Abstract
Home-monitoring of blood glucose concentrations has recently been introduced to owners. The objectives of this study were to investigate the feasibility of home-monitoring of blood glucose in diabetic cats by owners, the problems encountered and to compare glucose concentrations at home with those measured in the hospital. Twelve of 15 cat owners were able to generate glucose curves over the study period of 4 months. Most problems were related to restraining the cat, generating negative pressure with the lancing device and producing a blood drop. In the majority of cases, these problems could be resolved during the study. Blood glucose concentrations in the clinic tended to be lower than at home; some of the differences were significant. No association between tolerance of the procedure and blood glucose concentrations measured at home was found. We, therefore, assume that the lower glucose levels in the hospital were caused by lack of food intake. In 38% of cases, treatment based on hospital curves would have been different from that based on home curves. Home-monitoring appears to be a valuable tool in the management of cats with diabetes mellitus. One of its major advantages is that it enables frequent generation of blood glucose curves, which is of particular importance in cats that are difficult to regulate.
Serial blood glucose curves (BGCs) are necessary to assess insulin efficacy, glucose nadir, time of peak insulin effect, duration of the insulin effect and degree of fluctuations in blood glucose (BG) concentrations. Blood glucose curves are also required to recognise the Somogyi phenomenon (Feldman and Nelson 1996). Until recently, the vast majority of BGCs were performed in the hospital because most pet owners are unable to collect blood samples by venepuncture. However, a variety of problems are associated with the determination of BGCs in hospitalised patients. The process is time consuming and relatively expensive and therefore, is not performed as frequently as required. Stress or lack of food intake can markedly influence BG concentrations. Cats in particular are sensitive to stress caused by an unfamiliar environment or by veterinary manipulation. Therefore, especially in diabetic cats, in-hospital BGCs can be difficult to interpret or may even be useless.
In human medicine, it is standard procedure for diabetics to determine their own blood glucose concentrations with a portable blood glucose meter (PBGM). Blood is usually collected from a fingertip using a lancing device. This so called self-monitoring has become an integral part of the management of diabetes mellitus in humans (Cohen and Zimmet 1980, Bergman and Felig 1984, American Diabetes Association 1996, 1998, Foster et al 1999).
Several recent studies have shown that collection of capillary blood using a lancing device is possible in dogs and cats, and glucose concentrations determined using a PBGM and capillary blood from the ear are in agreement with those of venous blood (Wess and Reusch 2000a, Thompson et al 2002). Evaluation of various PBGMs showed that their accuracy and reliability were sufficient for the purpose of home-monitoring of diabetic dogs and cats. (Wess and Reusch 2000b, 2000c). A number of problems, mostly technical in nature, can arise during home-monitoring. However, with experience many pet owners overcome these difficulties and perform correct measurements (Casella and Reusch 2000, Casella et al 2002). A recent study performed over several months in diabetic dogs revealed that in 42% of glucose curves (total of 38 pairs), treatment decisions based on BGCs generated in the hospital differed from those generated by the owners at home. In 3% of cases, treatment decisions based on in-hospital BGCs completely contradicted those based on BGCs determined at home (Casella et al 2003).
Up till now no studies on home-monitoring in cats with diabetes mellitus have been published. Therefore, the objectives of this study were: (a) to determine whether owners of diabetic cats are willing and able to perform home-monitoring on a long-term basis, (b) to determine the types of possible problems encountered and (c) to compare BGCs generated in the hospital with those generated at home by the owner, using the same technique.
Materials and methods
Selection of cats
This prospective study was conducted during the years 1999 and 2000 at the Clinic of Small Animal Internal Medicine, Vetsuisse-Faculty University of Zurich, Switzerland. Cats with diabetes mellitus were included in the study if their owners were willing to learn home-monitoring and to return to our clinic for six re-evaluations of their cats over a 16-week period.
Diagnosis of diabetes mellitus was based on characteristic clinical signs, fasting hyperglycemia and elevated serum fructosamine (>340 μmol/l). Fifteen cats were included into the study. They ranged in age from 5 to 16 years (median 11 years) and weighted 3.2–11.3 kg (median 5.6 kg). In 13 cats, diabetes mellitus was diagnosed at our clinic. One cat had been treated with insulin for 3 days and one other for 2 months by the referring veterinarian prior to the study. There were four spayed female and 11 castrated male cats. Breeds included domestic shorthair cats (n=14) and one Maine Coon. Twelve were indoor cats and three were allowed to go outside. None of the owners was familiar with blood collection before the study. Informed owner consent was obtained in all cases.
Study design
A thorough physical examination, complete blood count, biochemical profile (including fructosamine and total T4) and urinanalysis were performed in all cats. Further examinations were performed when indicated. Cats received an intermediate-acting insulin (Caninsulin: Intervet, Boxmeer, the Netherlands) at a dosage of 0.25 IU/kg when fasting BG was <20 mmol/l SC bid, and 0.5 IU/kg, SC bid, when fasting BG was >20 mmol/l. The majority of cats remained in our clinic for 3 days. Blood glucose concentrations were determined before and every 3 h after administration of insulin for a 12-h period (five measurements). The dosage of insulin was adjusted only when hypoglycemia occurred (BG concentration <3 mmol/l). Six cats that had diabetic ketoacidosis and one cat with hypoglycaemia caused by insulin over dosage at the time of admission underwent intensive treatment; after stabilisation they were treated as described above. At discharge, owners received detailed information on various aspects of diabetes mellitus and were instructed on how to inject insulin. Also, the concept of home-monitoring was introduced to the owner for the first time.
