Abstract
A case of nasopharyngeal stenosis with secondary hiatal hernia is described. An 8-year-old castrated male domestic shorthair cat was referred for a chronic upper respiratory problem and presumptive vomiting. Despite conservative management by the primary care veterinarian, the cat's condition progressed. The cat was presented to an emergency facility prior to referral to a specialty hospital. On presentation, inspiratory stridor was evident. Thoracic radiography revealed a hiatal hernia. Computed tomography indicated pharyngeal edema and probable nasopharyngeal stenosis. Endoscopy confirmed the presence of nasopharyngeal stenosis consistent with either stricture or choanal atresia. Balloon dilation of the choana was performed. The hiatal hernia regressed spontaneously post-resolution of the nasopharyngeal stenosis. The cat remained asymptomatic at recheck 3 months later.
An 8-year-old 3.6 kg (7.9 lb) castrated male indoor only domestic shorthair cat was presented to an emergency care facility for dyspnea and hematemesis. The owners reported that the cat had an intermittent chronic upper respiratory problem since they first acquired him as a kitten, the etiology of which was never determined. Three months prior to presentation, the cat had experienced frequent episodes of presumptive vomiting after wheezing. Therapy with furosemide, metoclopramide and terbutaline had been instituted by the primary care veterinarian. Despite conservative management, the respiratory difficulties remained unchanged and the presumptive vomiting continued. Examination at the emergency facility revealed stridor with severe nasal congestion, wheezing, and dyspnea. No oral or nasal abnormality was apparent on physical exam. Thoracic radiographs, obtained with the patient conscious, revealed a soft tissue opacity over the caudal thoracic esophagus near the diaphragm. Cardiovascular and pulmonary structures were unremarkable. Barium administration detailed a dilated esophagus. A respiratory acidosis with metabolic compensation was detected on arterial blood gas analysis. A complete blood count was