Abstract
The feasibility of autologous intrarenal mesenchymal stem cell (MSC) therapy in cats with chronic kidney disease (CKD) was investigated. Six cats (two healthy, four with CKD) received a single unilateral intrarenal injection of autologous bone marrow-derived or adipose tissue-derived MSC (bmMSC or aMSC) via ultrasound guidance. Minimum database and glomerular filtration rate (GFR) via nuclear scintigraphy were determined pre-injection, at 7 days and at 30 days post-injection. Intrarenal injection did not induce immediate or long-term adverse effects. Two cats with CKD that received aMSC experienced modest improvement in GFR and a mild decrease in serum creatinine concentration. Despite the possible benefits of intrarenal MSC injections for CKD cats, the number of sedations and interventions required to implement this approach would likely preclude widespread clinical application. We concluded that MSC could be transferred safely by ultrasound-guided intrarenal injection in cats, but that alternative sources and routes of MSC therapy should be investigated.
Chronic kidney disease (CKD) is a major cause of morbidity and mortality in cats. At present, the only definitive treatment option for cats with CKD is renal transplantation. 1,2 However, renal transplantation is not a viable option for most CKD-affected cats and supportive care, designed to stabilize renal function and reverse metabolic complications is most common. 3,4 However, these supportive treatments do not address the underlying and often progressive disease process.
The effects of mesenchymal stem cell (MSC) therapy have been investigated in rodent chronic renal failure models including genetic disease, glomerulonephritis and experimentally-induced CKD. 5–18 In the majority of experimentally-induced CKD models investigated, MSC administration has resulted in beneficial changes, as evidenced by improvement in renal functional parameters and reduction of renal fibrosis and glomerulosclerosis. 5–8,10 Although the mechanisms underlying these effects are not yet fully understood, most investigators propose that paracrine effects from the injected MSC are more important than the effects of direct cellular incorporation into functional nephrons. 19,20
The purpose of this pilot study was to assess the feasibility of intrarenal MSC transfer in normal cats and in cats with CKD. Unilateral intrarenal transfer of MSC was chosen to allow internal comparison between the injected kidney and the non-injected kidney using glomerular filtration rate (GFR) determined by nuclear scintigraphy. The effects of MSC transfer on GFR, renal functional parameters, and overall animal health were assessed. This study was designed to test the hypothesis that MSC could be safely administered to cats with CKD and that MSC injection would result in improvement in function of the injected kidney.
Materials and methods
Healthy specific pathogen-free (SPF) cats
Two healthy SPF purpose-bred domestic shorthair cats (Andrea D Lauerman SPF Cat Colony Resource, Fort Collins, CO) were utilized for the study. Both cats were 1.5 years of age (one castrated male, one intact female). The cats were adopted into private homes at the conclusion of the study.
Study population of cats with stable CKD
Cats with stable CKD were recruited from the patient population at the Veterinary Teaching Hospital at Colorado State University. Cats were determined to have stable CKD based on two repeated biochemical evaluations performed at least 2 weeks apart. Pre-treatment evaluation included complete blood count (CBC), biochemistry profile, urinalysis, urine culture, blood pressure, total T4, urine protein–creatinine ratio (UPC), feline leukemia virus/feline immunodeficiency virus (FeLV/FIV) serology, abdominal radiographs, and a renal ultrasound. Cats were excluded from the study if they had evidence of ureteroliths, pyelonephritis, uncontrolled hypertension, or concurrent systemic disease. Administration of concurrent supportive therapies was allowed provided there were no changes in therapy during the study period. A summary of the demographics of participating cats is presented in Table 1. The study was approved by the Institutional Animal Care and Use Committee at Colorado State University, and all owners reviewed and signed consent forms prior to participation in the study.
Table 1.
Description of cats included in the study.
| Cat | Group | Signalment | IRIS stage and creatinine (mg/dl) | Treatment |
|---|---|---|---|---|
| 1 | Young healthy | 1.5-year MC DSH | IRIS: N/A Creatinine: 1.7 UPC: 0.1 | 1×105 bmMSC injected into the right kidney |
| 2 | Young healthy | 1.5-year FI DSH | IRIS: N/A Creatinine: 1.3 UPC: 0.1 | 1×105 bmMSC injected into the left kidney |
| 3 | CKD+bmMSC | 6-year-old MC DLH | IRIS: IV Creatinine: 5.4 UPC: 1.7 | 1×105 bmMSC injected into the left kidney |
| 4 | CKD+bmMSC | 15-year MC Tonkinese | IRIS: III Creatinine: 4.3 UPC: 0.3 | Unable to expand sufficient bmMSC for therapy |
| 5 | CKD+bmMSC | 17-year MC Siamese | IRIS: II Creatinine: 2.6 UPC: not performed | Unable to expand sufficient bmMSC for therapy |
| 6 | CKD+bmMSC | 14-year MC DSH | IRIS: II Creatinine: 2.0 UPC: 0.2 | Unable to expand sufficient bmMSC for therapy |
| 7 | CKD+aMSC | 9-year FS Siamese | IRIS: III Creatinine: 3.5 UPC: 0.1 | 1×106 aMSC injected into the left kidney |
| 8 | CKD+aMSC | 9-year MC DSH | IRIS: II Creatinine: 2.6 UPC: 0.2 | 2×106 aMSC injected into the left kidney |
| 9 | CKD+aMSC | 7-year MC DSH | IRIS: IV Creatinine: 6.5 UPC: 0.3 | 4×106 aMSC injected into the left kidney |
| 10 | CKD no MSC | 9-year MC DSH | IRIS: II Creatinine: 2.3 UPC: 0.3 | GFR repeatability only |
| 11 | CKD no MSC | 12-year FS DSH | IRIS: III Creatinine: 3.3 UPC: 0.1 | GFR repeatability only |
| 12 | CKD no MSC | 13-year MC DSH | IRIS: II Creatinine: 2.6 UPC: 0.2 | GFR repeatability only |
DSH=domestic shorthair, FS=female spayed, MC=male castrated; IRIS=Internation Renal Interest Society.
