entry. Cats had to have previously documented CKD which was not rapidly progressive and of at least 2 months duration. Cats had to have objective evidence of non-infectious CKD, as determined by elevated blood urea nitrogen (BUN) and creatinine with inappropriate urine concentrating ability (urine specific gravity (USG) <1.030). ‘Rapidly progressive CKD’ was defined as markedly worsening indices of renal function (increases of >50%) over the previous 2–6 months or development of uremia in the previous 2–6 months. Cats could be receiving additional medications or dietary modifications at the time of enrollment; however, these were to be kept constant throughout the study period.
Cats were excluded if they met the following criteria: CKD diagnosed less than 2 months prior to enrollment; cats with co-morbidities likely to impact clinical status or ability to complete the trial (eg, diabetes, hyperthyroidism, heart disease); cats with uncontrolled hypertension or untreated hypertension; cats with acute renal failure or renal failure of infectious or neoplastic causes.
Cats could be withdrawn from the study without prejudice at any time by the owners. Cats with substantial worsening in clinical status that precluded completion of the study were also withdrawn and not included in analysis.
Study design
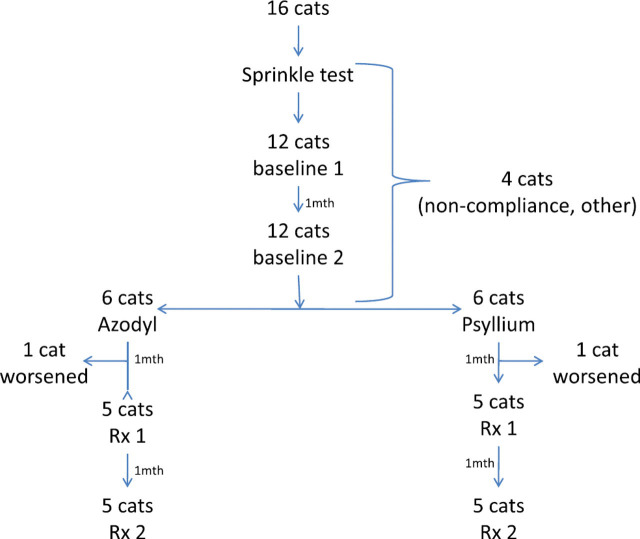
All cats were examined and treated by the enrolling primary care veterinarians through the entire study. Prior to enrollment, samples of the synbiotic were sent to owners to ensure compliance. Owners were instructed to sprinkle contents on food for 3–4 days to ensure that the patients would consume the diet. Once patient compliance was demonstrated, blood collection packs were distributed to the enrolling clinician. The study design is shown in Fig 1. Briefly, two baseline blood samples were collected by the primary care clinician at 30-day intervals to demonstrate disease stability. After analysis of the second baseline sample, patients were randomized to receive either placebo or synbiotic, which were then mailed on ice overnight to the owner with storage and administration instructions. Owners and primary care clinicians were masked as to the group assignment. Clinicians were provided with the clinical pathology reports after each sampling analysis. Two further blood samples were collected during the therapeutic period 30 and 60 days after initiating therapy.
Fig 1.
Study design.
Synbiotic dosing was based on manufacturer's recommendations: for cats weighing under 5 lb, owners sprinkled one capsule per day on to the food. For cats between 5 and 10 lb, owners sprinkled one capsule twice daily on to the food. For cats over 10 lb, owners sprinkled two capsules in the morning and one capsule in the evening on to food.
Owners could administer the synbiotic either by sprinkling onto food, mixing into the food, or mixing into a slurry and syringing this into the cat's mouth, but could not administer capsules intact.
Control capsules were provided by the manufacturer (Vetoquinol), and looked identical to active product, but contained only psyllium husk, which is the filler/prebiotic in the active product.
All samples were shipped overnight to the Animal Health Diagnostic Laboratory at Cornell University for analysis.