unremarkable. Exploratory laparotomy was performed due to a suspected esophageal foreign body. No foreign body was present and multiple biopsies were obtained, including liver, stomach, jejunum, pancreas and mesenteric lymph node. Recovery from anesthesia was uneventful. The patient was monitored closely and provided with supplemental oxygen as indicated overnight. The cat continued to decline and was referred for further diagnostics and intensive care.
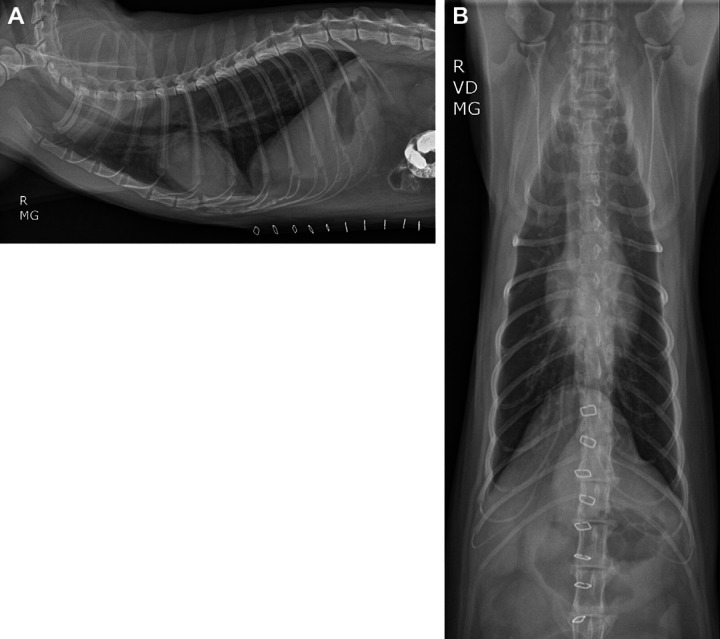
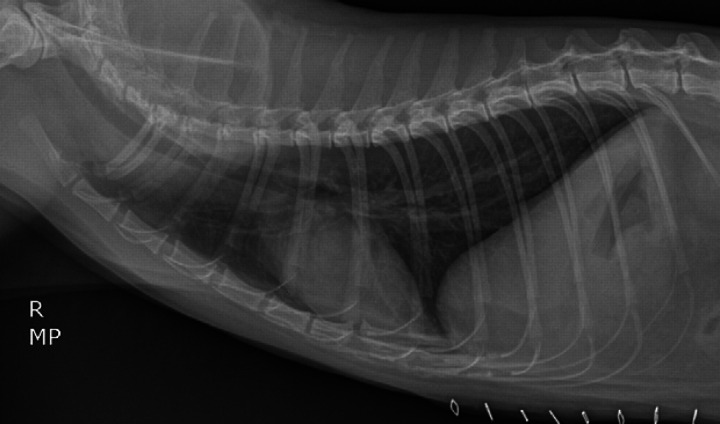
On presentation, the patient's vital signs were within normal limits and the cat exhibited an inspiratory stridor. A complete blood count revealed a mild anemia (hematocrit 28.1%; reference interval (RI) 29–45%) and a stress leukogram. Hypocalcemia (7.5 mg/dl; RI 8.2–11.8), hypophosphatemia (2.5 mg/dl; RI 3.0–7.0), hyponatremia (140 mEq/l; RI 147–156), hypochloremia (104 mEq/l; RI 111–125), hypokalemia (3.0 mEq/l; RI 3.9–5.3) and an increased aspartate aminotransferase (AST) (127 U/l; RI 5–55) were present as well. The cat tested feline leukemia virus antigen and feline immunodeficiency virus antibody negative. Thoracic radiographs revealed an ill-defined soft tissue opacity in association with the esophagus, just cranial to the diaphragm, along with gaseous dilation of the mid and caudal extent of the thoracic esophagus. The soft tissue opacity was consistent with a hiatal hernia (Fig 1).
Fig 1.
Right lateral (A) and ventrodorsal (B) radiographic views of the thorax of an 8-year-old castrated male domestic shorthair cat evaluated for inspiratory stridor and vomiting. There is gaseous dilation of the mid and caudal extent of the thoracic portion of the esophagus. An ill-defined soft tissue opacity is seen in association with the esophagus, just cranial to the crura of the diaphragm. Cardiovascular and pulmonary parenchyma are unremarkable. Skin staples are noted along the cranioventral abdominal wall. There is reduced serosal detail in the cranial ventral abdomen and previously administered barium is evident in the intestines.
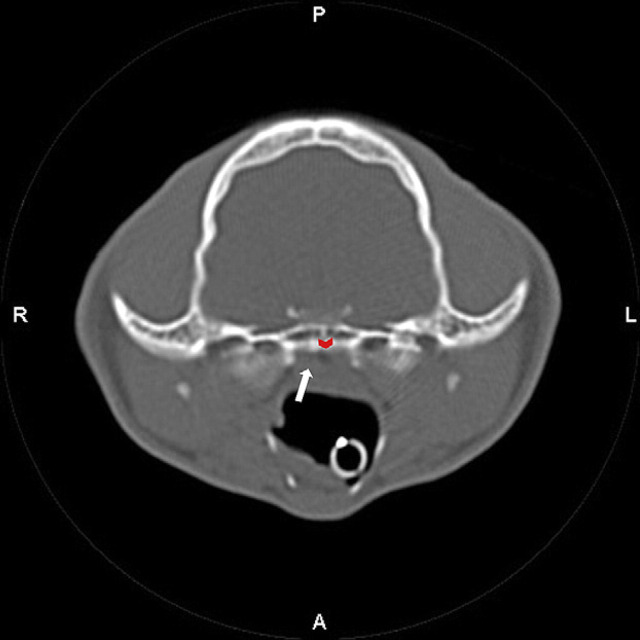
