Abstract
The objective of this study was to compare simpler indices of insulin sensitivity with the minimal model-derived insulin sensitivity index to identify a simple and reliable alternative method for assessing insulin sensitivity in cats. In addition, we aimed to determine whether this simpler measure or measures showed consistency of association across differing body weights and glucose tolerance levels. Data from glucose tolerance and insulin sensitivity tests performed in 32 cats with varying body weights (underweight to obese), including seven cats with impaired glucose tolerance, were used to assess the relationship between Bergman's minimal model-derived insulin sensitivity index (SI), and various simpler measures of insulin sensitivity. The most useful overall predictors of insulin sensitivity were basal plasma insulin concentrations and the homeostasis model assessment (HOMA), which is the product of basal glucose and insulin concentrations divided by 22.5. It is concluded that measurement of plasma insulin concentrations in cats with food withheld for 24 h, in conjunction with HOMA, could be used in clinical research projects and by practicing veterinarians to screen for reduced insulin sensitivity in cats. Such cats may be at increased risk of developing impaired glucose tolerance and type 2 diabetes mellitus. Early detection of these cats would enable preventative intervention programs such as weight reduction, increased physical activity and dietary modifications to be instigated.
Impaired tissue sensitivity to the action of insulin, termed insulin resistance, is one of the major metabolic abnormalities in obesity and type 2 diabetes in humans and cats (DeFronzo et al 1978, Kolterman et al 1980, Feldhahn et al 1999a, Appleton et al 2001a). Insulin resistance also appears to be the primary defect that leads to impaired glucose tolerance in humans (Sheen 1992). We have previously shown that non-obese cats with insulin sensitivity values below the population median for healthy cats, have a threefold higher risk of developing impaired glucose tolerance with weight gain (Appleton et al 2001a). High basal insulin concentrations also increase the risk for developing impaired glucose intolerance in cats (Appleton et al 2001a). This suggests that some cats have an underlying predisposition to develop glucose intolerance as a result of intrinsic insulin resistance. If these cats become obese, they may, therefore, be at greater risk of developing overt type 2 diabetes.
Evidence suggests that by the time glucose tolerance becomes impaired, or fasting glucose concentrations increase, significant β cell destruction may have already occurred (Ferrannini 1997). It is important that cats at risk of β cell failure from underlying insulin resistance be detected early. Therefore, the ability to easily assess a cat's insulin sensitivity status is an important clinical tool. Programs aimed at preventing weight gain and type 2 diabetes would be most effective if directed at cats with underlying low insulin sensitivity, before glucose intolerance develops.
Currently, the lack of a simple method of measuring insulin sensitivity in veterinary practice prevents the routine identification of these cats. In humans, insulin resistance is measured using the glucose clamp technique which is considered the ‘gold standard’, or by minimal model analysis of the frequently-sampled intravenous glucose tolerance test (Bergman et al 1979, DeFronzo et al 1979). Both the minimal model and clamp techniques assess peripheral tissue insulin resistance (Ferrannini and Mari 1998). While the minimal model insulin sensitivity test is useful for research purposes in cats, neither technique is practical for use in veterinary practice for client-owned cats. Both require multiple blood sampling and injections of glucose and insulin, and the minimal model technique requires sophisticated mathematical computer modeling software. Identification of a simpler, less labor-intensive and time-consuming method of assessing insulin sensitivity would be advantageous.
Several simplified measures of insulin sensitivity have been proposed that are easily calculable from plasma concentrations of insulin, either alone, or in combination with glucose concentrations, at baseline or after a glucose challenge (Anderson et al 1995). These measures include basal insulin concentration, the basal insulin to glucose concentration ratio, the homeostasis model assessment (HOMA), the quantitative insulin check index (QUICKI), the Bennett index, insulin concentrations at 60- and 120-min after a glucose injection and their corresponding insulin to glucose ratios, area under the insulin concentration curve during a glucose tolerance test, and finally the ratio of area under the insulin concentration curve to area under the glucose concentration curve during a glucose tolerance test.
The purpose of this study was to evaluate a variety of simpler, less time-consuming methods of assessing insulin sensitivity than the minimal model technique. We aimed to identify a practical, safe and reliable test, which would be useful both in general veterinary practice and in clinical research, to assess insulin sensitivity in cats.