Re-evaluations were scheduled 1, 3, 6, 9, 12, and 16 weeks after the first evaluation and included a detailed updated history, physical examination and measurement of haematocrit and concentrations of serum fructosamine, albumin and total protein. A blood glucose curve was obtained by measuring BG concentration before and every 2 h after administration of insulin for a 12-h period (seven measurements).
At the second re-evaluation, each owner was taught the technique of home-monitoring. This process took a minimum of 30 min and consisted of repeated demonstrations. The owners then performed the technique once or twice on their pet. Each owner was also taught how to calibrate the PBGM, how to check its accuracy using the control strip and how to record BG concentrations on prepared forms.
Owners received written instructions, which included pictures of the home-monitoring technique, a PBGM, test strips, a lancing device, a form to record BG measurements and a questionnaire. They were asked to perform a blood glucose curve at home within 1 week before the next re-evaluation. Blood glucose concentrations were to be determined before and every 2 h after administration of insulin for a 12-h period. Owners were also asked to fill in a questionnaire after each BGC (Casella et al 2002). At the third re-evaluation (6 weeks after the first evaluation), a veterinarian (MC) evaluated and, if necessary, corrected the owner's technique of capillary blood collection. When required, other aspects of the home-monitoring technique were also discussed at this time. Insulin dosage was adjusted based on the glucose nadir: when the nadir was <5 mmol/l, from 5 to <9 mmol/l, or ≥9 mmol/l, the insulin dosage was decreased (1 IU/cat), remained unchanged or was increased (1 IU/cat), respectively.
Owners performed a total of four BGCs, which were referred to as home curves. A BGC was performed at our clinic within 1 week after each of the home curves; these curves were referred to as hospital curves. Each of the four home curves was compared to the corresponding hospital curve. The comparisons were referred to as the first curve comparison (fifth and sixth weeks after first evaluation), second curve comparison (eighth and ninth weeks after first evaluation), third curve comparison (11th and 12th weeks after first evaluation) and fourth curve comparison (15th and 16th weeks after first evaluation). The dosage of insulin administered was identical for each pair of corresponding curves.
Equipment
The PBGM and lancing device used in this study were the Glucometer Elite and the Microlet Vaculance (both from Bayer Diagnostics, Zurich, Switzerland).
Collection of capillary blood and measurement of blood glucose concentration
Capillary blood was collected from the inner pinna using the lancing device. Blood glucose concentrations were measured using the PBGM. The technique was performed as previously described (Wess and Reusch 2000a, Reusch et al 2001, Casella et al 2002, 2003). For all blood glucose measurements (hospital and home), the same blood collecting technique and the same type of PBGM were used (Fig 1).
Fig 1.
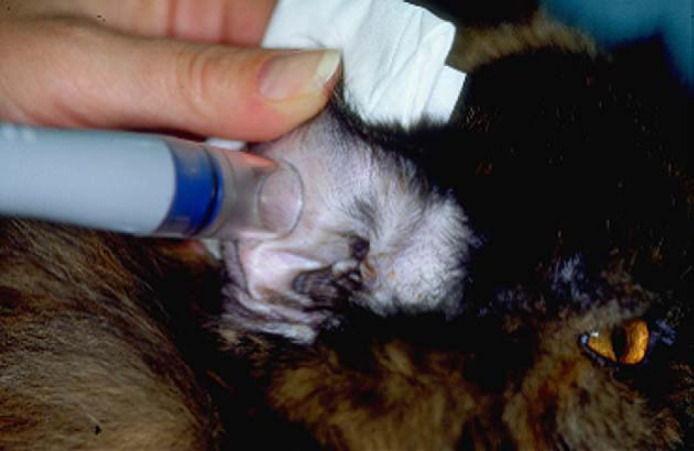
Capillary blood sampling from the pinna of a cat by use of a lancing device (Microlet Vaculance).
Data analysis
A descriptive data analysis was used for describing technical problems encountered during blood collection. The general condition of each cat and clinical variables were used to assess the efficacy of therapy. A cat was considered stable if it was healthy and interactive at home, had a normal appetite, normal water intake and a stable body weight and if the BG concentrations during the day were between 4.5 and 26 mmol/l. A difference of 50 μmol/l between the serum fructosamine concentration at the beginning and end of the study was defined as clinically relevant, eg, relevant increase or relevant decrease (Reusch et al 1995).
The other results were analysed by means of non-parametric statistical methods (SPSS/PC V 10.0. base manual, SPSS Inc, Chicago, USA). Ranges and median values are reported. Differences were tested using Friedman ANOVA and the Wilcoxon signed rank test for matched pairs. The χ2 test was used to test for association between tolerance of the procedure and blood glucose concentrations. Differences were considered significant at P<0.05. Box-and-whisker plots were used for graphical representation of glucose concentrations. The horizontal lines of the box represent, from bottom to top, the 25th, 50th (median) and the 75th percentiles. The ends of the whiskers represent the 10th and the 90th percentiles. Outlying data points are represented by open circles.