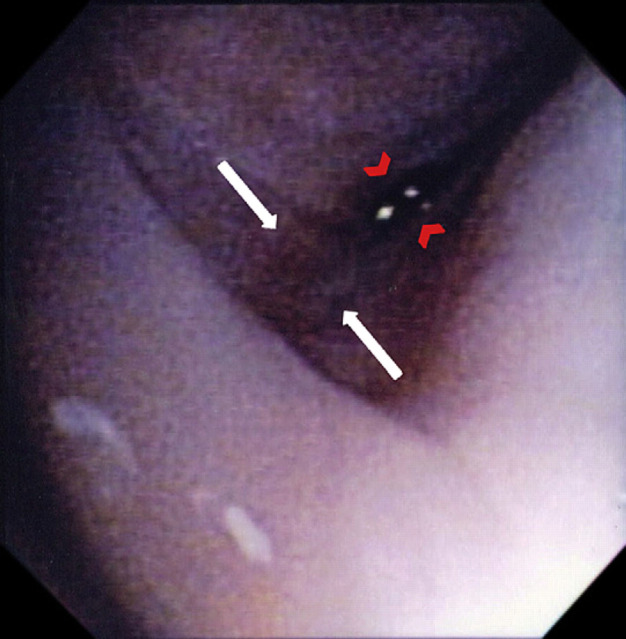
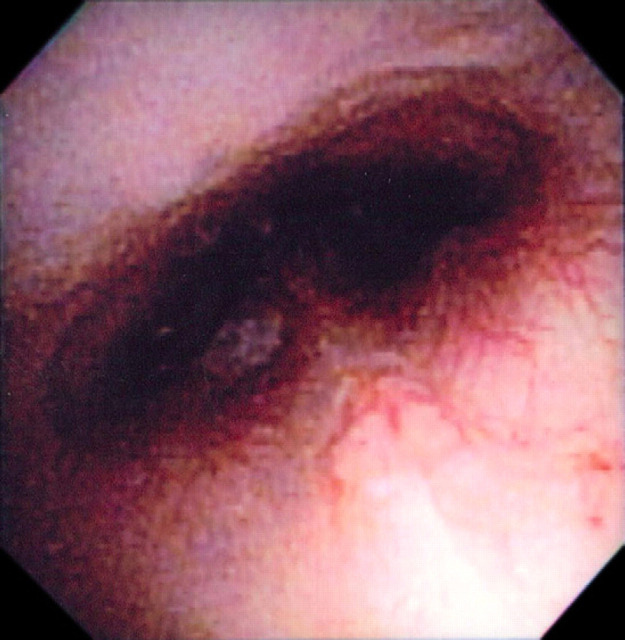
The differential diagnoses of a nasopharyngeal polyp or stenosis were considered and computed tomography (CT) of the nasopharyngeal region was performed. The cat was premedicated with buprenorphine (0.02 mg/kg IV), acepromazine (0.02 mg/kg IV) and atropine (0.02 mg/kg IV). Anesthesia was induced with propofol (5 mg/kg IV) and was maintained with isoflurane and oxygen. Cefazolin (22 mg/kg IV) was administered at the start of the CT scan. Head CT evaluation, including helical series and reconstructing transverse images at 2 mm slice thickness, was performed. The images depicted no thickening of either tympanic bulla and normal external ear canals. However, there was an ill-defined soft tissue opacity in the dorsal aspect of the pharyngeal region and caudal aspect of the nasopharynx along with an unusual associated gas opacity (Fig 2). The findings were consistent with pharyngeal edema and suspected nasopharyngeal stenosis. Nasopharyngoscopy confirmed severe narrowing of the nasopharyngeal region consistent with either stricture or choanal atresia (Fig 3). Balloon dilation of the choana was subsequently performed and observed to dilate on post-ballooning nasopharyngoscopy (Fig 4). Recovery from anesthesia was routine and uneventful. Postoperative treatments included IV administration of isotonic fluids (12 ml/h), IV cefazolin (22 mg/kg IV q8h), and buprenorphine (0.02 mg/kg IV q8h).
Fig 2.
A CT evaluation of the skull was performed acquiring helical series and reconstructing transverse images at 2-mm slice thicknesses. There is an ill-defined soft tissue opacity in the dorsal aspect of the pharyngeal region and caudal aspect of the nasopharynx (white arrow) along with an unusual associated gas opacity (red arrowhead).
Fig 3.
Retroflexed endoscopic view of the nasopharyngeal opening with nasopharyngeal stenosis (white arrows). The opening is reduced to a small hole (red arrowheads).
Fig 4.
Retroflexed endoscopic view of the nasopharyngeal opening after balloon dilation of nasopharyngeal stenosis.