Statistics
Patients were randomized by use of an online virtual coin toss (http://www.random.org/coins/?num=1&cur=60-aud.1dollar). Once all assignments within one group were filled, all subsequent patients were assigned to the other group.
Only cats that completed the study were included in the analysis. Cats that were withdrawn from the study were not included.
We acquired patient BUN and creatinine concentrations at each time-point. To allow for potential effects of dehydration on BUN, we further visually examined serum albumin concentrations. In order to optimize the possibility for detecting an effect of the synbiotic, the percentage change in BUN and creatinine was calculated by using the highest baseline value and the lowest post-treatment value for each variable. Furthermore, alpha was set at 0.2 (increasing the risk of a type 1 error), while maintaining power at 0.8. We chose a difference in % reduction of 30% between control and synbiotic, based on data obtained in a minipig model of kidney disease, and because we felt that a reduction of this magnitude would be clinically meaningful. 10 We estimated the sample population standard deviation at 10%. Using these values, we calculated a sample size of five patients in each group would be necessary to demonstrate a 30% difference in mean reduction of azotemia.
Differences in the percentage change of clinical variables were compared by Mann–Whitney U test.
Results
Sixteen cats were recruited into the study; however, six subsequently failed to complete the study and were not included in analysis. Reasons for removal from the study included compliance (two cats), failure to complete blood sampling (one), death or euthanasia for worsening renal failure (two; one placebo, one probiotic–prebiotic combination) or other causes (one cat). Ten cats completed the study.
Only one cat required capsule contents to be administered by the syringe method. All other cats consumed the capsule contents with their regular diet.
Table 1 details the results of the study. Neither the percentage change in BUN nor percentage change in creatinine differed between groups (P=0.841 for each analyte) (Fig 2). Albumin concentrations remained within reference intervals at all times for all cats (Table 1).
Table 1.
BUN, creatinine and albumin concentrations in cats treated with azodyl sprinkled onto food.
| Placebo | Azodyl | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cat | IRIS | B1 | B2 | T1 | T2 | % Change | Cat # | IRIS | B1 | B2 | T1 | T2 | % Change |