Autologous MSC collection and culture
For collection of bone marrow or adipose tissue biopsies, cats were sedated with ketamine (Fort Dodge) 3.3–4.8 mg/kg IV, once (dose repeated once if needed), midazolam (Baxter HealthCare, Deerfield, IL) 0.1 mg/kg IV once and butorphanol (Fort Dodge) 0.1 mg/kg IV once. Intravenous fluids were administered during sedation at 5 ml/kg/h and blood pressure, pulse and respiration were monitored. Approximately 1 ml of bone marrow was collected from the proximal humerus and placed into plastic tissue culture flasks (BD Biosciences, San Jose, CA) in MSC medium (low-glucose Dulbecco's modified Eagle's medium (DMEM), 100 U/ml penicillin, 100 μg/ml streptomycin: Invitrogen/Gibco, Carlsbad, CA) plus 15% fetal bovine serum (Cell Generation, Fort Collins, CO). The bone marrow-derived MSC (bmMSC) were incubated until approximately 70% confluent with media changes every 2–3 days. The cells were harvested with trypsin (Invitrogen/Gibco, Carlsbad, CA) and passaged until adequate cell numbers were obtained for injection.
Adipose tissue was obtained from a subcutaneous site on the ventral abdomen just caudal to the umbilicus. For preparation of the adipose tissue for culture, the tissue was minced and digested with 1 mg/ml collagenase (Sigma Aldrich, St Louis, MO) for 30 min at 37°C. The sample was centrifuged and the stromal vascular fraction was washed, plated in MSC medium and expanded as described above for bmMSC.
Characterization of MSC
Adipose tissue-derived MSC (aMSC) and bmMSC were characterized by surface marker expression using flow cytometry and a panel of feline-specific and cross-reactive antibodies specific for surface determinants expressed by MSC from other species. 21−24 Specifically, feline MSC were analyzed for surface expression of CD44 (anti-feline antibody clone:IM7, eBioscience, San Diego, CA), CD105 (antibody clone:SN6, eBioscience, San Diego, CA), and CD90 (antibody clone:eBio5E10, eBioscience, San Diego, CA). MSC were also assessed for expression of CD4 (anti-feline antibody clone:3-4F4, Southern Biotech, Birmingham, AL) and feline MHC class II (antibody clone: TÜ39, BD Biosciences, San Jose, CA). Samples were analyzed using a Cyan ADP flow cytometer (Beckman Coulter, Brea, CA).
In vitro differentiation assays were conducted to confirm the multipotency of feline MSC, as assessed by their ability to differentiate into three cell lineages (osteoblasts, chondrocytes, and adipocytes) that are characteristic of MSC. 25 Assays were performed by incubating confluent MSC with medium supplemented with factors to stimulate differentiation. 25 At the end of the differentiation period, cells were fixed with 10% neutral buffered formalin and stained with Oil red O (Sigma Aldrich, St Louis, MO) for presence of lipid, with toluidine blue (Richard-Allan Scientific, Kalamazoo, MI) for cartilage matrix, or with Alizarin red (Sigma Aldrich, St Louis, MO) for the presence of calcium. 25 MSC cultured in MSC media alone under identical conditions were used as differentiation controls.
Intrarenal injection of MSC
Nine cats (two healthy cats, seven cats with CKD) were enrolled in this study. Two healthy cats and four CKD-affected cats were ultimately treated with MSC in the study. Table 1 summarizes the treatments received for all cats. Of the seven CKD cats were enrolled in the study initially, bmMSC could not be cultured to produce sufficient treatment quantity in three of the cats. Increasing doses of MSC were administered to the enrolled cats. Each recipient cat was sedated using intravenous administration of ketamine 3.3–4.8 mg/kg, once (dose repeated once if needed), midazolam 0.1 mg/kg, and butorphanol 0.1 mg/kg, placed in lateral recumbency, and monitored as previously described. Harvested MSC suspended in PBS were divided into three 150–200 μl aliquots and injected using a 25-gauge needle into three sites in the renal cortex under ultrasound guidance. The cortical injection sites were assessed for hemorrhage 1 h and 24 h after injection using ultrasonography.