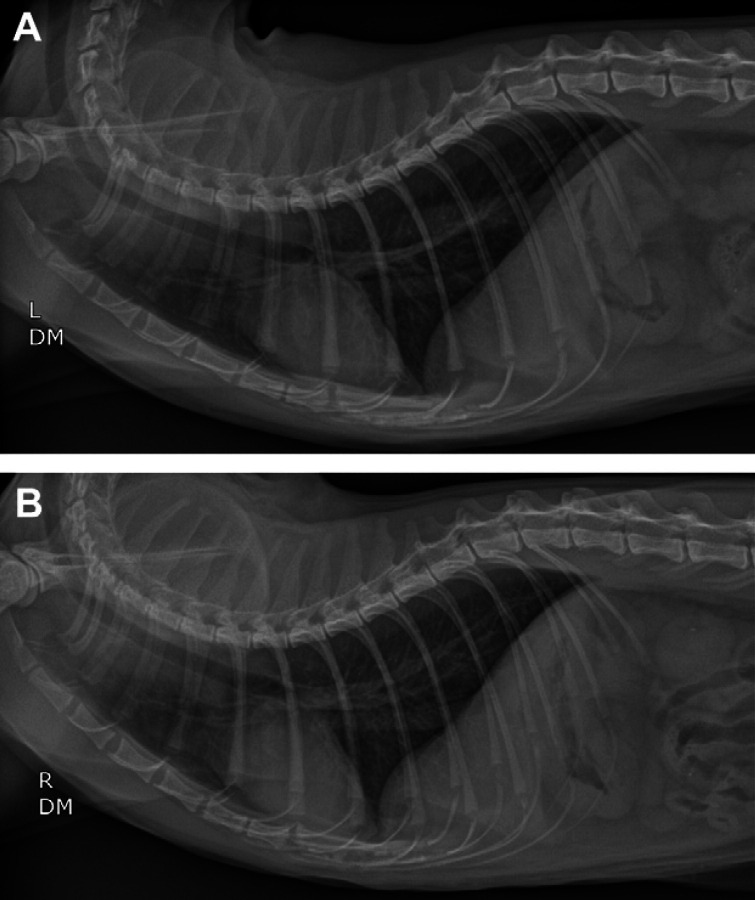
The following morning, the patient's respiratory character was markedly improved with no inspiratory or expiratory effort. Thoracic radiographs revealed resolution of the hiatal hernia and esophageal dilation (Fig 5). The patient was discharged from the hospital 3 days following presentation and prescribed a tapering anti-inflammatory dose of prednisone (0.5 mg/kg PO q24h for 7 days then 0.1 mg/kg PO q24h for 10 days then 0.1 mg/kg PO q24h every other day for 10 doses), amoxicillin–clavulanate (17.4 mg/kg PO q12h, Clavamox; Pfizer) as well as buprenorphine (0.02 mg/kg PO q8–12h as needed). Ten days later, at re-evaluation and staple removal, the patient remained asymptomatic with normal respiratory sounds and no episodes of vomiting or regurgitation. The patient was re-evaluated 3 months later. The owner reported that the cat was much more energetic. The cat was not experiencing any difficulty breathing and was eating well. Repeat thoracic radiographs were within normal limits (Fig 6).
Fig 5.
A right lateral radiographic view of the thorax post-balloon dilation of the choanae. The previously noted esophageal dilation and increased opacity at the caudal aspect of the esophagus, cranial to the diaphragmatic crura, are no longer evident. Cardiovascular and pulmonary parenchyma are unremarkable.
Fig 6.
Left lateral (A) and right lateral (B) radiographic views of the thorax at 3 months post-balloon dilation of the choanae. There is no evidence of hiatal hernia or esophageal dilation. A small amount of esophageal fluid is evident on the left lateral radiograph. The cardiovascular and pulmonary structures are unremarkable.
To our knowledge, this is the only reported case of nasopharyngeal stenosis with secondary hiatal hernia in a cat. Nasopharyngeal stenosis can occur as a congenital or acquired anomaly with the latter being more common.
Choanal atresia is a congenital malformation of the posterior nasal cavity resulting in nasopharyngeal stenosis. Choanal atresia is rare in the dog and even more rare in the cat, with the first reported cases in 1998 and 2007, respectively. 4,17 The choana is obliquely positioned, oval paired openings between the nasopharyngeal meatuses and the nasopharynx. 7 The stenosis affects the rostral area of the nasopharynx, limiting or preventing the airflow from the nasal cavity to the nasopharynx. 19 The malformation results in either unilateral or bilateral partial or complete choanal occlusion. The defect can be bony, membranous or a mixture of both. Dogs and cats most often have bilateral partial and/or complete membranous involvement.
