Abstract
A 15-year-old, spayed female domestic shorthair cat was evaluated for 1-year duration of cyclic intermittent estrous behavior. Diagnostic testing performed before referral, including baseline progesterone concentration, human chorionic gonadotropin (hCG) hormone stimulation test and surgical exploratory laparotomy, had remained inconclusive for a remnant ovary. Evaluation of sex hormones before and after adrenocorticotropic hormone (ACTH) administration revealed increased basal concentrations of androstenedione, estradiol, progesterone, and 17α−hydroxyprogesterone and normal ACTH-stimulated hormone concentrations. Enlargement of the right adrenal gland was identified by abdominal ultrasound. The cat underwent an adrenalectomy and histopathology of the excised adrenal gland was consistent with an adrenocortical carcinoma. Clinical signs resolved immediately following surgery, and most hormone concentrations declined to within or below the reference interval (RI) by 2 months after surgery.
A 15-year-old, female, spayed domestic shorthair cat was referred to the Purdue University Veterinary Teaching Hospital (PUVTH) for recurrence of cyclic estrous behavior which had started 1 year prior to presentation. Clinical signs included posturing, licking the vulva, vocalizing, rolling on the ground and head rubbing, and occurred regularly every 2 weeks. The clinical signs were reported to be similar to the period of estrous the cat had exhibited at 6 months of age prior to ovariohysterectomy. The owner reported no possible exposure to exogenous estrogens. An initial diagnostic evaluation including vaginal cytology, human chorionic gonadotropin (hCG) stimulation test, and an exploratory laparotomy had failed to reveal evidence of an ovarian remnant.
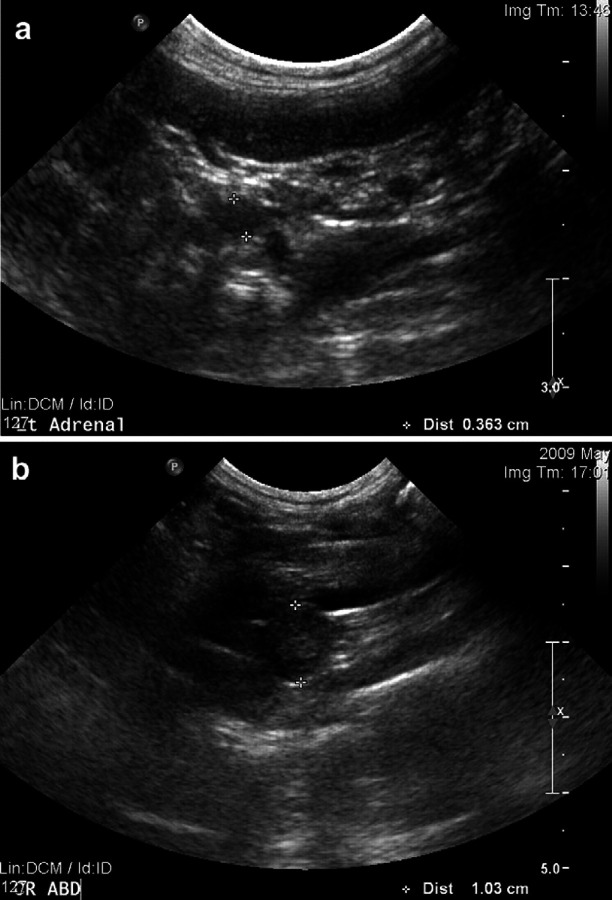
Upon presentation at the PUVTH, the cat was bright, alert and responsive. The owner reported estrous behavior at home at the time of presentation. Physical examination was normal except for a decreased body condition score (2 out of 5) and aggressive behavior. A complete blood count, serum biochemical profile, and urinalysis were unremarkable apart from mild hyperglobulinemia (4.2 g/dl; reference interval (RI) 2.3–3.8 g/dl), hypokalemia (3.3 mmol/l; RI 3.5–5.1 mmol/l) and thrombocytopenia (209×103/μl; RI 300–700×103/μl). The specific gravity of the urine was 1.015. Vaginal cytology showed the presence of some superficial cells. A modified hCG stimulation test was performed while the cat was exhibiting signs of estrous using 500 IU of hCG injected intramuscularly. Blood samples were collected for measurement of progesterone concentration at baseline and day 15, and measurement of estradiol concentration at 30, 60, 120 and 240 min after hCG administration. Baseline progesterone concentration was 0.5 ng/ml (RI<0.35 ng/ml) and 0.6 ng/ml 2 weeks after hCG injection which was inconsistent with luteal activity. Baseline estradiol concentration was high at 97.2 pg/ml (RI 56–74 pg/ml) and estradiol levels did decrease