Determination of GFR by nuclear scintigraphy
GFR was assessed in each kidney of cats injected with MSC using nuclear scintigraphy and was performed just prior to injection of MSC, 7 days after injection, and 30 days after injection. Cats were sedated (ketamine 3.3–4.8 mg/kg and butorphanol 0.1 mg/kg IV once) at a standard time before the procedure. A technician performed all of the procedures for each particular cat. For each procedure 1.0 mCi of Tc99m-labeled DTPA (Cardinal Health, Dublin, OH) was injected intravenously via a catheter placed in a standard location in each cat. Images were obtained using GE Millenium SPECT system (GE Healthcare, Waukesha, WI). Three independent radiologists evaluated the GFR data, and a mean GFR value for each kidney as well as a global value was determined.
Assessment of GFR variability
To assess the degree of intra-patient variability in repeated GFR measurements as determined by nuclear scintigraphy, three cats with CKD that did not receive MSC underwent nuclear scintigraphy GFR assessments on two occasions, 1 week apart. Data from these cats was used to provide information on inherent GFR variability and help interpret the results of GFR measurements made in cats with CKD treated with MSC.
Clinical monitoring of treated cats
Each treated cat underwent physical examination and routine blood work (CBC, serum biochemistry, urinalysis, and determination of UPC) immediately prior to MSC injection and on day 7, day 30, and day 60 after MSC injection.
Histopathology
Two cats with stage VI CKD were humanely euthanased in consultation with their owners due to progressive renal failure. The tissues of both the MSC-injected and non-injected kidneys were examined histopathologically using hematoxylin and eosin stained sections.
Statistical analysis of data
Changes in GFR, serum creatinine, blood urea nitrogen (BUN) and packed cell volume (PCV) data over time in the MSC-injected cats were evaluated by repeated measures ANOVA, followed by Bonferroni's correction. Values were considered statistically different for P<0.05. Statistical analyses were done using Prism5 software (GraphPad, San Diego, CA).
Results
Autologous MSC culture
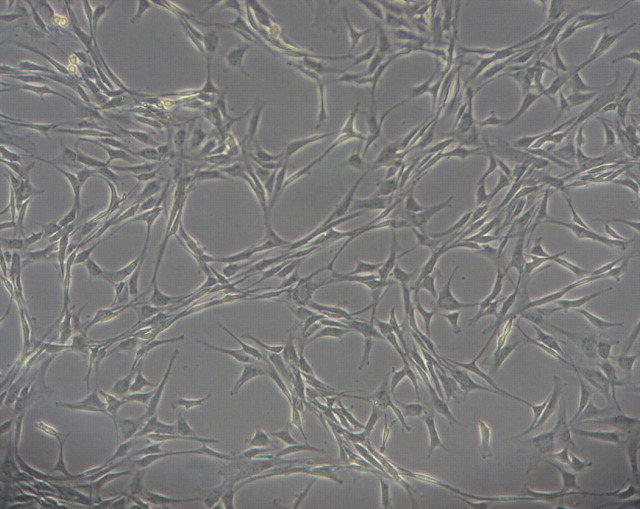
Feline MSC developed over a 1- to 2-week period into a relatively homogeneous population of plastic-adherent cells with fibroblast-like morphology (Fig 1). BmMSC from CKD cats were difficult to expand to obtain sufficient quantities for injection, and for three cats that were initially enrolled in the study, adequate cells could not be obtained. In contrast, it was observed that aMSC were easier to culture and expand, and sufficient cells for treatment were easily obtained from all three CKD cats enrolled.
Fig 1.
Phenotype of feline aMSC in culture. Feline adipose-derived MSC were established from adipose tissue biopsies, as described in the materials and methods. Cells assumed a typical elongated morphology, as described previously for feline MSC. 26 (Feline adipose MSC, magnification 10×.)
Characterization of feline MSC
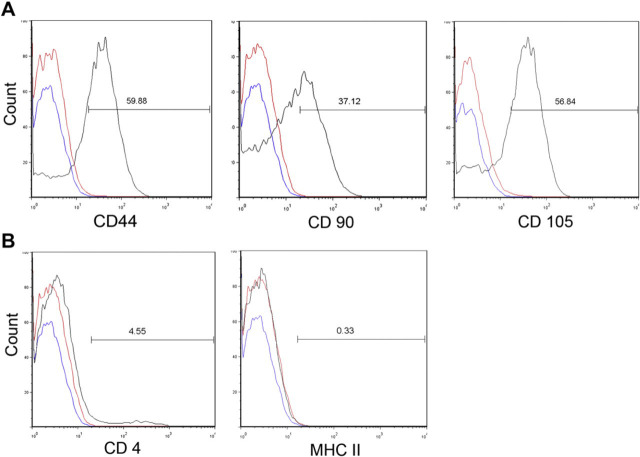
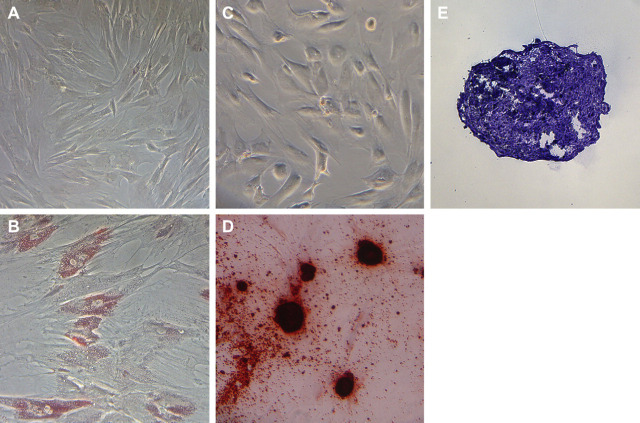
Both bmMSC and aMSC expressed high levels of CD44, CD90 and CD105 and were negative for expression of CD4 and MHC class II (Fig 2), which was consistent with the phenotype described previously for feline MSC. 26 Both bmMSC and aMSC were capable of trilineage differentiation (Fig 3).