Acquired nasopharyngeal stenosis can result secondary to a space-occupying lesion, trauma, or after an inflammatory disease of the upper respiratory tract. Differential diagnoses for space-occupying etiologies affecting the feline nasopharynx include foreign bodies, neoplasia (predominately lymphosarcoma), and inflammatory polyps. Inflammation secondary to chronic upper respiratory disease can be a result of bacterial rhinitis, viral rhinitis or cryptococcosis. A retrospective study evaluating 77 cats with nasal disease, ranked neoplasia as the most common diagnosis, followed by chronic rhinitis. 12 Additionally, eight cats were diagnosed with nasal foreign bodies, five with nasopharyngeal stenosis, two with rhinitis secondary to Actinomyces species infection, two with nasal polyps, two with stenotic nares and one with rhinitis secondary to trauma.
Diagnosis of nasopharyngeal stenosis is based on clinical signs, physical examination, radiographic imaging, and endoscopic examination of the nasal cavity and nasopharynx. Clinical signs depend on the extent of the lesion and may include inappetence, nasal discharge, sneezing, wheezing, stertor, open-mouth breathing and decreased nasal airflow unilaterally or bilaterally with the mouth closed. Radiographs of the nasal passages and nasopharynx can be difficult to assess due to superimposition of adjacent structures. 18 CT is the imaging modality of choice for diagnosing nasopharyngeal stenosis. 15 CT can provide pertinent information with regard to the extent of the stenosis, tissue type involved in the stenosis and other concurrent abnormalities such as a deviated nasal septum. 1
The flexible endoscope is an invaluable modality for diagnosing nasopharyngeal stenosis, determining the extent of the obstruction and suspected cause. It is also a pertinent modality to incorporate when performing balloon dilation, laser ablation or stent placement in the stenotic region.
Reported techniques to achieve resolution of nasopharyngeal stenosis include transpalatal reconstruction, mucosal flap rotation, laser ablation, balloon dilation, and intraluminal stenting. 2,3,8–10,15,20,21 Relapse of nasopharyngeal stenosis following these interventions is not uncommon. Thus, several attempts at one or more of these techniques may be necessary to achieve resolution of clinical signs. If there is a failure to achieve resolution, permanent tracheostomy may be performed as a salvage procedure.
Transpalatal reconstruction has been applied alone and in conjunction with other modalities for the treatment of nasopharyngeal stenosis. 4,20,21 A ventral rhinotomy is performed by making a midline incision through the soft palate. The stenotic opening is enlarged via excision of the obstructing membrane. The soft palate defect is either sutured closed or left to heal by second intention. Long-term success with transpalatal resection alone has not been reported. Complications include breakdown of the repair due to excessive tension from primary closure and reoccurrence of scar tissue from second intention healing.
Transpalatal reconstruction in combination with mucosal flap advancement decreases the need for surgical revision. Mucosal flap advancement for the treatment of nasopharyngeal stenosis was first reported in the cat in 2000. 10 Surgery included a midline incision through the soft palate from its free border to a level just caudal to the hard palate. The scar tissue, between the soft palate and the dorsal nasopharynx, causing the nasopharyngeal stenosis was resected. Resection of the scar tissue resulted in a 1 cm wide defect. A flap was generated by elevating the mucosa and submucosa caudal to the defect in the dorsal nasopharynx. The flap was advanced rostrally and then secured with a two-layer suture closure.
Laser ablation for treatment of nasopharyngeal stenosis has been reported in horses. 13,14 Laser ablation is a less invasive procedure that is performed with endoscopic guidance. Ablation of obstructing tissue has been reported using the carbon dioxide laser and the neodymium:yttrium–aluminum–garnett laser. 13,14 Sole use of the laser to ablate tissue has required revision. However, its combined use with mucosal flap advancement and intraluminal stenting has not been reported in cats. 5
Balloon dilation for the treatment of nasopharyngeal stenosis in the cat was first reported in 1999. 21 This study showed only transient improvement in clinical signs. A second study from 2002 used the same procedure but with a different approach and different sized catheter. 9 In this endoscopic guided study, a 15 mm balloon dilation catheter was placed in an orthograde fashion through the ventral nasal canal into the stenosis and then inflated. After two interventions combined with glucocorticoids, long-term resolution of clinical signs was achieved. A more recent study showed long-term resolution of clinical signs in six cats diagnosed with nasopharyngeal stenosis post-balloon dilation. 8 Besides recurrence, the only reported complication of balloon dilation has been vagally-mediated bradycardia associated with distention of the nasopharynx and suspected compression of the carotid bodies. Atropine was administered and the bradycardia resolved. 3 Overall, balloon dilation is a relatively simple and non-invasive procedure that can result in resolution of clinical signs secondary to nasopharyngeal stenosis.