Materials and methods
Insulin sensitivity values determined from minimal model analysis of the frequently-sampled intravenous glucose tolerance test were correlated quantitatively with a variety of simpler measures derived from the glucose tolerance test and basal concentrations of insulin and glucose. The strength of association between the alternative measures and the minimal model-derived insulin sensitivity index were tested across a range of body weights and degrees of glucose tolerance. The protocol for this study and the care and handling of cats were approved by the Animal Experimentation Ethics Committee of the University of Queensland.
Animals
Data from 25 cats with a body condition score of 3, were analysed to determine reference ranges for each of the simplified measures in clinically healthy, ideal-weight cats. Accurate ages of the cats were unknown, however, all were estimated by visual assessment and examination of dentition to be between 1 and 5 years of age.
Combined data from 32 adult cats that were involved in two studies (18 castrated males and 14 spayed females) were then analysed to determine correlations between insulin sensitivity measured by the minimal model and the simpler measures. Initial data were collected from 16 cats with varying body conditions including underweight, ideal-weight and overweight. Data from 11 of these cats were included in the data used to establish the reference ranges. Data from another 16 overweight and obese cats, which were part of a second trial, were obtained approximately 10 months later. These cats had been allowed free access to energy dense diets over this period, during which they gained an average of 44% of their body weight. Data from these 16 overweight and obese cats have been previously reported (Appleton et al 2001a). Seven of these cats became glucose intolerant with weight gain. Impaired glucose tolerance was defined as being present when one or more of the values for glucose T1/2, or plasma glucose concentrations at 0, 60, 90 or 120 min during a glucose tolerance test exceeded the upper limit of the population 95% confidence intervals (Appleton et al 2001a).
All cats were healthy except for body condition, based on clinical examination and routine hematological and serum biochemical analyses. Based on body condition score using a 5 point scale, cats were classed as underweight (score=2; 2 cats), ideal-weight (score=3; 12 cats), overweight (score=4; 12 cats) and obese (score=5; 6 cats) (Sunvold and Bouchard 1998a). Body weights of the combined group of 32 cats ranged from 3.3 kg to 8.6 kg (average 5.4±1.37), (Table 1). Ages were assessed as being between 1 and 5 years as previously described (Appleton et al 2001a).
Table 1.
Clinical characteristics and basal glucose concentrations of 32 mixed-weight cats and in seven overweight and obese cats with impaired glucose tolerance
| Ideal-weight cats | Mixed-weight cats | Overweight and obese glucose intolerant cats | |
|---|---|---|---|
| Number of cats (male/female) | 25 (10/15) | 32 (14/18) | 7 (2/5) |
| Weight (kg) | 4.3±0.56 (3.35–5.2) | 5.4±1.37 (3.3–8.6) | 6.45±1.61 (4.4–8.6) |
| Body condition score* | 3 | 3.77±0.93 (2.0–5.0) | 4.64±0.48 (4.0–5.0) |
| Basal glucose concentration (mmol/l) | 3.91±0.75 (2.39–5.23) | 3.93±0.76 (2.21–4.99) | 4.51±0.50 (3.52–4.99) |
Mean±SD (Range).
Body condition was scored on a scale of 1–5 with 5 representing obese.
The testing protocol was identical for all cats. In summary, cats were individually housed and acclimatized to the facilities for a minimum of 2 weeks before testing. Body weight and body condition scores were recorded in all cats. All blood samples were collected via a jugular catheter inserted under general anesthesia, 24 h before testing (Appleton et al 2001b). An insulin-modified, frequently-sampled, intravenous glucose tolerance test (FSIVGTT) with minimal model analysis, and an intravenous glucose tolerance test were performed on each cat on separate days during the same week with a minimum of 18 h between tests. Baseline glucose and insulin concentrations were determined as part of each test.
Minimal model analysis
Insulin sensitivity was determined using Bergman's MINMOD computer program where a mathematical model of glucose disappearance is used to estimate insulin sensitivity (Bergman et al 1979). This method is based on the frequently-sampled intravenous glucose tolerance test (FSIVGTT), modified to include an injection of both glucose and insulin (Finegood et al 1990, Welch et al 1990).
After an overnight fast, an insulin-modified FSIVGTT was performed in each cat as previously described (Appleton et al 2001b). Briefly, four baseline blood samples were collected over 15 min immediately preceding an intravenous bolus injection of glucose (0.3 g/kg) over 30 s. Twenty-seven blood samples were collected at various time points over the next 3 h. Twenty minutes after the glucose injection, regular human insulin (0.05 U/kg) was injected intravenously via the jugular catheter.