slightly after injection of hCG (concentrations at 30, 60, 120 and 240 min were 101.6, 78.3, 61.8 and 72.5 pg/ml, respectively). However, the negative response of progesterone to hCG stimulation indicates that an ovarian remnant was not present. Thoracic and abdominal radiographs were unremarkable. Abdominal ultrasound revealed that the right adrenal gland was larger than the left (right adrenal thickness 0.70 cm; left adrenal thickness 0.36 cm) (Fig 1a). Reported normal values for cat adrenal thickness are between 0.30 cm and 0.55 cm. 1 The right adrenal gland was rounded in appearance with a hyperechoic echotexture. No evidence for an ovarian remnant was found. Concentrations of cortisol, androstenedione, estradiol, progesterone, 17α−hydroxyprogesterone, aldosterone, and testosterone were measured before and after intravenous administration of 125 μg of synthetic adrenocorticotropic hormone (ACTH) (Table 1) 5 . Abnormalities included suppression of the post-ACTH cortisol concentration and increased baseline concentrations of estradiol, androstenedione, progesterone and 17α−hydroxyprogesterone (sex hormones). Post-ACTH concentrations of these hormones were within the RI. A low-dose dexamethasone suppression test (using 0.1 mg/kg dexamethasone IV) was normal [basal cortisol 2.54 μg/dl (RI 1.8–3.7 μg/dl) and 8 h post-dexamethasone injection less than 1.00 μg/dl (RI 0.0–1.9 μg/dl)]. Endogenous ACTH concentration was within the RI (33.4 pg/ml; RI 10–45 pg/ml). 2
Fig 1.
Ultrasonographic evaluation of the adrenal glands. (a) Ultrasound image of the left adrenal gland. The left adrenal appeared normal shape, echogenicity and size (0.36 cm). (b) Ultrasound image of the right adrenal gland. The right adrenal gland appeared rounded and its thickness was 1.03 cm [0.30–0.55 cm being the normal range in cats].
Table 1.
Results of the measurement of adrenal hormone concentrations before and after adrenocorticotropin hormone stimulation test before (T0), 1 month (T1) and 2 months after surgery.* Abnormal values in excess are in bold.
| Test | Result (baseline) | RI | Result (post-ACTH) | RI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t 0 | t 0+30 min | t 0 + 60 min | ||||||||||
| Hormone | Unit | T0 | T1 | T2 | T0 | T1 | T2 | T0 | T1 | T2 | ||
| Cortisol | ng/ml | 29.8 | 24.4 | 36.0 | 8.7–34 | 40.5 | 27.5 | 40.1 | 38.3 | 23.6 | 40.6 | 106–150 |
| Androstenedione | ng/ml | 6.1 | 0.51 | 0.44 | 1.4–3.2 | 6.9 | 2.0 | 0.76 | 6.5 | 1.7 | 1.11 | 4.4–20 |
| Estradiol | pg/ml | 87.1 | 94.1 | 61.2 | 56–74 | 70.9 | 109.6 | 74.4 | 62.6 | 84.2 | 78.4 | 51–77 |
| Progesterone | ng/ml | 0.36 | 0.23 | 0.11 | 0.07–0.3 | 1.06 | 1.06 | QNS | 0.85 | 1.4 | 1.02 | 1.3–5.2 |
| 17OH Progesterone | ng/ml | 0.25 | 0.25 | 0.20 | 0.08–0.2 | 0.65 | 0.7 | 0.58 | 0.66 | 1.0 | 0.88 | 0.5–1.6 |
| Aldosterone | pg/ml | 175.7 | 67.2 | 135.6 | 11.3–294.3 | 193.1 | 86.1 | QNS | 168.0 | 107.0 | QNS | No values |
| Testosterone | ng/ml | 0.05 | 0.02 | 0.17–0.3 | 0.04 | QNS | 0.05 | 0.02 | 0.2–0.4 | |||
QNS = Quantity Not Sufficient.