Fig 2.
Expression of cell surface markers by feline adipose-derived MSC. Primary cultures of feline MSC obtained from cats 3, 7 and 8 were passaged three to five times in culture, then collected by trypsinization and immunostained for assessment of cell surface marker expression by flow cytometry, as described in the materials and methods. Feline adipose MSC expressed high surface levels of CD44, CD90, and CD105 (panel A), but did not express CD4 or MHC class II (panel B). Isotype controls are represented in red and unstained MSC are represented in blue. Similar results were obtained with adipose MSC from two additional young healthy research cats (not described in this study).
Fig 3.
Trilineage differentiation of feline aMSC. (A) Control aMSC incubated in standard media stained with Oil red O. (B) aMSC produced intracellular lipid vacuoles when incubated in adipocytic differentiation media for 21 days. (C) Control aMSC incubated in standard media stained with Alizarin red. (D) aMSC stained positive for calcium with Alizarin red following differentiation into osteocytic phenotype after 21 days of incubation in differentiation media. (E) Cryosection of pellets of cartilage matrix (stained with toluidine blue) formed by aMSC when exposed to chondrocytic differentiation media for 21 days.
Short-term safety of intrarenal injection of MSC in cats
Cats enrolled in the study were observed for adverse effects immediately following intrarenal MSC injection, including physical examination to assess pulse, respiration, mucus membrane color and abdominal or renal discomfort. By ultrasound examination, we did not observe hemorrhage 1 h or 24 h post-injection in any of the treated cats. The cats appeared clinically normal, and renal discomfort was not elicited on abdominal palpation. One of the healthy young cats developed transient, microscopic hematuria 24 h after MSC injection, but was not otherwise clinically affected.
Assessment of GFR variability
GFR values were determined on two separate occasions for three cats with CKD that did not receive MSC injection. These studies revealed that the mean total GFR for the three untreated cats was 1.46±0.28 ml/kg/min on week 1 and 1.55±0.08 ml/kg/min on week 2. The mean calculated variation between repeated GFR values for the three untreated cats with CKD between week 1 and week 2 was 9.6% (data not shown).
Effects of MSC injection on GFR
In the two healthy, young cats that received intrarenal MSC injection, the mean pre-treatment global GFR (sum of both kidneys) as determined by nuclear scintigraphy was 3.3±0.57 ml/kg/min (mean and SD), the mean 7-day GFR was 2.7±0.86 ml/kg/min, and the mean 30-day GFR was 3.6±1.5 ml/kg/min.
Renal function in three cats with CKD that received unilateral intrarenal injection of MSC (one bmMSC, two aMSC) was evaluated immediately prior to MSC injection, on day 7 post-injection, and on day 30 post-injection. A fourth cat was unable to undergo GFR monitoring via nuclear scintigraphy due to fractious nature. The mean pre-treatment global GFR for the three cats with CKD was 0.88±0.53 ml/kg/min, (median 0.72 ml/kg/min) which was 35% of the reported normal global GFR value for healthy adult cats (2.5 ml/kg/min). 27 Following MSC transfer, mean global GFR value for the three MSC-injected cats was 1.1±0.59 ml/kg/min (median 0.74 ml/kg/min) at 30 days, which though numerically increased was not significantly different from the pre-treatment global GFR value. While GFR variability in non-treated cats was assessed to be approximately 9.6%, CKD cats 7 and 8 had global GFR increases of 16% and 55% from baseline, respectively. In cat 3, who received bmMSC, no change from baseline was observed.
GFR values were also determined for individual kidneys of cats with CKD injected with MSC (Fig 4). In the MSC-injected kidney of all three cats, the mean pre-treatment GFR value was 0.45±0.39 ml/kg/min, (median 0.26 ml/kg/min) compared with a mean value of 0.58±0.54 ml/kg/min (median 0.28 ml/kg/min) determined on day 30. For the non-injected kidney, the mean pre-treatment value was 0.42±0.14 ml/kg/min, (median 0.46 ml/kg/min) compared to 0.47±0.05 ml/kg/min (median 0.46 ml/kg/min) on day 30. These data suggested a trend towards increasing GFR values for the injected kidney.