Intraluminal stenting is commonly used in conjunction with balloon dilation or transpalatal resection. 2,17,21 Intraluminal stenting can be applied temporarily or permanently. Temporary stenting was reported in a cat following surgical repair via the transnasal route and balloon dilation. 17 The stent was left in place for 7 days and all clinical signs resolved. The stent was well tolerated and no complications were reported. Permanent stent placement has been reported using several different types of stents. A Wallstent wire-braided endoprosthesis was placed within the nasopharynx in a cat with nasopharyngeal stenosis. 21 There was no evidence of implant migration. However, proliferative granulation tissue formed at the pharyngeal end of the wire endoprosthesis, creating a narrowing of the lumen. A 2008 retrospective study evaluated balloon-expandable metallic stents in dogs and cats for nasopharyngeal stenosis. 2 One cat showed exaggerated swallowing when eating. Oral examination revealed a palpable bulge dorsal to the soft palate. Rhinoscopy revealed that the distal aspect of the stent had collapsed, was not incorporated into the nasopharyngeal epithelium, and was filled with entrapped hair. Overall, intraluminal stenting can be an effective modality but complications can include granulation tissue formation in the stent lumen, stent migration, stent collapse, dysphagia, and foreign body entrapment.
To date, there has been no report of a hiatal hernia secondary to nasopharyngeal stenosis in the cat. However, hiatal hernia has been reported secondary to congenital narrowing of the intrapharyngeal region in the dog. 6 Two types of hiatal hernias have been identified in dogs and cats. The most common type is a sliding hiatal hernia. 23 Paraesophageal hiatal hernia is less common and has been reported in two dogs. Hiatal hernias can be either congenital or acquired, with the latter being more common. A retrospective case-control study involving Bulldogs, investigated the association between upper airway obstructive disease and hiatal hernia. 11 This study concluded that Bulldogs with hiatal hernia were more likely to have at least one diagnosis associated with brachycephalic syndrome than were Bulldogs without hiatal hernia. 11 It is proposed that increased inspiratory effort exhibited with upper airway obstructive disease causes an increase in negative intra-esophageal and intrapleural pressure resulting in hiatal hernia. 16 Clinical signs of hiatal hernia are a result from reflux esophagitis and obstruction and include regurgitation, vomiting, hematemesis and hypersalivation. 22 Specifically, regurgitation and hypersalivation result from the chemical effects of gastric fluid on the esophageal mucosa, whereas vomiting can result from the obstructive effects of the hernia. 16 Megaesophagus is also commonly reported secondary to esophageal obstruction from the hiatal hernia. When abnormal esophageal motility is present, aspiration may develop, resulting in dysphagia and dyspnea. Diagnosis of a hiatal hernia is made by survey radiography of the thorax and positive-contrast radiography of the esophagus, with the latter being more sensitive. 22
Considering the patient's signalment and history, acquired nasopharyngeal stenosis was the primary differential diagnosis. Although the definitive cause is unknown, it is suspected that the cat's chronic rhinitis is the cause of the acquired nasopharyngeal stenosis. The vomiting and megaesophagus are presumed to be secondary to the obstructive effects of the hiatal hernia. In this case report, the cat's hiatal hernia and megaesophagus reduced spontaneously on radiographs 1-day post-resolution of the nasopharyngeal stenosis. This indicates an obstructing sliding hiatal hernia that was secondary to the nasopharyngeal stenosis. It can be speculated that the inspiratory dyspnea caused an increased negative intrathoracic pressure, thus resulting in the hiatal hernia. 6
References
- 1. Berent A.C., Kinns J., Weisse C. Balloon dilation of nasopharyngeal stenosis in a dog, J Am Vet Med Assoc 229, 2006, 385–388. [DOI] [PubMed] [Google Scholar]