Sex-hormones concentrations were measured using the hormone assays previously validated for use in cats. 5
An estradiol secreting adrenal tumor was suspected as a cause for the clinical signs. However, due to the lack of support in the literature for cyclic estrous-like signs related to adrenal tumors in cats, and the absence of life-threatening illness, re-evaluation in 2 months was recommended. Two months later, the clinical signs had become more severe and were now persistent. Re-evaluation of the right adrenal gland by ultrasonography showed increased adrenal gland size (1.03 cm) (Fig 1b). An exploratory laparotomy and adrenalectomy were performed.
At laparotomy, no evidence of an ovarian remnant was identified. The right adrenal gland was enlarged and rounded while the left adrenal gland was small and irregular. A right adrenalectomy was performed (Fig 2a and b). Histopathology revealed a large nodule almost completely replacing the normal adrenal architecture (Fig 3a). Neoplastic cells formed disorganized cords surrounded by delicate stroma and numerous capillaries. Tumor cells multifocally infiltrated normal adrenal tissue (Fig 3b). Neoplastic cells were pleomorphic with abundant eosinophilic or vacuolated cytoplasm, and large round vesicular nuclei with prominent central nucleoli. Karyomegalic cells were common and there were rare multinucleate giant cells (Fig 3c). There was one mitotic figure per 400× field. A diagnosis of adrenocortical carcinoma was established. Postoperatively the cat was treated with one dose of dexamethasone (0.1 mg/kg IV) and cefazolin (22 mg/kg IV q12 h). The rationale for the administration of glucocorticoid was the blunted cortisol response after ACTH stimulation. The postoperative recovery was uneventful and