Fig 4.
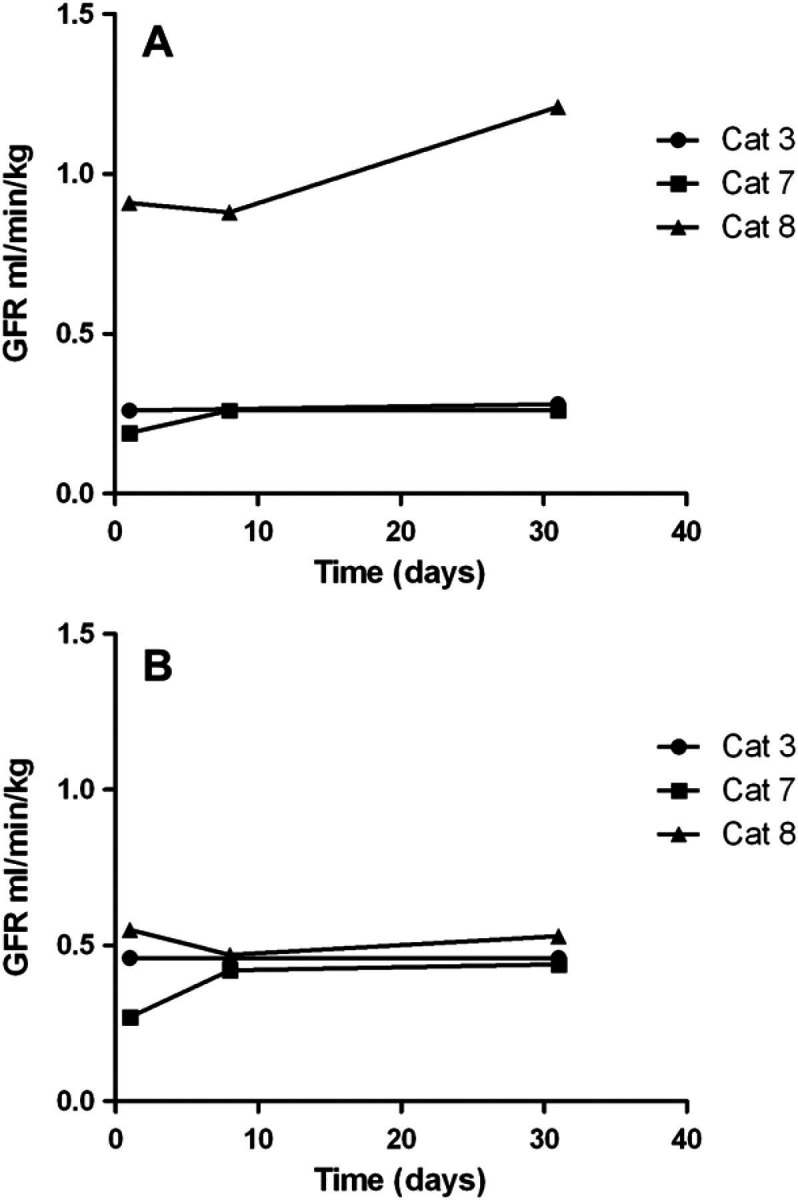
Changes in GFR over time in cats with CKD that received unilateral intrarenal MSC injections. GFR via nuclear scintigraphy was evaluated prior to treatment, on day 7 and day 30 in three cats for the injected kidney (A) and non-injected kidney (B). Modest improvement in GFR in the injected kidney was seen in one cat. Mild improvement in GFR of both kidneys was seen in one cat. While GFR variability in non-treated cats was assessed to be approximately 9.6%, CKD cats 7 and 8 had global GFR increases of 16% and 55% from baseline, respectively.
Effects of MSC injection on clinical parameters of renal function and outcome
In the two healthy cats that received intrarenal injections with bmMSC, we did not observe substantial changes in relevant laboratory values (serum creatinine, BUN, PCV, UPC ratio) measured before and after MSC injection (data not shown).