| BUN (mg/dl) | |||||||||||||
| C1 | 4 | 140 | 112 | 89 | 149 | −36.4 | A1 | 2 | 46 | 47 | 44 | 43 | −8.5 |
| C2 | 3 | 51 | 53 | 68 | 56 | 5.7 | A2 | 4 | 79 | 78 | 116 | 143 | 46.8 |
| C3 | 3 | 47 | 50 | 51 | 65 | 2 | A3 | 3 | 78 | 70 | 66 | 64 | −17.9 |
| C4 | 2 | 38 | 40 | 39 | 53 | −2.5 | A4 | 3 | 55 | 64 | 66 | 63 | −1.6 |
| C5 | 3 | 54 | 47 | 52 | 55 | −3.7 | A5 | 2 | 37 | 43 | 44 | 49 | 2.3 |
| Creatinine (mg/dl) | |||||||||||||
| C1 | 4 | 7.3 | 4.4 | 6 | 5.5 | −24.7 | A1 | 2 | 2.5 | 2.4 | 2.5 | 2.5 | 0 |
| C2 | 3 | 3.1 | 3.3 | 3.3 | 2.7 | −18.2 | A2 | 4 | 6 | 6.5 | 10.1 | 9.9 | 52.3 |
| C3 | 3 | 3.3 | 3.6 | 3.5 | 3.2 | −11.1 | A3 | 3 | 3.8 | 3.3 | 2.8 | 2.7 | −28.9 |
| C4 | 2 | 2.5 | 2.6 | 2.7 | 3.1 | 3.8 | A4 | 3 | 4.3 | 4.1 | 4.2 | 4.2 | −2.3 |
| C5 | 3 | 3.2 | 3.6 | 4.2 | 4.3 | 16.7 | A5 | 2 | 2.3 | 2.7 | 2.6 | 2.7 | −3.7 |
| Albumin (g/dl) | |||||||||||||
| C1 | 4 | 3.6 | 3 | 3.1 | 3.1 | −13.9 | A1 | 2 | 3.7 | 4.0 | 4.2 | 3.9 | −2.5 |
| C2 | 3 | 3.0 | 3.4 | 3.4 | 3.3 | −2.9 | A2 | 4 | 2.9 | 3.2 | 3.5 | 3.2 | 0 |
| C3 | 3 | 3.4 | 3.4 | 3.4 | 3.3 | −2.9 | A3 | 3 | 3.3 | 3.4 | 3.0 | 3.1 | −11.8 |
| C4 | 2 | 3.4 | 3.5 | 3.6 | 3.2 | −8.6 | A4 | 3 | 3.9 | 3.9 | 4.0 | 3.7 | −5.1 |
| C5 | 3 | 3.8 | 3.9 | 3.6 | 3.5 | −10.3 | A5 | 2 | 3.4 | 3.4 | 3.0 | 3.1 | −11.8 |
B1, B2=baseline sample values, T1, T2=treatment sample values. Numbers in bold represent the baseline (maximum) and treatment (minimum) values used to calculate the % change. Underlined albumin values represent the values of albumin corresponding to the maximal baseline and minimal treatment BUN concentrations for each cat. IRIS—International Renal Interest Society Classification.
Fig 2.
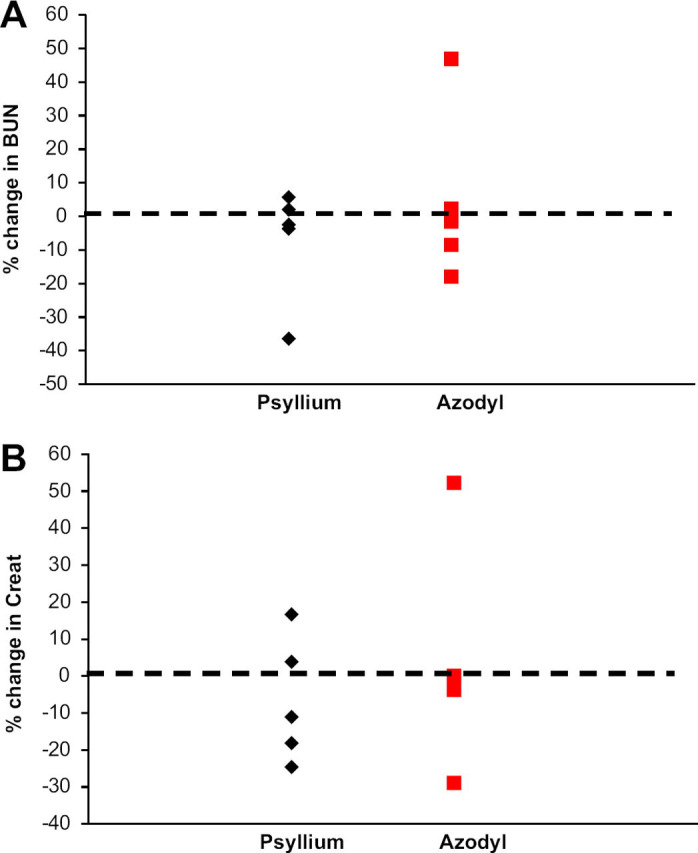
Percentage change in (A) BUN and (B) creatinine in cats with CKD treated with psyllium or Azodyl. A negative value indicates a decrease in the measured analyte; a positive value indicates an increase in the measured analyte.
Discussion
Our study shows that cats, administered this synbiotic for 2 months by sprinkling onto or mixing with food, fail to demonstrate an improvement in their azotemia, as measured by either a decrease in BUN or creatinine. Therefore, owners and clinicians electing to treat cats with this synbiotic should do so in the manner advocated by the manufacturer. However, whether administration in the prescribed manner would result in a decrease in BUN or creatinine remains unknown, as our study did not address this question.