Results
Feasibility of home-monitoring
Twelve of 15 owners were able to determine BG concentrations. Seven of them completed all four BGCs. The other five owners encountered problems with blood collection: two completed only the last three of four BGCs, two only the last two BGCs and one owner performed only the first, third and fourth BGC. Three of 15 owners were unable to perform any BG measurements because their cats did not tolerate the procedure.
Problems encountered initially included: producing negative pressure with the lancing device (11/12), producing a blood drop (10/12), restraining the cat (8/12), absorption of the blood drop (4/12) and correct use of the test strips (2/12) and the PBGM (2/12). At the end of the study, there were still problems involving restraint of the cat (8/12), production of a blood drop (3/12) and the generation of negative pressure using the lancing device (2/12). Of the 12 owners who completed the study, home-monitoring at the time of the first curve comparison was considered not feasible by four, difficult by another four, mostly straightforward by three and straightforward by one. At the end of the study, home-monitoring was considered difficult by only one owner, mostly straightforward by six and straightforward by five (Table 1). Ten of the 12 owners performed BG measurements without help and two owners always required another person for help. Of the 12 cats, two tolerated the procedure very well from the start of the study, five became accustomed to the procedure during the first BGC, in another five problems increased during the first day. At the end of the study, four tolerated the procedure very well, six became accustomed to the procedure after the first or second blood sample of every BGC and two continued to cause problems during the collection of blood.
Table 1.
Technical problems encountered during blood collection from the ear and general judgement of cat owners on feasibility of home-monitoring
| Beginning home-monitoring | End home-monitoring | |
|---|---|---|
| Technical problems | ||
| Negative pressure | 11/12 | 2/12 |
| Generation of blood drop | 10/12 | 3/12 |
| Restraint of cat | 8/12 | 8/12 |
| Absorption of blood | 4/12 | 0/12 |
| Correct use of test strip | 2/12 | 0/12 |
| Correct use of PBGM | 2/12 | 0/12 |
| General judgement of owner | ||
| Not feasible | 4/12 | 0/12 |
| Difficult | 4/12 | 1/12 |
| Mostly straightforward | 3/12 | 6/12 |
| Straightforward | 1/12 | 5/12 |
PBGM=portable glucose meter.
In the clinic, two of 12 cats were aggressive making completion of all BGCs difficult. In one, the first two BGCs and in the other, the last two BGCs were not performed.
Comparison of home and hospital blood glucose curves
A total of four comparisons between home and hospital BGCs were made. The maximum BG concentrations (mmol/l) in BGCs performed in the clinic and at home, respectively, were 17.6–29.4 (median 25.6) and 12.2–31.2 (median 25.8) for the first curve comparison, 13.3–29.1 (median 26.4) and 13.2–32.3 (median 25.9) for the second curve comparison, 10.1–32.2 (median 23.6) and 9.7–33.3 (median 22.8) for the third curve comparison, 10.8–30.6 (22.2) and 6.5–33.3 (median 24.8) for the fourth curve comparison.
The nadirs (mmol/l) of the curves performed in the clinic and at home, respectively, were 5.4–24.2 (median 13.1) and 5.1–25.8 (median 13.8) for the first curve comparison, 1.6–23.4 (median 11.6) and 2.3–17.4 (median 8.3) for the second curve comparison, 1.4–16.2 (median 3.9) and 2.0–19.9 (median 9.4) for the third curve comparison, 1.6–13.9 (median 7.3) and 2.7–18.2 (median 13.5) for the fourth curve comparison.
The mean (mmol/l) of the curves performed in the hospital and at home, respectively, were 13.7–26.0 (median 18.7) and 7.7–22.9 (median 20.2) for the first curve comparison, 5.3–25.6 (median 17.6) and 7.8–24.4 (median 21.2) for the second curve comparison, 4.5–21.3 (median 13.1) and 5.6–26.7 (median 17.1) for the third curve comparison, 5.7–21.6 (median 13.1) and 4.3–26.6 (median 19.2) for the fourth curve comparison (Fig 2A and B).
Fig 2.