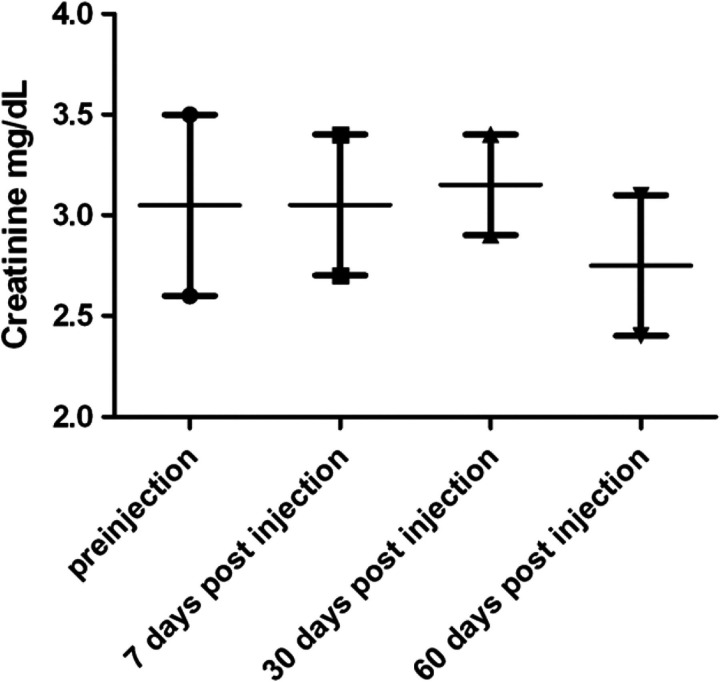
In CKD cats 7 and 8, that received aMSC, a modest though statistically insignificant improvement in serum creatinine concentration was noted, particularly at the 60-day time point (Fig 5). The PCV, BUN, and UPC ratio values were unchanged following treatment. Cat 7 was euthanased for acute lymphocytic leukemia 4 months after completing the MSC study, an outcome which was considered unrelated to the study itself. Cat 8 was still alive with stable CKD 10 months after MSC injection.
Fig 5.
Changes in serum creatinine concentration over time in cats with CKD that received unilateral, intrarenal MSC injections. Serum creatinine concentrations were evaluated prior to treatment, on day 7, day 30 and on day 60 in two cats that completed a 60-day trial of MSC injection therapy. There was a 9.8% overall decline in mean serum creatinine at day 60 compared to pre-treatment value in the two cats.
In CKD cat 3 (stage IV: creatinine 5.4 mg/dl) that received bmMSC, a small improvement in serum creatinine was observed on day 7 (creatinine 5.1 mg/dl) and day 30 (creatinine 5.2 mg/dl), and the UPC value increased over the same time period (initial UPC 1.1; 30-day UPC 3.3). This cat traveled some distance for its 60-day recheck (800 miles) and although the owners perceived the cat was doing well at home before travel, renal function in this cat had declined substantially upon arrival (creatinine 8.2 mg/dl), and the cat was subsequently euthanased at day 100.
In CKD cat 9 (stage IV: creatinine 6.5 mg/dl) that received aMSC, a small change in creatinine was seen at day 7 (creatinine 6.1 mg/dl) but by day 30 this cat was clinically less stable (creatinine 8.4) and was subsequently euthanased at day 42.
Histopathology
Histopathologic examination of the kidneys was performed in both CKD cats 3 and 9. In cat 3, there were abundant numbers of lymphocytes and plasma cells found multifocally, expanding the interstitium as well as extensive interstitial fibrosis. Rare subcapsular cysts were noted. Tubules were frequently lost, atrophied and ectatic with some evidence of regeneration and frequently contained luminal protein casts and scattered calcium oxalate crystals. There was mineralization of tubules within the cortex and medulla. Periglomerular fibrosis, glomerulosclerosis, mineralization and atrophy were present in 50% of glomeruli. In cat 9 there were lymphocytes, plasma cells and lesser neutrophils multifocally throughout the interstitium. There was tubular loss, atrophy and regeneration often associated with fibrosis. Numerous tubules contained sloughed epithelial cells, as well as, cellular, protein and waxy casts. Multifocally, tubules contained intraluminal crystals that were crescent or circular with radiating spokes and polarize. There were crystals present within the pelvis, which was lined by hyperplastic epithelium. Periglomerular fibrosis, glomerulosclerosis and a thickened basement membrane were present in 95% of glomeruli. Examination of the MSC-injected kidney of either cat did not show evidence of pathologic changes (vascular anomalies, tissue disruption, abnormal cell type) at the presumed injection sites.
Discussion
CKD is a major cause of morbidity and mortality in cats. In this study, intrarenal injection of autologous MSC was well tolerated in IRIS stages II and III CKD cats and may have induced mild improvement in renal function. Two cats with CKD exhibited mild improvement in creatinine and GFR values after intrarenal injection of MSC, though the differences were not statistically significant, which can be attributed in part to the small numbers of animals enrolled in this study. As this was a pilot feasibility and safety study, an untreated group was not included. Two cats with IRIS stage VI did not experience improvement in renal values and both cats were subsequently euthanased for progression of disease. Both cats had end-stage disease at enrollment, with a predicted median survival time of 44 days. 28 Histopathology in cat 9 revealed the presence of crystals, consistent with melamine–cyanuric acid, and this could have affected the cat's ability to respond to MSC therapy. Although it is possible the intrarenal MSC injections may have precipitated decline in these cats, we believe this unlikely as the decompensation occurred sometime after intrarenal injection.