the cat was discharged 36 h after surgery on a tapering dose of prednisone (0.2 mg/kg/d for 3 days then 0.1 mg/kg/d for 3 days and 0.1 mg/kg every other day for four doses) and buprenorphine (0.01 mg/kg PO q6 h for 3 days). Clinical signs of estrous resolved within 24 h after surgery and did not recur.
Fig 2.
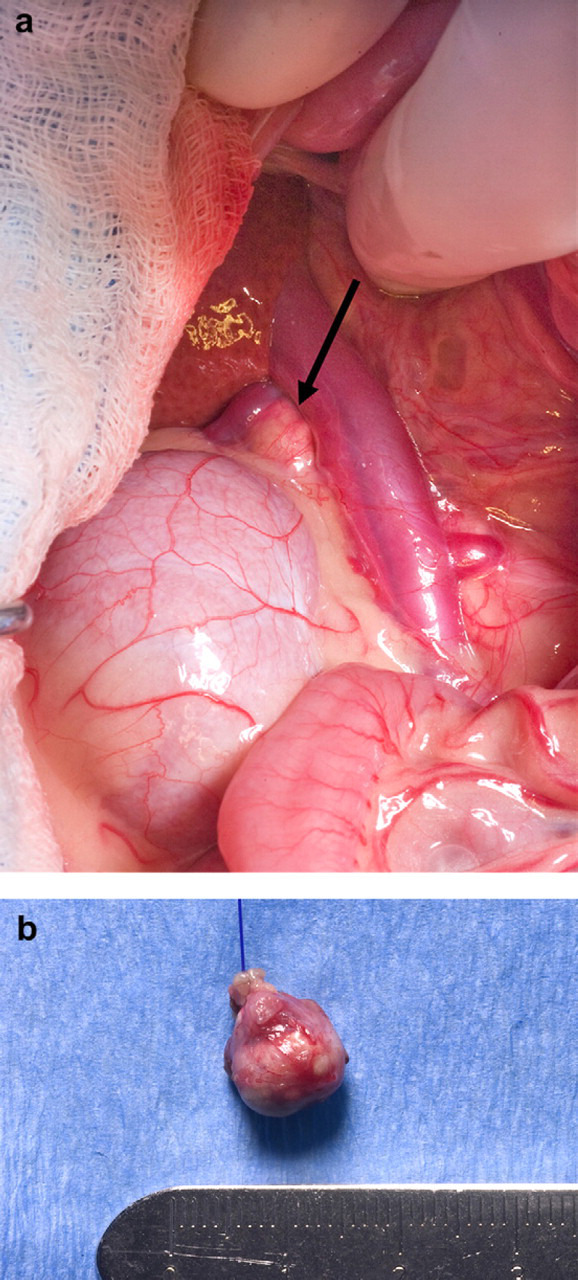
Photograph of the right adrenal gland at the time of surgery. (a) The right adrenal gland in its anatomical position. The adrenal gland (arrow) appeared as a rounded structure craniomedially to the right kidney and adjacent to the caudal vena cava. (b) The excised right adrenal gland. The excised adrenal gland appeared rounded and larger than normal, around 1 cm in diameter.
Fig 3.
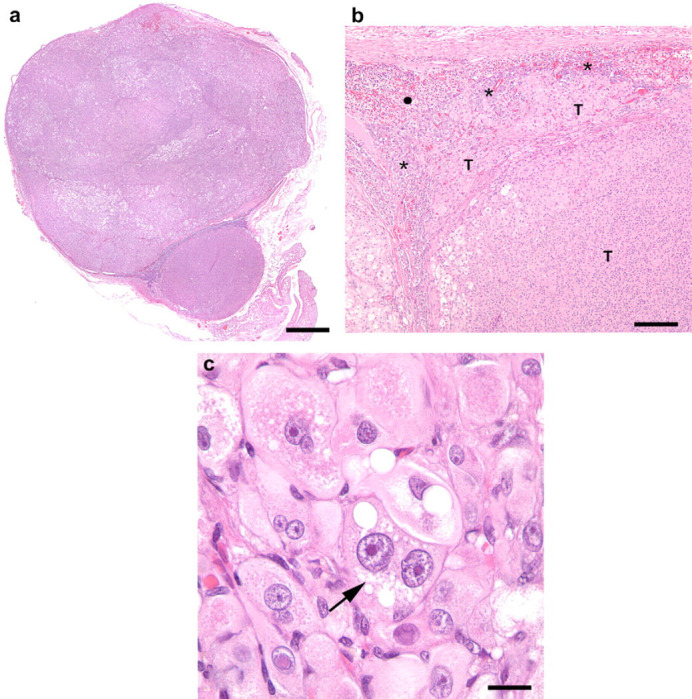
Histopathologic images of the excised right adrenal gland. (a) Histopathologic image of the adrenocortical carcinoma, right adrenal gland. Subgross appearance. Most of the normal adrenal tissue has been replaced by neoplastic cells. Hematoxylin and eosin. Bar=1.28 mm. (b) Histopathologic image of the adrenocortical carcinoma, right adrenal gland. Neoplastic cells (T) infiltrate normal adrenal medulla (asterisk). Normal adrenal cortex (solid circle). Hematoxylin and eosin. Bar=250 μm. (c) Histopathologic image of the adrenocortical carcinoma, right adrenal gland. Marked atypia of neoplastic cells characterized by anisocytosis and anisokaryosis, proeminent central nucleolus, and binucleation (arrow). Hematoxylin and eosin. Bar=25 μm.
Sex-hormone concentrations were re-evaluated 4 and 8 weeks after surgery (Table 1). Basal and post-ACTH estradiol concentrations were still increased above the reference interval (RI) at 4 weeks but had declined to near the RI by 8 weeks after surgery.