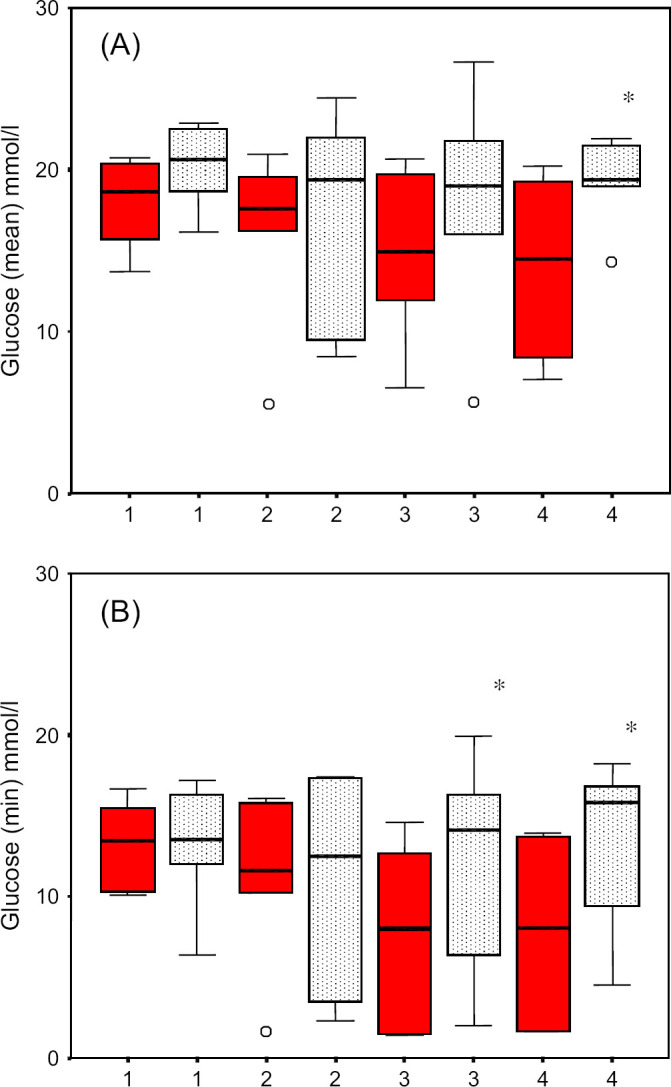
(A) Comparison of mean blood glucose concentrations of home and hospital blood glucose curves of 12 cats with diabetes mellitus. (B) Comparison of glucose nadirs of home and hospital blood glucose curves of 12 cats with diabetes mellitus. The mean blood glucose concentrations of the fourth hospital curve and the minimal blood glucose concentrations of the third and fourth hospital curves were significantly lower compared to the corresponding home curves. Blood glucose concentrations determined in the clinic=black; at home=stippled. *P<0.05.
The nadirs in the third and fourth curve comparisons and the mean glucose concentration in the fourth curve comparison were significantly lower for curves performed in the hospital than for those performed at home. There was no difference between maximum glucose concentrations measured in the hospital and at home.
For further analyses, tolerance of the procedure for each curve at home was categorised as good, moderate and poor. There was no association between maximum, minimum and mean blood glucose concentrations measured at home and tolerance of the procedure.
Comparison of blood glucose nadirs and potential treatment decisions
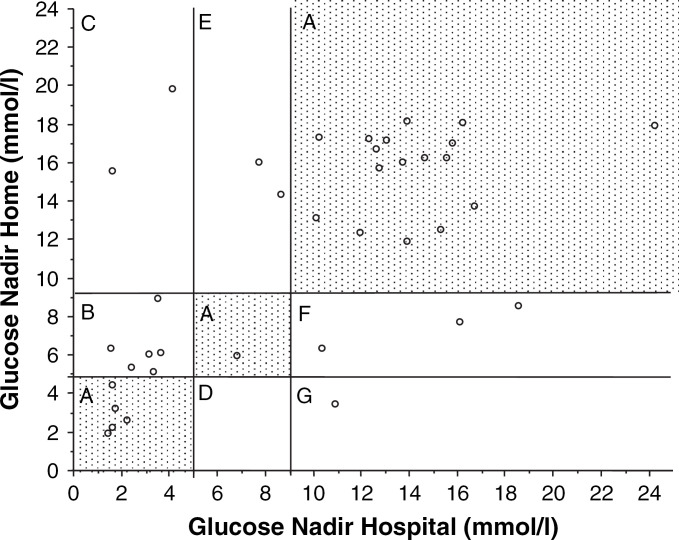
A total of 37 pairs of BGCs (hospital and home) were available to determine whether the two corresponding curves would have led to the same or to a different clinical decision with regard to adjustments of insulin dosage. For this comparison it was assumed that decisions would have been based on the respective blood glucose nadirs of the hospital and home curves. Decisions regarding insulin dosage would have been in agreement in 23 pairs of BGCs (Fig 3A) and would have differed in 14. Based on the results of home BGCs, no change in insulin dosage would have been instituted in nine of 14 cats (Fig 3B and F), whereas on the basis of hospital curves, the insulin dosage would have been decreased in six cats (Fig 3B) and increased in three (Fig 3F). In four other cases, the insulin dosage would have been increased based on home BGCs (Fig 3C and E), whereas on the basis of hospital BGCs, the insulin dosage would have remained unchanged in two cats (Fig 3E) and decreased in two (Fig 3C). In the one remaining case, the insulin dosage would have been decreased based on the home BGC and increased based on the hospital BGC (Fig 3G).
Fig 3.
Graphic representation of the glucose nadirs for all blood glucose comparisons. The x-axis represents values from hospital curves and the y-axis represents values from home curves. In 23 of 37 blood glucose pairs, the decisions regarding treatment were in agreement (zone A). In 14 of 37 pairs, the decision regarding treatment differed in one of five different ways: zone B—hospital curve: decrease insulin; home curve: insulin unchanged; zone C—hospital curve: decrease insulin; home curve: increase insulin; zone D—hospital curve: insulin unchanged; home curve: decrease insulin; zone E—hospital curve: insulin unchanged; home curve: increase insulin; zone F—home curve: increase insulin; home curve: insulin unchanged.