The difficulty experienced in expanding autologous bmMSC caused us to abandon these cells in favor of aMSC for the remainder of the study. The use of aMSC in lieu of bmMSC is advantageous for several reasons. Collection of aMSC was less technically challenging and larger numbers of MSC could be obtained from the fat biopsy. Age and disease status may have also reduced the ability to establish and expand bmMSC cultures to a greater degree than with aMSC cultures, a phenomenon that has been observed in other species. 29
As this was a pilot study, with no previous safety data for MSC intrarenal injection to rely on, a dose-escalation study was conducted. Potentially, the use of dose-escalation as opposed to a single dose of MSC is a limitation of this study and could affect the ability to compare results between enrolled cats. Though the intrarenal injection method for MSC treatment of CKD in cats was feasible and appeared safe, our experience with this study leads us to believe that this approach is unlikely to be readily applicable clinically. During the study we observed that the multiple sedations required for obtaining bone marrow or adipose tissue samples, injections, and monitoring of renal function all contributed to substantial stress for the animals that over time could have adversely affected renal function. For example, the owners of all three cats with CKD noted that the hospital visits and sedation events affected the normal behavior of the cats and that it was generally 1–2 days before the cats resumed their normal behavior. Therefore, despite the potential benefits of intrarenal MSC injections for CKD in cats, we believe the large number of sedations and interventions required to implement this approach would preclude widespread clinical application.
Additionally, recent literature suggests that the intrarenal injection model utilized in this project may not be necessary, and this is a potential limitation of this pilot study. It is now generally thought that paracrine mechanisms are responsible for the therapeutic benefit seen in renal disease and that MSC do not need to be injected into the site of interest due to their migratory capabilities. 7,19,20 This is a potential explanation for the mild bilateral increase in GFR in cat 7; MSC could have also affected the non-injected kidney.
Thus, it may be preferable to consider alternative MSC sources and routes of administration for treatment of cats with CKD. Compelling support for intravenous administration of MSC comes from a recent study by Semedo et al. 7 In this study, it was reported in a rodent model of CKD that repeated intravenous delivery of relatively small numbers of MSC could elicit a significant positive impact on renal function and renal inflammation. Additionally, use of allogeneic instead of autologous MSC would eliminate the need to sedate the animals to obtain tissue samples and accelerate treatment administration. Allogeneic MSC have been widely used in experimental stem cell transfer investigations, including clinical trials in humans, and thus would not be expected to elicit unexpected adverse effects relative to autologous MSC therapy. 30−32
In summary, the results of the pilot study reported here lead us to conclude that MSC transfer by the intrarenal route is feasible in cats with CKD. Unilateral injection of the MSC did not adversely affect renal function in cats with CKD and may have elicited modest improvement in renal function in two of the four treated cats. Despite the possible benefits of intrarenal MSC injections for CKD in cats, we believe the large number of sedations and interventions required to implement this approach preclude widespread clinical application. Continued evaluation of the efficacy of MSC therapy in cats with CKD is warranted, but alternative sources and routes should be explored.
Acknowledgements
The authors wish to acknowledge the assistance of Drs A Marolf, D Gall and A Valdez for analyzing nuclear scintigraphy results and Dr Michael Lappin for providing SPF cats used in this study. This study was supported in part by grants from the Winn Feline Foundation and the Morris Animal Foundation.
References
- 1. Adin C.A., Gregory C.R., Kyles A.E., Cowgill L. Diagnostic predictors of complications and survival after renal transplantation in cats, Vet Surg 30, 2001, 515–521. [DOI] [PubMed] [Google Scholar]
- 2. Schmiedt C.W., Holzman G., Schwarz T., McAnulty J.F. Survival, complications, and analysis of risk factors after renal transplantation in cats, Vet Surg 37, 2008, 683–695. [DOI] [PubMed] [Google Scholar]
- 3. Roudebush P., Polzin D.J., Ross S.J., et al. Therapies for feline chronic kidney disease. What is the evidence?, J Feline Med Surg 11, 2009, 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Plotnick A. Feline chronic renal failure: long-term medical management, Compend Contin Educ Vet 29, 2007, 342–344, 46–50; quiz 51 [PubMed] [Google Scholar]
- 5. Choi S., Park M., Kim J., et al. The role of mesenchymal stem cells in the functional improvement of chronic renal failure, Stem Cells Dev 18, 2009, 521–529. [DOI] [PubMed] [Google Scholar]
- 6. Kirpatovskii V.I., Kazachenko A.V., Plotnikov E.Y., et al. Functional aftereffects of intraparenchymatous injection of human fetal stem and progenitor cells to rats with chronic and acute renal failure, Bull Exp Biol Med 141, 2006, 500–506. [DOI] [PubMed] [Google Scholar]