Our results differ from those obtained by a previous investigator. 11 However, in that study, no control group was included, and the range of reductions in BUN ranged from 4.7% to 36.5%, while reductions in creatinine ranged from 0% to 51.9%. Our study design allowed us to determine that the CKD in our patients was stable at enrollment, rather than recovering from an acute insult. Additionally, we compared outcomes with a control group that received the prebiotic, but not the probiotic component of the therapy.
Our results also differ from studies in humans, pigs or rats with either spontaneous or experimentally induced kidney disease. 6,7,9,10 However, in those studies, the synbiotic was administered as an intact capsule, as recommended by the manufacturer. Furthermore, the changes, while statistically significant for some analytes, varied by a much smaller concentration than we sought (10–30% difference between placebo and probiotic). Therefore, whether a larger study could have detected a smaller difference in azotemia between control and synbiotic groups, is unknown.
We examined the non-standard method of administration because owners of feline patients are often unable to administer capsules or medications intact to their pets. This was illustrated in the previously published case series, where all owners resorted to administration by sprinkling capsule contents onto food. Thus, if this proved to be an acceptable method of administration, it would allow owners to more easily administer the medication to their pets.
We set a fairly large reduction in azotemia a priori as a target (30% reduction). We did this for three reasons: (1) we felt that a reduction of this magnitude would be clinically relevant (from a perspective of patient improvement), (2) reductions substantially less than this could be due to day-to-day variability and variability in sample analysis and (3) prior studies in experimental models suggested a 30% reduction after 2 months of therapy was achievable. 10 Examination of the raw data would suggest that even a substantially lower target value would be unlikely to yield a significant difference, although larger sample population would be required to determine if this was true. We offset the large target reduction (30%) by accepting a less stringent alpha value (0.2) and examining the biggest difference between pre- and post-therapy analyte concentrations.
We examined albumin concentrations in each cat to account for the possibility that incidental dehydration during the treatment period could result in a pre-renal component to the azotemia that would mask the effect of the synbiotic on the renal component. All albumin concentrations were within reference intervals at all time-points. The greatest within-cat difference between albumin concentrations was 0.6 g/dl (2.9–3.5 g/dl) in one cat receiving synbiotic (cat A2), and 0.5 g/dl (3.6–3.1 g/dl) in one cat receiving psyllium husk (cat C1). These two cats had the biggest percentage increase and percentage decrease in BUN and creatinine respectively, and these changes occurred at the same time as the maximal change in albumin concentration (Table 1). Thus, it is possible that the most dramatic responses seen in each group with respect to changes in BUN and creatinine concentrations could be related to subclinical changes in hydration status. However, even if these two cats are removed from analysis, no difference between groups exists. Furthermore, BUN concentration in cat C1 returned to the maximal baseline concentration at the second treatment time-point without a concomitant increase in albumin concentration, suggesting that the observed change in BUN was independent of changes in albumin or hydration (Table 1). Similarly, cat A2 had an increase in albumin during the two baseline measurements of 0.3 g/dl without any change in BUN, and a subsequent 2 month post-treatment measurement 0.3 g/l lower than the 1 month measurement, despite a concurrent increase in BUN, suggesting a progression of the renal disease, rather than any effect of dehydration (Table 1).
We used psyllium husk as the control therapy in this study. Psyllium husk is the ‘filler’ in this synbiotic preparation and could be considered a placebo. However, considerable evidence exists that psyllium husk is not inert, but might affect endogenous microfloral composition. 12–14 Nevertheless, one small study examining the ability of psyllium husk to reduce azotemia failed to find any effect. 15 Our data support these findings, because we failed to observe any significant change in BUN or creatinine in the control group.
In conclusion, our study suggests that administration of this synbiotic by sprinkling capsule contents into food or by mixing with food does not reduce azotemia in cats with stable CKD. Additional studies are required to determine whether administration of intact capsules reduces azotemia in these patients.