Quality of glycaemic control
In the 12 cats of the present study, treatment decisions with regard to adjustment of insulin dosage were based on the nadir of the home curves starting 6 weeks after the first evaluation (at the beginning of home-monitoring). At this time, general condition had improved compared to initial evaluation. Water intake was judged to be normal in one cat, moderately elevated in nine cats and severely elevated in another two cats. At the end of the study (week 16) general condition was judged to be normal in all 12 cats. In seven of the above-mentioned nine cats water intake had normalised, in two of the nine cats water intake was unchanged (remaining moderately elevated). In the two cats with previously severely elevated water intake improvement was seen. The one cat with normal water intake at week 6 was also normal at week 16. Fructosamine concentrations decreased in the same period in seven cats, remained unchanged in two and increased in three.
At the end of the study eight of the 12 cats were judged to be well regulated with regard to clinical signs, in four cats glycaemic control was judged to be moderate.
Discussion
In the present study 80% (12 of 15) of the owners of diabetic cats were willing and able to perform a series of BGCs at home over a period of 4 months. In a previous study, only 43% (3/7) of owners of healthy cats were able to perform the same tasks during a shorter study period (Casella et al 2002). Possibly, owners of ill cats are more motivated to overcome the problems associated with home-monitoring with the hope that their pet will benefit from their efforts. For example, many owners devised strategies for easier restraint of their cat and reported that the cat tolerated blood collection better when placed in a favourite spot, such as a windowsill or bed or in a confined area such as a sink. The present study lasted longer and possibly gave owners more time to become proficient at the technique of home-monitoring. Furthermore, in comparison to the healthy cats, the diabetic cats seemed to cooperate substantially better during the procedure of blood collection. This may be because the diabetic cats were older than the healthy cats and with the exception of one, were exclusively indoor cats.
The success of home-monitoring hinges greatly on careful preparation and instruction of the owner. The technique of home-monitoring was first introduced to all owners 3 weeks after the initiation of treatment in our clinic. This time frame allowed owners to become familiar with the disease and with administration of insulin. Re-assessments were carried out frequently (every 3 or 4 weeks) so that the owners' technique could be evaluated often and corrected when necessary.
Three of 12 owners were immediately able to generate BGCs at home. Technical errors were corrected via telephone for two owners. However, additional demonstrations of the technique in our clinic were necessary for seven owners. In human medicine, the importance of patient education with repeated demonstrations and observation of patient technique is emphasised in a study of The National Steering Committee for Quality Assurance in Capillary Blood Glucose Monitoring (1993). It has been shown that most errors in self-monitoring of blood glucose concentration are associated with blood collection or use of the PBGM (Fleming 1994). It is also important to minimise any technical difficulties for pet owners. In the present study, the Glucometer Elite was selected as the easiest PBGM on the market to operate: it has no buttons to press, turns on automatically when the test strip is inserted and requires only a small amount of blood (2 μl), which is automatically aspirated into the reaction chamber after contacting the test strip. Studies in humans have shown that the accuracy of PBGMs can vary greatly (Trajanoski et al 1996, Chan et al 1997, Brunner et al 1998). In the past few years, several groups evaluated PBGMs for use in animals using a variety of different statistical procedures (Joseph et al 1987, Link et al 1997, Cohn et al 2000, Wess and Reusch 2000b, 2000c). In human medicine, error grid analysis has gained widespread acceptance for the assessment of blood glucose measurements (Clarke et al 1987, Kabadi et al 1994, Brunner et al 1998). Two recent veterinary studies that used error grid analysis demonstrated that glucose measurements of canine and feline blood samples using different PBGMs were in clinically acceptable ranges (Wess and Reusch 2000b, 2000c). In contrast to other PBGMs, which over- or underestimated glucose concentrations, the one used in the present study consistently underestimated blood glucose concentrations (Wess and Reusch 2000b, 2000c). This is considered an advantage because the errors are more predictable. Because collection of blood via venepuncture is beyond the scope of an average pet owner, a method for collection of capillary blood using a special lancing device (Microlet Vaculance) was recently developed. This device creates a negative pressure, which facilitates collection of an adequate amount of blood from the ear of cats and dogs (Wess and Reusch 2000a). An important prerequisite for blood collection is correct handling of the lancing device so that it actually creates a vacuum. This can be difficult and is linked to most of the technical problems (Casella and Reusch 2000, Casella et al 2002). In our experience, this problem can be overcome by repeated demonstrations and practise.