- 7. Semedo P., Correa-Costa M., Cenedeze M. Antonio, et al. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model, Stem Cells 27, 2009, 3063–3073. [DOI] [PubMed] [Google Scholar]
- 8. Cavaglieri R.C., Martini D., Sogayar M.C., Noronha I.L. Mesenchymal stem cells delivered at the subcapsule of the kidney ameliorate renal disease in the rat remnant kidney model, Transplant Proc 41, 2009, 947–951. [DOI] [PubMed] [Google Scholar]
- 9. Caldas H.C., Fernandes I.M., Gerbi F., et al. Effect of whole bone marrow cell infusion in the progression of experimental chronic renal failure, Transplant Proc 40, 2008, 853–855. [DOI] [PubMed] [Google Scholar]
- 10. Ninichuk V., Gross O., Segerer S., et al. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice, Kidney Int 70, 2006, 121–129. [DOI] [PubMed] [Google Scholar]
- 11. Kunter U., Rong S., Boor P., et al. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes, J Am Soc Nephrol 18, 2007, 1754–1764. [DOI] [PubMed] [Google Scholar]
- 12. Ezquer F., Ezquer M., Simon V., et al. Endovenous administration of bone-marrow-derived multipotent mesenchymal stromal cells prevents renal failure in diabetic mice, Biol Blood Marrow Transplant 15, 2009, 1354–1365. [DOI] [PubMed] [Google Scholar]
- 13. Wong C.Y., Cheong S.K., Mok P.L., Leong C.F. Differentiation of human mesenchymal stem cells into mesangial cells in post-glomerular injury murine model, Pathology 40, 2008, 52–57. [DOI] [PubMed] [Google Scholar]
- 14. Prodromidi E.I., Poulsom R., Jeffery R., et al. Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome, Stem Cells 24, 2006, 2448–2455. [DOI] [PubMed] [Google Scholar]
- 15. Imasawa T., Utsunomiya Y. Stem cells in renal biology: bone marrow transplantation for the treatment of IgA nephropathy, Exp Nephrol 10, 2002, 51–58. [DOI] [PubMed] [Google Scholar]
- 16. Imasawa T., Nagasawa R., Utsunomiya Y., et al. Bone marrow transplantation attenuates murine IgA nephropathy: role of a stem cell disorder, Kidney Int 56, 1999, 1809–1817. [DOI] [PubMed] [Google Scholar]
- 17. Sugimoto H., Mundel T.M., Sund M., et al. Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease, Proc Natl Acad Sci U S A 103, 2006, 7321–7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guillot P.V., Cook H.T., Pusey C.D., et al. Transplantation of human fetal mesenchymal stem cells improves glomerulopathy in a collagen type I alpha 2-deficient mouse, J Pathol 214, 2008, 627–636. [DOI] [PubMed] [Google Scholar]
- 19. Togel F., Yang Y., Zhang P., Hu Z., Westenfelder C. Bioluminescence imaging to monitor the in vivo distribution of administered mesenchymal stem cells in acute kidney injury, Am J Physiol Renal Physiol 295, 2008, F315–F321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Togel F., Weiss K., Yang Y., et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury, Am J Physiol Renal Physiol 292, 2007, F1626–F1635. [DOI] [PubMed] [Google Scholar]
- 21. Avery P.R., Lehman T.L., Hoover E.A., Dow S.W. Sustained generation of tissue dendritic cells from cats using organ stromal cell cultures, Vet Immunol Immunopathol 117, 2007, 222–235. [DOI] [PubMed] [Google Scholar]
- 22. Kolf C.M., Cho E., Tuan R.S. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation, Arthritis Res Ther 9, 2007, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Locke M., Windsor J., Dunbar P.R. Human adipose-derived stem cells: isolation, characterization and applications in surgery, ANZ J Surg 79, 2009, 235–244. [DOI] [PubMed] [Google Scholar]
- 24. Vieria N.M., Brandalise V., Zucconi E., et al. Isolation, characterization and differentiation potential of canine adipose-derived stem cells, Cell Transplant Transplant 19, 2010, 279–289. [DOI] [PubMed] [Google Scholar]
- 25. Reger R.L., Tucker A.H., Wolfe M.R. Differentiation and characterization of human MSCs. Prockop D.J., Phinney D.J., Bunnell B.A. Methods in molecular biology; mesenchymal stem cells: methods and protocols, 2008, Humana Press: Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 26. Martin D.R., Cox N.R., Hathcock T.L., Niemeyer G.P., Baker H.J. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow, Exp Hematol 30, 2002, 879–886. [DOI] [PubMed] [Google Scholar]
- 27. Kerl M.E., Cook C.R. Glomerular filtration rate and renal scintigraphy, Clin Tech Small Anim Pract 20, 2005, 31–38. [DOI] [PubMed] [Google Scholar]
- 28. Boyd L.M., Langston C., Thompson K., Zivin K., Imanishi M. Survival in cats with naturally occurring chronic kidney disease (2000–2002), J Vet Intern Med 22, 2008, 1111–1117. [DOI] [PubMed] [Google Scholar]
- 29. Stenderup K., Justesen K., Clausen C., Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells, Bone 33, 2003, 919–926. [DOI] [PubMed] [Google Scholar]
- 30. Togel F., Cohen A., Zhang P., et al. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury, Stem Cells Dev 18, 2009, 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wolf D., Reinhard A., Seckinger A., et al. Regenerative capacity of intravenous autologous, allogeneic and human mesenchymal stem cells in the infarcted pig myocardium-complicated by myocardial tumor formation, Scand Cardiovasc J 43, 2009, 39–45. [DOI] [PubMed] [Google Scholar]
- 32. McTaggart S.J., Atkinson K. Mesenchymal stem cells: immunobiology and therapeutic potential in kidney disease, Nephrology (Carlton) 12, 2007, 44–52. [DOI] [PubMed] [Google Scholar]