The cat was diagnosed with chronic renal failure 10 months after surgery and was euthanased after progression of clinical signs. Abdominal ultrasound at that time showed no evidence for tumor regrowth and the left adrenal gland was unchanged ultrasonographically. An ACTH stimulation test performed at the time of that evaluation revealed normal estradiol concentrations and normal resting cortisol. The post-ACTH cortisol was low (41.1 ng/ml).
Differential diagnoses for hyperestrogenism in cats include an ovarian remnant, estrogen-secreting tumor or exposure to exogenous estrogens. To the authors’ knowledge this case is the first report of cyclic clinical signs associated with an estrogen secreting adrenal tumor in a cat.
Functional adrenal tumors are rare in cats. Fifty percent of these tumors are adrenocortical adenocarcinomas and 50% are adrenocortical adenomas. 2 The majority of functional adrenal tumors in cats are associated with excessive secretion of glucocorticoids and result in clinical signs of hyperadrenocorticism (insulin resistant diabetes mellitus, cutaneous fragility, alopecia, muscle weakness and pot-bellied appearance). Excessive production of other adrenal hormones such as mineralocorticoids (aldosterone) and sex hormones (estradiol, testosterone, progesterone) either alone or in combination has also been reported in association with functional adrenal tumors in cats. 3 The first case report of an isolated progesterone-secreting adrenal gland was published in 1999. 4 The reason for presentation in this cat was non-pruritic bilateral truncal progressive alopecia. Since then, five other reports of sex-hormone overproduction by the adrenal glands have been published. 5–9 Reported clinical signs included signs of hypercortisolism, weight loss, palpable abdominal mass, and urine spraying and aggressive behavior in both a male and a female cat. The male cat with aggression and urine spraying had increased baseline concentrations of all measured sex hormones except for estradiol and the female cat had increased baseline concentrations of estradiol and testosterone. Post-ACTH stimulation test results in the male cat (not available for the female cat) showed persistent elevation of all sex hormones except for estradiol and progesterone which were within normal limits. Out of these five cases, a histologic diagnosis was obtained by surgical removal of the abnormal adrenal gland in two cases and at necropsy in one case. In two of the three cases, an adrenocortical adenocarcinoma was identified, and in the third one, an adrenocortical adenoma. 5,6,9 In the cat of this report, the association of hyperestrogenism with the adrenal tumor was demonstrated by resolution of clinical signs and normalization of estradiol concentrations after surgical removal of the tumor.
The case described in this report has some similarities with the adrenocortical disease described in ferrets. 10 Most ferrets with hyperadrenocorticism produce sex hormones (mainly androstenedione, 17α−hydroxyprogesterone, dehydroepiandrosterone sulfate and estradiol) rather than cortisol. 11 Depending on the study, adrenocortical adenoma represents 15–65% of the cases; nodular hyperplasia 25–55% of cases, and adrenocortical carcinoma 10–25% of the cases. 12 The first report of an estradiol secreting adrenocortical tumor in a ferret was published in 1993. 13 The clinical signs exhibited by this spayed female were an enlarged vulva, generalized alopecia and estrous behavior toward the male ferret of the household; these signs were initially cyclic (every year in the spring) and became continuous the third spring. Interestingly, the cat in this report initially showed intermittent cyclic signs that also became continuous over a 1-year period but there were no signs of vulvar enlargement or signs of hypercortisolism such as alopecia. The current hypotheses for the high prevalence of adrenal disease in ferrets are early-age gonadectomy, an artificially prolonged photoperiod and a possible genetic predisposition. Ferrets in the United States that are neutered at an early age (around 6 weeks of age) have a higher incidence of adrenal disease than ferrets in the United Kingdom that are not systematically neutered. The lack of negative feedback exerted by the sex hormones on the pituitary gland induces an increased production of luteinizing hormone (LH). The overstimulation of the adrenal tissue by LH may stimulate cellular proliferation resulting in adrenocortical hyperplasia and potentially neoplasia. 14,15 Shoemaker et al (2002) using histochemical techniques, identified LH receptors in the hyperplastic and neoplastic adrenal tissues of ferrets suffering from hyperadrenocorticism. 16 Moreover, increased concentrations of androstenedione and 17α-hydroxyprogesterone were measured in ferrets with hyperadrenocorticism after stimulation by gonadotropin-releasing hormone (GnRH), supporting the role of the GnRH-LH axis. Others have shown an association between early-age neutering and adrenocortical neoplasia in both ferrets and rodents. 15 Recently, LH receptors have been found in dogs on cells of both normal adrenal tissue and adrenocortical tumors. Although LH receptors did not appear to be overexpressed in adrenocortical tumors compared to normal adrenals, the pattern obtained on histochemistry appeared different. 17 The relationship of these receptors to the high LH hormone level identified in neutered dogs and sex-hormone secretion by the adrenal glands is still unknown. However, to suspect the implication of early spaying and neutering as a possible triggering factor for the development of adrenocortical tumors in dogs, as it is true in ferrets, sounds reasonable and warrants further studies.