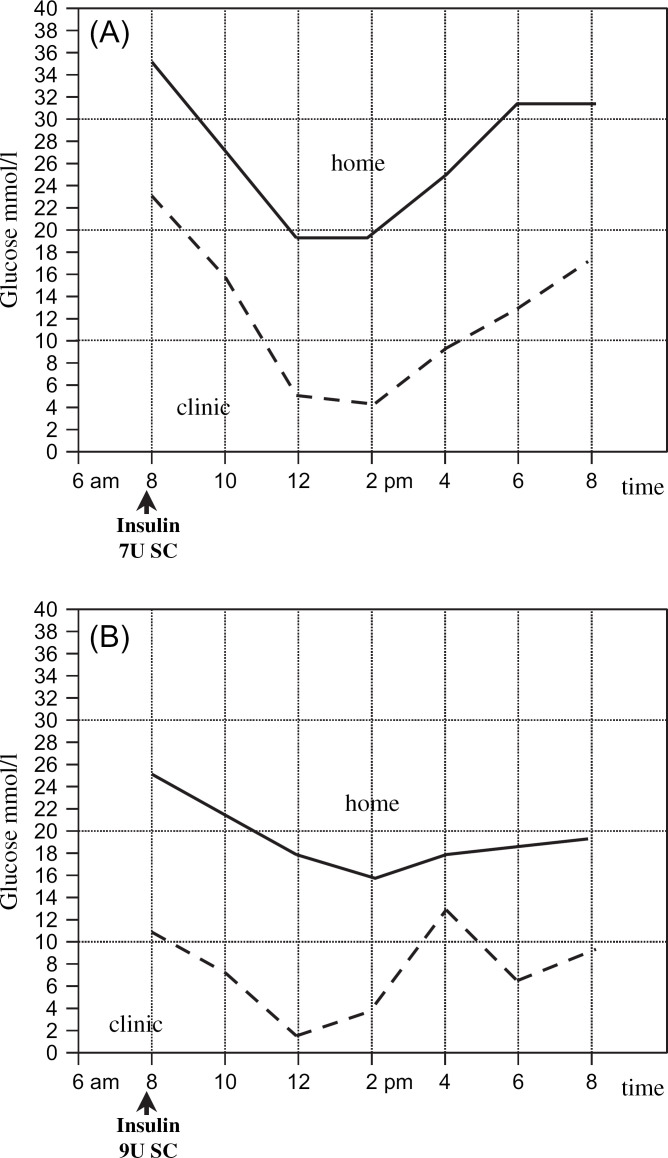
Interestingly, glucose concentrations measured in the hospital had the tendency to be lower than those measured at home and the minimum glucose concentrations in the third and fourth curve comparisons and the mean glucose concentration in the fourth curve comparison were significantly lower for hospital curves. In fact, we had assumed that due to stress caused by an unfamiliar environment, veterinary manipulation and reduced physical activity during the stay in the clinic, the BG concentrations would be elevated. Cats are considered particularly sensitive to stress (Leidinger et al 1989, Opitz 1990, Feldman and Nelson 1996). There are several possible explanations for our finding. First, one could argue that the differences were caused by technical errors made by owners at home. However, this appears to be unlikely because an inadequate amount of blood would have resulted in an erroneous low BG measurement (Wess and Reusch 2000b, 2000c). Second, one has to consider the possibility that the values at home were higher than those in the hospital because the cats were actually more stressed when untrained owners collected capillary blood. To investigate this possibility tolerance of the procedure at home was classified for each BGC as good, moderate and poor. Statistically no association was found between blood glucose levels and tolerance of the procedure. Therefore, we are hesitating to attribute the higher concentrations to the stress of home-monitoring. We can of course not exclude that some cats were stressed but tolerated the procedure well. Third, it has to be considered that the glucose levels at home reflected the true glucose concentrations and that, false low readings were obtained in hospital. A plausible reason could be that hospitalised cats frequently refused to eat. In the present study in 10 pairs of BGCs obtained from seven cats during the third and fourth curve comparison the nadir was lower in the hospital than at home. We are aware that at least five of the seven cats refused to eat, the rest ate only very little. In a similar study of diabetic dogs, a decrease in food intake was believed to be the most likely explanation for lower BG concentrations in hospitalised dogs compared to dogs evaluated at home (Casella et al 2003). We did not take exact notes on the amount of food our cats ate in the hospital compared to what they were used to eat at home. Therefore, this information was not included into the result section and was not used for any further statistical analysis (Fig 4).
Fig 4.
Two blood glucose curves (BGCs), generated either at home (broken line) or in the clinic (solid line) in a 7-year-old, weighing 7.6 kg, castrated male, domestic shorthair cat. For each BGC, the blood glucose concentration was measured before and every 2 h after administration of insulin during a 12-h period for a total of seven measurements. The two BGCs, generated within one day of each other, differ. (A) Third re-evaluation. The cat is on 7 IU Caninsulin bid and still showed polydipsia and polyuria. The home BGC has a nadir of 19.9 mmol/l, which is too high, and thus, an increase in the insulin dosage would be advised. The hospital BGC has a nadir of 4.1 mmol/l, which is considered borderline, and a reduction in the insulin dosage might be advised. The final decision was based on the home curve and the insulin dosage was increased to 9 IU bid. (B) Forth re-evaluation. The cat is on 9 IU Caninsulin bid. Polydipsia and polyuria are still present but have improved. The home BGC had a nadir of 15.6 mmol/l and the hospital BGC a nadir of 1.6 mmol/l. Based on the hospital and home curves, the insulin dosage would be reduced and increased, respectively. The insulin dosage was kept unchanged and the owner was asked to generate another BGC a week later. The low glucose nadirs in the hospital BGC were attributed to decreased food intake during hospitalisation. The home BGC one week later (not shown) had again a nadir >9 mmol/l and the owner increased the insulin dosage to 10 IU bid. Since then the cat is doing well and clinical signs have markedly improved.