- 2. Berent A.C., Weisse C., Todd K., et al. Use of a balloon-expandable metallic stent for treatment of nasopharyngeal stenosis in dogs and cats: six cases (2005–2007), J Am Vet Med Assoc 233, 2008, 1432–1440. [DOI] [PubMed] [Google Scholar]
- 3. Boswood A., Lamb C.R., Brockman D.J., et al. Balloon dilation of nasopharyngeal stenosis in a cat, Vet Radiol Ultrasound 44, 2003, 53–55. [DOI] [PubMed] [Google Scholar]
- 4. Coolman B.R., Maretta S.M., McKiernan B.C., et al. Choanal atresia and secondary nasopharyngeal stenosis in a dog, J Am Anim Hosp Assoc 34, 1998, 497–501. [DOI] [PubMed] [Google Scholar]
- 5. Dedo H.H. Transnasal mucosal flap rotation technique for repair of posterior choanal atresia, Otolaryngol Head Neck Surg 124, 2001, 674–682. [DOI] [PubMed] [Google Scholar]
- 6. Dvir E., Spotswood T.C., Lambrechts N.E., et al. Congenital narrowing of the intrapharyngeal opening in a dog with concurrent oesophageal hiatal hernia, J Small Anim Pract 44, 2003, 359–362. [DOI] [PubMed] [Google Scholar]
- 7. Evans H.E. Miller's anatomy of the dog, 3rd edn, 1993, WB Saunders: Philadelphia. [Google Scholar]
- 8. Glaus T.M., Tomsa G.K., Keiser M., et al. Reproducible and long-lasting success of balloon dilation of nasopharyngeal stenosis in cats, Vet Rec 157, 2005, 257–259. [DOI] [PubMed] [Google Scholar]
- 9. Glaus T.M., Tomsa K., Reusch C.E. Balloon dilation for the treatment of chronic recurrent nasopharyngeal stenosis in a cat, J Small Anim Pract 43, 2002, 88–90. [DOI] [PubMed] [Google Scholar]
- 10. Griffon D.J., Tasker S. Use of a mucosal advancement flap for the treatment of nasopharyngeal stenosis in a cat, J Small Anim Pract 41, 2000, 71–73. [DOI] [PubMed] [Google Scholar]
- 11. Hardie E.M., Ramirez O., Clary E.M., et al. Abnormalities of the thoracic bellows: stress fractures of the ribs and hiatal hernia, J Vet Intern Med 12, 1998, 279–287. [DOI] [PubMed] [Google Scholar]
- 12. Henderson S.M., Bradley K., Day M.J., et al. Investigation of nasal disease in the cat – a retrospective study of 77 cases, J Fel Med Surg 6, 2004, 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hogan P.M., Embertson R.M., Hunt R.J. Unilateral choanal atresia in a foal, J Am Vet Med Assoc 207, 1995, 471–473. [PubMed] [Google Scholar]
- 14. James F.M., Parente E.J., Palmer J.E. Management of bilateral choanal atresia in a foal, J Am Vet Med Assoc 11, 2006, 1784–1789. [DOI] [PubMed] [Google Scholar]
- 15. Jimenez D.A., Berry C.R., Ferrell E.A., et al. Imaging diagnosis – choanal atresia in a dog, Vet Radiol Ultrasound 48, 2007, 135–137. [DOI] [PubMed] [Google Scholar]
- 16. Keeley B., Puggioni A., Pratschke K. Congenital oesophageal hiatal hernia in a pug, Ir Vet J 61, 2008, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khoo A.M.L., Marchevsky A.M., Barrs V.R., et al. Choanal atresia in a Himalayan cat – first reported case and successful treatment, J Feline Med Surg 9, 2007, 346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lamb C.R., Richbell S., Mantis P. Radiographic signs in cats with nasal disease, J Feline Med Surg 5, 2003, 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lopez J.T., del Palacio M.J. Fernandez, Cano F.G., et al. Nasopharyngeal stenosis secondary to soft palate dysgenesis in a cat, Vet J 181, 2009, 200–204. [DOI] [PubMed] [Google Scholar]
- 20. Mitten R.W. Nasopharyngeal stenosis in four cats, J Small Anim Pract 29, 1988, 341–345. [Google Scholar]
- 21. Novo R.E., Kramek B. Surgical repair of nasopharyngeal stenosis in a cat using a stent, J Am Anim Hosp Assoc 35, 1999, 251–256. [DOI] [PubMed] [Google Scholar]
- 22. Pyrmak C., Saunders H.M., Washabau R.J. Hiatal hernia repair by restoration and stabilization of normal anatomy, an evaluation in four dogs and one cat, Vet Surg 18, 1989, 386–391. [DOI] [PubMed] [Google Scholar]
- 23. Sivacolundhu R.K., Read R.A., Marchevsky A.M. Hiatal hernia controversies – a review of pathophysiology and treatment options, Aust Vet J 80, 2002, 48–53. [DOI] [PubMed] [Google Scholar]