The model provides an index of insulin sensitivity (SI), defined as the dependence of fractional glucose disappearance on plasma insulin concentration (Bergman et al 1979). This index is a measure of how a given change in plasma insulin concentration can increase the clearance of plasma glucose (Beard et al 1986). A decreasing index value indicates that the subject is less sensitive to insulin.
Intravenous glucose tolerance test
On a separate day and after withholding food overnight, a glucose tolerance test was performed in each cat as previously as described (Appleton et al 2001b). In summary, basal plasma glucose and insulin concentrations were measured before an intravenous glucose injection (0.5 g/kg). Further blood samples were taken at various times over the 120-min duration of the test. Total areas under the glucose and insulin curves (AUC) were calculated by the trapezoidal for 120-min after glucose injection (Rowland and Tozer 1989).
Simplified insulin sensitivity measurements
Simplified estimates of insulin sensitivity were calculated from insulin and glucose concentrations obtained at baseline, and during the glucose tolerance test (Tables 2 and 3). These simplified measures were then compared with the minimal model-derived insulin sensitivity index (Table 4).
Table 2.
A summary of simplified measures of insulin sensitivity and their relevant references
| Measure | Definition | Reference |
| Basal insulin | I0 (single sample) | Laakso 1993 |
| I0 (mean of two samples) | Laakso 1993 | |
| Fasting SI1 | I0/G0 (single sample) | Caro 1991 |
| I0/G0 (mean of two samples) | ||
| Bennett index | Anderson 1995 | |
| HOMA | Matthews 1985 | |
| QUICKI | Katz 2000 | |
| Insulin after glucose injection | 120-min | Laakso 1993 |
| 60-min | ||
| Insulin/glucose ratio after glucose injection | 120-min | Caro 1991 |
| 60-min | ||
| Area under the insulin curve | AUCins | Anderson 1995, Reaven 1974 |
| Ratio of area under the insulin to glucose curve | AUCins/AUCglu | Caro 1991, Cederholm 1990 |
I0=basal insulin; G0=basal glucose; Fasting SI1=basal insulin to glucose ratio; AUC=area under the curve.
Table 3.
Mean (±SD), reference range and median calculated in ideal-weight cats (n=25), and mean (±SD) and observed ranges calculated in mixed weight cats (n=32), and in overweight and obese cats with impaired glucose tolerance (n=7), for the minimal model-derived insulin sensitivity index and all other simplified measures of insulin sensitivity
| Measure of insulin sensitivity | Abbreviations | Ideal weight cats* | Mixed weight cats | Overweight and obese glucose intolerant cats |
| Insulin sensitivity index † (×10−4min/μU/ml) | S I | 3.38±1.85 (0.92–9.82) 2.54 § | 2.11±1.61 (0.34–8.47) | 1.26±0.55 (0.34–2.23) |
| Basal insulin ‡ (μU/ml) | I0 (one sample) | 7.74±2.79 (0.8–14.7) 7.22 § | 9.39±4.61 (4.11–28.3) | 11.7±2.63 (6.71–15.31) |
| I0 (two samples) | 10.7−4.26 (0.04–21.3) 9.93 | 11.28±5.06 (4.63–28.8) | 12.7±3.57 (7.5–18.9) | |
| Fasting SI1 ‡ | I0/G0 (one sample) | 2.04±0.84 (0–4.14) 1.94 | 2.46±1.18 (1.03–5.89) | 2.61±0.63 (3.51–4.98) |
| I0/G0 (two samples) | 2.49±0.91 (0.24–4.76) 2.29 | 2.65±1.09 (1.29–6.04) | 2.68±0.71 (1.51–3.73) | |
| Bennett index † | 0.41±0.15 (0.19–0.82) | 0.40±0.15 (0.19–1.05) | 0.28± 0.04 (0.23–0.34) | |
| HOMA ‡ | 1.34±0.59 (0–2.84) 1.18 § | 1.67±1.04 (0.66–6.03) | 2.34±0.64 (1.38–3.36) | |
| QUICKI † | 0.31±0.05 (0.22–0.39) 0.30 | 0.67±0.09 (0.47–0.85) | 0.59±0.04 (0.53–0.67) | |
| Insulin after glucose injection ‡ (μU/ml) | 60-min | 20.8±6.85 (3.73–37.9) 20.1 | 23.4±7.02 (8.21–37.0) | 27.4±2.96 (24.4–32.1) |
| 120-min | 8.6±3.63 (2.98–21.4) 6.93 | 13.6±8.88 (4.1–37.0) | 25.7±7.46 (15.4–37) | |
| Insulin/glucose ratio after glucose injection ‡ | 60-min | 2.91±1.16 (1.04–7.04) 2.63 | 2.81±1.22 (0.92–5.81) | 2.36±0.75 (1.79–3.96) |
| 120-min | 2.34±1.04 (0.84–5.62) 2.14 | 3.12±1.8 (1.04–8.25) | 4.42±2 (2.37–8.25) | |
| Area under the insulin curve ‡ (μU/ml/min) | AUCins | 1967±378 (1026–2908) 1958 | 2019±953 (418–4006) | 2983±953 (2313–3363) |
| Ratio of area under the insulin to glucose curve ‡ | AUCins/AUCglu | 2.07±0.54 (0.71–3.42) 2.06 | 1.80±0.95 (0.34–4.53) | 2.12±0.56 (1.52–3.23) |
Reference ranges shown are the 95% confidence interval of data obtained from 25 ideal weight cats.