We do not know why there was no such difference between the BGCs in the first two curve comparisons, but we hypothesise that it was due to stress-related hyperglycaemia overriding a decrease of BG because of lack of food intake at the beginning of the study in cats in the clinic. During the course of study, the stress caused by the blood collection in the hospital may have diminished as cats became accustomed to the procedure, although not to the point where they felt comfortable enough to eat. However, it is impossible to determine the role of stress in this study. Physiological stress is difficult to define in animals. Recently, a study attempted to characterise the changes in BGCs in cats exposed to an acute stressor and to determine the associations between BG and behavioural indicators of stress in stress hyperglycaemia (Rand et al 2002). It was demonstrated that there is a strong relationship between increased BG and fear responses of cats and that the nature of the stressor influences the severity of hyperglycaemia. That study, however, was performed in healthy cats, and it is currently not known whether the findings also apply to sick or diabetic cats.
In addition to comparing home and hospital curves statistically, we also compared the potential treatment decisions based on BGCs of each cat. As the cats were also part of another multicentre study we were obliged to base the decisions exclusively on the nadir of the BGCs. In the majority of cases, 23/37 (62%), treatment decisions would have been the same. In 14/37 (38%) of the cases (case=one pair of BGCs), treatment decisions based on the nadir of the hospital curves differed from those of the home curves; in three cases, the treatment decisions would have been completely contrary (increase vs decrease of insulin dosage) and in the remaining 11 cases, they would have differed, although probably with little clinical consequence. So far we do not know the reproducibility of blood glucose curves generated at home. Blood glucose curves are known to vary from day to day (Feldman and Nelson 1996). Similarly, Fleeman and Rand (2003) reported that in diabetic dogs, BGCs determined in the clinic on successive days varied widely. Studies are currently underway in our clinic to compare the reproducibility of BGCs performed at home and in the clinic.
At the moment we are unable to prove whether hospital or home curves reflect the ‘true’ blood glucose concentrations. In the present study, the adjustments of insulin dosage were based on the results of home BGCs. Since, at the end of the study, eight cats were judged to be well regulated and four cats showed marked improvement we assume that the correct decision was made most of the time.
The results of this study indicate that owners of diabetic cats are willing and able to perform long-term monitoring of BG concentrations. At the end of the study, the majority of owners could not only evaluate the results of testing but also dose the insulin correctly. All 12 owners involved in this study elected to keep the PBGM. They communicated with the clinic on a regular basis, and presently, discuss treatment based on home BGCs via telephone or fax at least once a month. Periodic evaluations of the owners' technique are required and the targeted blood glucose concentrations should be explicitly defined to avoid confusion and to provide a better understanding of the management goals.
We think that home-monitoring of blood glucose is a valuable tool in the management of cats with diabetes mellitus. One of its major advantages is that it enables frequent generation of BGCs, which may be of particular importance in cats that are difficult to regulate. In complicated cases, more than one BGC can be performed at home before a decision is made concerning therapy.
Further studies in a larger group of cats are needed to evaluate if metabolic control in cats managed by home-monitoring is better than in those cats in which blood glucose levels are only measured in the hospital.
Acknowledgments
We wish to express our thanks to Bayer Diagnostics (Switzerland) for providing equipment (PBGM, lancing device and test strips) and financial support. We also thank our cat owners for their cooperation.
This work represents part of the dissertation of Martina Casella (MC) at the Clinic for Small Animal Internal Medicine, Vetsuisse-Faculty University of Zurich, Switzerland.
References
- American Diabetes Association Self-monitoring of blood glucose (consensus statement), Diabetes Care 19 (Suppl. 1), 1996, 62–66. [Google Scholar]
- American Diabetes Association Standards of medical care for patients with diabetes mellitus (position statement), Diabetes Care 21 (Suppl. 1), 1998, 69–71.9538972 [Google Scholar]
- Bergman M., Felig P. Self-monitoring of blood glucose levels in diabetes, Archive of Internal Medicine 144, 1984, 2029–2033. [PubMed] [Google Scholar]
- Brunner G.A., Ellmerer M., Sendlhofer G., Wutte A., Trajanoski Z., Schaupp L., Quehenberger F., Wach P., Krejs G.J., Pieber T.R. Validation of home blood glucose meters with respect to clinical and analytical approaches, Diabetes Care 21, 1998, 585–590. [DOI] [PubMed] [Google Scholar]
- Casella M., Reusch C.E. Home monitoring of capillary blood glucose in dogs and cats: technical aspects, Journal of Veterinary Internal Medicine Abstract, 2000, 754.