In dogs with atypical Cushing's disease, cortisol levels are often normal or low while sex-hormone levels are increased. Excessive sex hormone production by adrenocortical tumors has already been reported in this species; however, none of the cases described in the literature had a predominance of estrogen production nor were there any reports of estrous behavior. 18,19
In humans, androgens appear to be the predominant hormones produced in females diagnosed with sex hormone secreting adrenocortical tumors; 20 in males, feminization due to excessive adrenal estrogen secretion is rare. There is one report describing estrogen synthesis by human adenocortical carcinoma cells. 21 In this study of human H295 adrenocortical carcinoma cells, adrenal tumor cells had increased aromatase and 17β-hydroxysteroid dehydrogenase expression both of which are necessary enzymes for the synthesis of estradiol. Development of aberrant biosynthetic pathways may be the mechanism by which adrenocortical neoplasms produce estrogens. 22
Ovarian tumors in cats such as sex cord-stromal tumors (granulosa-cell tumor and interstitial gland tumors), germ cell tumors (dysgerminoma, teratoma) and epithelial tumors (cystadenoma) may secrete estrogens and result in signs of estrous. 23 Continuous signs of estrous have been reported with granulosa-cell tumors, dysgerminomas and luteomas. 23,24 One case report described cyclic regular estrous behavior in a previously neutered cat diagnosed with a teratoma of the right ovary. Resolution of clinical signs was associated with excision of the tumor. 25 The reason for the cyclic pattern in that case or in the cat described in this report is unclear. In ferrets that are susceptible to photoperiod, it can be hypothesized that the pineal gland and melatonin play a role in the pathophysiology of the intermittent signs. 10
Remnant ovarian syndrome relates to the persistence of functional ovarian tissue in a neutered animal; most commonly in cats. Most cases are related to incomplete removal of an ovary. 26 The hCG stimulation test has been reported to be useful for diagnosis of ovarian remnants in cats. 27 hCG has LH-like activity that induces ovulation of mature follicles. In the presence of ovarian tissue, hCG results in decreased estradiol and increased progesterone concentrations. In this case, the test was useful to exclude an ovarian remnant as a cause of the cyclic estrous behavior.
In the cat of this report, the estradiol concentration remained markedly high pre- and post-ACTH administration 1 month after tumor resection, although clinical signs had resolved. Reasons for the persistence of high estradiol concentrations include a false positive test due to cross-reactivity or plasma interference with estradiol assay, inter-assay variability, binding of estradiol to serum binding proteins, tissue estradiol sequestration and slow release or less likely production by other sources such as adipocytes or hair follicles. The half-life of estradiol in cats is unknown. In humans, the half-life of estradiol is 2.7–13 h; however, this value doesn't take into consideration the protein-bound fraction and possible tissue sequestration and gradual release. Clinical experience with cases of exposure to estrogen-containing cream in dogs suggests that the serum estradiol concentrations remain high for weeks to months (Jack Oliver, personal communication, Professor and Director, Clinical Endocrinology Services, College of Veterinary Medicine, The University of Tennessee). In the cat of this report, baseline estradiol concentration had returned to within the RI by 2 months after surgery.