Acknowledgements
This study was funded by the VIN Foundation. Azodyl and control capsules were provided by Vetoquinol USA.
References
- 1. White J.D., Norris J.M., Baral R.M., Malik R. Naturally-occurring chronic renal disease in Australian cats: a prospective study of 184 cases, Aust Vet J 84, 2006, 188–194. [DOI] [PubMed] [Google Scholar]
- 2. Boyd L.M., Langston C., Thompson K., Zivin K., Imanishi M. Survival in cats with naturally occurring chronic kidney disease (2000–2002), J Vet Intern Med 22, 2008, 1111–1117. [DOI] [PubMed] [Google Scholar]
- 3. Plotnick A. Feline chronic renal failure: long-term medical management, Compend Contin Educ Vet 29, 2007, 342–350, quiz 351 [PubMed] [Google Scholar]
- 4. Elliott D.A. Nutritional management of chronic renal disease in dogs and cats, Vet Clin North Am Small Anim Pract 36, 2006, 1377–1384, viii. [DOI] [PubMed] [Google Scholar]
- 5. Ross S.J., Osborne C.A., Kirk C.A., Lowry S.R., Koehler L.A., Polzin D.J. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats, J Am Vet Med Assoc 229, 2006, 949–957. [DOI] [PubMed] [Google Scholar]
- 6. Ranganathan N., Ranganathan P., Friedman E.A., et al. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease, Adv Ther 27, 2010, 634–647. [DOI] [PubMed] [Google Scholar]
- 7. Ranganathan N., Friedman E.A., Tam P., Rao V., Ranganathan P., Dheer R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: a 6-month pilot scale trial in Canada, Curr Med Res Opin 25, 2009, 1919–1930. [DOI] [PubMed] [Google Scholar]
- 8. Ranganathan N., Patel B.G., Ranganathan P., et al. In vitro and in vivo assessment of intraintestinal bacteriotherapy in chronic kidney disease, ASAIO J 52, 2006, 70–79. [DOI] [PubMed] [Google Scholar]
- 9. Ranganathan N., Patel B., Ranganathan P., et al. Probiotic amelioration of azotemia in 5/6th nephrectomized Sprague-Dawley rats, Scientific World Journal 5, 2005, 652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ranganathan N, Patel B, Ranganathan P, et al. Probiotics reduce azotemia in Gottingen minipigs. Poster presentation at the 3rd World Congress of Nephrology, June 26–30, 2005, Singapore. [accessed 01.11.10]: http://www.vetoquinolusa.com/Studies/Azotemia/PosterPresentation.pdf
- 11. Palmquist R. A preliminary clinical evaluation of Kibow Biotics, a probiotic agent, on feline azotemia, J Am Holist Vet Med Assoc 24, 2006, 23–27. [Google Scholar]
- 12. Kanauchi O., Mitsuyama K., Araki Y., Andoh A. Modification of intestinal flora in the treatment of inflammatory bowel disease, Curr Pharm Des 9, 2003, 333–346. [DOI] [PubMed] [Google Scholar]
- 13. Fujimori S., Gudis K., Mitsui K., et al. A randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis, Nutrition 25, 2009, 520–525. [DOI] [PubMed] [Google Scholar]
- 14. Elli M., Cattivelli D., Soldi S., Bonatti M., Morelli L. Evaluation of prebiotic potential of refined psyllium (Plantago ovata) fiber in healthy women, J Clin Gastroenterol 42 (Suppl 3 Pt 2), 2008, S174–S176. [DOI] [PubMed] [Google Scholar]
- 15. Dessau R.B., Olsen O.B., Frifelt J.J., Skott H. Influence of psyllium seed husk on azotemia, electrolytes, and bowel regulation in patients on CAPD, Perit Dial Int 9, 1989, 351. [PubMed] [Google Scholar]