- Casella M., Wess G., Reusch C.E. Measurement of capillary blood glucose concentrations by pet owners: a new tool in the management of diabetes mellitus, Journal of the American Animal Hospital Association 38, 2002, 239–245. [DOI] [PubMed] [Google Scholar]
- Casella M., Wess G., Hässig M., Reusch C.E. Home monitoring of blood glucose concentration by owners of diabetic dogs, Journal of Small Animal Practice 44, 2003, 298–305. [DOI] [PubMed] [Google Scholar]
- Chan J.C., Wong R.Y., Cheung C.K., Lam P., Chow C.C., Yeung V.T., Kan E.C., Loo K.M., Mong M.Y., Cockram C.S. Accuracy, precision and user-acceptability of self blood glucose monitoring machines, Diabetes Research Clinical Practice 36, 1997, 91–104. [DOI] [PubMed] [Google Scholar]
- Clarke W.L., Cox D., Gonder-Frederick L.A., Carter W., Pohl S.L. Evaluating clinical accuracy of systems for self-monitoring of blood glucose, Diabetes Care 15, 1987, 622–628. [DOI] [PubMed] [Google Scholar]
- Cohen M., Zimmet P.Z. Home blood-glucose monitoring. A new approach to the management of diabetes mellitus, Medical Journal of Australia 2, 1980, 713–716. [PubMed] [Google Scholar]
- Cohn L.A., McCaw D.L., Tate D.J., Johnson J.C. Assessment of five portable blood glucose meters, a point-of-care analyzer, and color test strips for measuring blood glucose concentration in dogs, Journal of American Veterinary Medical Association 216 (2), 2000, 198–202. [DOI] [PubMed] [Google Scholar]
- Feldman E.C., Nelson R.W. Diabetes mellitus. Feldman E.C., Nelson R.W. Canine and Feline Endocrinology and Reproduction, 1996, W.B. Saunders: Philadelphia, 392–421. [Google Scholar]
- Fleeman L.M., Rand J.S. Evaluation of day-to-day variability of serial blood glucose concentration curves in diabetic dogs, Journal of American Veterinary Medical Association 222 (3), 2003, 317–321. [DOI] [PubMed] [Google Scholar]
- Fleming D.R. Accuracy of blood glucose monitoring for patients: what it is and how to achieve it, Diabetes Education 20, 1994, 495–500. [DOI] [PubMed] [Google Scholar]
- Foster S.A., Goode J.R., Small R.E. Home blood glucose monitoring, The Annals of Pharmacotherapy 33, 1999, 355–363. [DOI] [PubMed] [Google Scholar]
- Joseph R.J., Allyson K., Graves T.K., Rondeau M.J., Peterson M.E. Evaluation of two reagent strips and three reflectance meters for rapid determination of blood glucose concentrations, Journal of Veterinary Internal Medicine 1, 1987, 170–174. [DOI] [PubMed] [Google Scholar]
- Kabadi U.M., O'Connel K.M., Johnson J., Kabadi M. The effect of recurrent practice at home on the acceptability of capillary blood glucose readings, Diabetes Care 17 (10), 1994, 1110–1114. [DOI] [PubMed] [Google Scholar]
- Leidinger K.I., Nolte I., Eigenbrodt E. Klinische und Labordiagnostische Untersuchungen zum Phänomen der Hyperglykämie der Katze, Kleintierpraxis 34, 1989, 421–488. [Google Scholar]
- Link K.R., Rand J.S., Hendrikz J.K. Evaluation of a simplified intravenous glucose tolerance test and a reflectance glucose meter for use in cats, Veterinary Record 140, 1997, 253–256. [DOI] [PubMed] [Google Scholar]
- Opitz M. Zur Stresshyperglykämie der Katze, Berliner und Muenchener Tieraerztliche Wochenschrift 103, 1990, 151–158. [PubMed] [Google Scholar]
- Rand J.S., Kinnaird E., Baglioni A., Blackshaw J., Priest J. Acute stress hyperglycemia in cats is associated with struggling and increased concentration of lactate and norepinephrine, Journal of Veterinary Internal Medicine 16, 2002, 121–122. [DOI] [PubMed] [Google Scholar]
- Reusch C.E., Dloughy U., Heusner A.A. Evaluation of statistically significant changes in the level of glycosylated hemoglobin and serum fructosamine, Journal of Veterinary Internal Medicine 9, 1995, 186. [Google Scholar]
- Reusch C.E., Wess G., Casella M. Home monitoring of blood glucose concentration in the management of diabetes mellitus, Compendium 23 (6), 2001, 544–553. [Google Scholar]
- The National Steering Committee for Quality Assurance in Capillary Blood Glucose Monitoring Proposed strategies for reducing user error in capillary blood glucose monitoring, Diabetes Care 16, 1993, 493–498. [DOI] [PubMed] [Google Scholar]
- Thompson M.D., Taylor S.M., Adams V.J., Waldner C.L., Feldman E.C. Comparison of glucose concentrations in blood samples obtained with a marginal ear vein nick technique versus from a peripheral vein in healthy cats and cats with diabetes mellitus, Journal of the American Veterinary Medical Association 221 (3), 2002, 389–392. [DOI] [PubMed] [Google Scholar]
- Trajanoski Z., Brunner G.A., Gfrerer R.J., Wach P., Pieber T.R. Accuracy of home blood glucose meters during hypoglycemia, Diabetes Care 19, 1996, 1412–1415. [DOI] [PubMed] [Google Scholar]
- Wess G., Reusch C.E. Capillary blood sampling from the ear of dogs and cats and use of portable meters to measure glucose concentration, Journal of Small Animal Practice 43, 2000a, 60–66. [DOI] [PubMed] [Google Scholar]
- Wess G., Reusch C.E. Evaluation of five portable blood glucose meters for use in dogs, Journal of American Veterinary Medical Association 216 (2), 2000b, 203–209. [DOI] [PubMed] [Google Scholar]
- Wess G., Reusch C.E. Laboratory assessment of five portable blood glucose meters for use in cats, American Journal of Veterinary Research 61, 2000c, 1587–1592. [DOI] [PubMed] [Google Scholar]