The blunted cortisol response to ACTH stimulation in this case was highly suggestive of excessive sex-hormone production. This has been previously described in dogs and cats with adrenocortical tumors and excessive sex-hormone production. 18 The pathophysiologic mechanism underlying this decreased cortisol production is hypothesized to be that the excessive sex-hormone production has a negative feedback on the hypothalamic-pituitary axis. Another possible explanation would include an altered cortisol synthesis pathway. As previously discussed, some enzymes, such as aromatase and 17β-hydroxysteroid dehydrogenase, can be upregulated in adrenal tumor tissue, but others, such as 21β-hydroxylase or 11β-hydroxylase which are required for normal steroid synthesis, can be deficient. 18 The reason why the blunted cortisol response was persistent at the 2-month re-check is unknown.
In conclusion, adrenocortical neoplasia should be considered in the differential list of diseases for a spayed cat with cyclic estrous behavior.
References
- 1. Zatelli A., D'Ippolito P., Fiore I., Zini E. Ultrasonographic evaluation of the size of the adrenal glands of 24 diseased cats without endocrinopathies, Vet Rec 160, 2007, 658–660. [DOI] [PubMed] [Google Scholar]
- 2. Feldman E.C., Nelson R.W. Canine and feline endocrinology and reproduction, 3rd edn, 2004, WB Saunders: Saint-Louis, 1089. [Google Scholar]
- 3. Chiaramonte D., Greco D.S. Feline adrenal disorders, Clin Tech Small Anim Pract 22, 2007, 26–31. [DOI] [PubMed] [Google Scholar]
- 4. Boord M., Griffin C. Progesterone secreting adrenal mass in a cat with clinical signs of hyperadrenocorticism, J Am Med Vet Assoc 214, 1999, 666–669. [PubMed] [Google Scholar]
- 5. Rossmeisl J.H., Scott-Moncrieff J.C., Siems J., et al. Hyperadrenocorticism and hyperprogesteronemia in cat with an adrenocortical adenocarcinoma, J Am Anim Hosp Assoc 36, 2000, 512–517. [DOI] [PubMed] [Google Scholar]
- 6. Boag A.K., Neiger R., Church D.B. Trilostane treatment of bilateral adrenal enlargement and excessive sex steroid hormone production in a cat, J Small Anim Pract 45, 2004, 263–266. [DOI] [PubMed] [Google Scholar]
- 7. DeClue A.E., Breshears L.A., Pardo I.D., Kerl M.E., Perlis J., Cohn L.A. Hyperaldosteronism and hyperprogesteronism in a cat with an adrenal cortical carcinoma, J Vet Intern Med 19, 2005, 355–358. [DOI] [PubMed] [Google Scholar]
- 8. Briscoe K., Barrs V.R., Foster D.F., Beatty J.A. Hyperaldosteronism and hyperprogesteronism in a cat, J Feline Med Surg 11, 2009, 758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Millard R.P., Pickens E.H., Wells K.L. Excessive production of sex hormones in a cat with an adrenocortical tumor, J Am Med Vet Assoc 234, 2009, 505–508. [DOI] [PubMed] [Google Scholar]
- 10. Simone-Freilicher E. Adrenal gland disease in ferrets, Vet Clinics North Am Exot Anim Pract 11, 2008, 125–137. [DOI] [PubMed] [Google Scholar]
- 11. Rosenthal K.L., Peterson M.E. Evaluation of plasma androgen and estrogen concentrations in ferrets with hyperadrenocorticism, J Am Vet Med Assoc 209, 1996, 1097–1102. [PubMed] [Google Scholar]
- 12. Rosenthal K.L., Peterson M.E., Quesenberry K.E., et al. Hyperadrenocorticism associated with adrenocortical tumor or nodular hyperplasia of the adrenal gland in ferrets: 50 cases (1987–1991), J Am Med Vet Assoc 203, 1993, 271–275. [PubMed] [Google Scholar]
- 13. Lipman N.S., Marini R.P., Murphy J.C., Zhibo Z., Fox J.G. Estradiol-17 beta-secreting adrenocortical tumor in a ferret, J Am Med Vet Assoc 203, 1993, 1552–1555. [PubMed] [Google Scholar]
- 14. Shoemaker N.J., Schuurmans M., Moorman H., Lumeij J.T. Correlation between age at neutering and age at onset of hyperadrenocorticism in ferrets, J Am Med Vet Assoc 216, 2000, 195–197. [DOI] [PubMed] [Google Scholar]
- 15. Bielinska M., Kiiveri S., Parviainen H., Mannisto S., Heikinheimo M., Wilson D.B. Gonadectomy-induced adrenocortical neoplasia in the domestic ferret (Mustela putorius furo) and laboratory mouse, Vet Pathol 43, 2006, 97–117. [DOI] [PubMed] [Google Scholar]
- 16. Schoemaker N.J., Teerds K.J., Mol J.A., Lumeij J.T., Thijssen J.H., Rijnberk A. The role of luteinizing hormone in the pathogenesis of hyperadrenocorticism in neutered ferrets, Mol Cell Endocrinol 197, 2002, 117–125. [DOI] [PubMed] [Google Scholar]
- 17. Galac S., Kars V.J., Klarenbeek S., Teerds K.J., Mol J.A., Kooistra H.S. Expression of receptors for luteinizing hormone, gastric-inhibitory polypeptide, and vasopressin in normal adrenal glands and cortisol-secreting adrenocortical tumors in dogs, Domest Anim Endocrinol 39, 2010, 63–75. [DOI] [PubMed] [Google Scholar]
- 18. Syme H.M., Scott-Moncrieff J.C., Treadwell N.G., et al. Hyperadrenocorticism associated with excessive sex hormone production by an adrenocortical tumor in two dogs, J Am Med Vet Assoc 219, 2001, 1725–1728. [DOI] [PubMed] [Google Scholar]
- 19. Hill K.E., Scott-Moncrieff J.C., Koshko M.A., et al. Secretion of sex hormones in dogs with adrenal dysfunction, J Am Med Vet Assoc 226, 2005, 556–561. [DOI] [PubMed] [Google Scholar]
- 20. d'Alva C.B., Abiven-Lepage G., Viallon V., et al. Sex steroids in androgen-secreting adrenocortical tumors: clinical and hormonal features in comparison with non-tumoral causes of androgen excess, Eur J Endocrinol 159, 2008, 641–647. [DOI] [PubMed] [Google Scholar]
- 21. Nicol M.R., Papacleovoulou G., Evans D.B., et al. Estrogen biosysnthesis in human H295 adrenocortical carcinoma cells, Mol Cell Endocrinol 300, 2009, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Orth D.N., Kovacs W.J., DeBold C.R. The adrenal cortex. Wilson J.D., Forster D.W. Williams textbook of endocrinology, 9th edn, 1998, WB Saunders: Philadelphia, 590–595. [Google Scholar]
- 23. Gelberg H.B., McEntee K. Feline ovarian neoplasm, Vet Pathol 22, 1985, 572–576. [DOI] [PubMed] [Google Scholar]
- 24. Choi U.S., Seo K.W., Oh S.Y., Kim D.Y., Youn H.Y., Lee C.W. Intra-abdominal mass aspirate from a cat in heat, Vet Clin Pathol 34, 2005, 275–277. [DOI] [PubMed] [Google Scholar]
- 25. Kustritz M.V. Root, Rudolph K.D. Theriogenology question of the month, J Am Med Vet Assoc 219, 2001, 1065–1066. [DOI] [PubMed] [Google Scholar]
- 26. Wallace M.S. The ovarian remnant syndrome in the bitch and queen, Vet Clinics North Am Small Anim Pract 21, 1991, 501–507. [DOI] [PubMed] [Google Scholar]
- 27. England G.C. Confirmation of ovarian remnant syndrome in the queen using hCG administration, Vet Rec 141, 1997, 309–310. [DOI] [PubMed] [Google Scholar]