The lower the value, the lower the insulin sensitivity.
The higher the value, the lower the insulin sensitivity.
It is recommended that preventative programs be instigated for cats with basal insulin concentrations or HOMA values above the mean of the reference range.
Table 4.
Correlation between simpler insulin sensitivity measures and the minimal model-derived insulin sensitivity index in mixed-weight cats (n=32) and in overweight and obese cats with glucose intolerance (n=7)
| Measure of insulin sensitivity | Abbreviations | Mixed weight cats | Overweight and obese glucose intolerant cats |
| Basal insulin | I0 (single sample) | P<0.001, r=−0.59 | P=0.002, r=−0.93 |
| I0 (mean of two samples) | P=0.01, r=−0.45 | P=0.009, r=−0.88 | |
| Fasting SI1 | I0/G0 (single sample) | P=0.002, r=−0.52 | P=0.04, r=−0.76 |
| I0/G0 (mean of two samples) | P=0.02, r=−0.41 | P=0.02, r=−0.85 | |
| Bennett index | N/S | P=0.03, r=−0.79 | |
| HOMA | P=0.007, r=−0.47 | P=0.009, r=−0.88 | |
| QUICKI | P=0.02, r=0.42 | P=0.01, r=0.87 | |
| Insulin after glucose injection | 60-min | P=0.002, r=−0.53 | N/S |
| 120-min | P=0.04, r=−0.37 | N/S | |
| Insulin/glucose ratio after glucose injection | 60-min | N/S | N/S |
| 120-min | P=0.05, r=−0.35 | N/S | |
| Area under the insulin curve | AUCins | P=0.01, r=−0.44 | P=0.01, r=−0.84 |
| Ratio of area under the insulin to glucose curve | AUCins/AUCglu | N/S | N/S |
N/S=not significant.
Basal insulin and glucose concentrations, and insulin to glucose ratios were determined from a single blood sample before the glucose tolerance test (‘single sample’), and also as the mean of two basal samples drawn on different days before the glucose tolerance and the insulin sensitivity tests (‘mean of two samples’) (Caro 1991, Laakso 1993). Insulin and glucose concentrations and insulin to glucose ratios were also calculated 60 and 120 min after glucose injection during the glucose tolerance test (Laakso 1993, Ferrannini and Mari 1998). Additional indices obtained from the glucose tolerance test data included area under the insulin curves (Reaven and Olefsky 1974, Anderson et al 1995) and ratios of area under the insulin to glucose curves (Cederholm and Wibell 1990, Caro 1991).
Various transformations of basal insulin and glucose concentrations were also used including the homeostasis model assessment (HOMA), quantitative insulin sensitivity check index (QUICKI) and the Bennett index (Table 2). The homeostasis model assessment (HOMA) uses the product of basal insulin and glucose concentrations divided by a constant (Matthews et al 1985). The quantitative insulin check index (QUICKI) is derived from calculating the inverse of the sum of logarithmically expressed values of basal insulin and glucose (Katz et al 2000). The Bennett index is also derived similarly from the inverses of the product of log transformed basal insulin and glucose concentrations (Anderson et al 1995). Because the basal insulin concentration has a skewed distribution, transformation of the data is thought to produce a closer relationship to the minimal model-derived insulin sensitivity index (Katz et al 2000).
Sample handling and analysis
Blood samples from each of the tests were handled similarly. Samples were placed into sterile EDTA vacuettes containing the proteinase inhibitor, aprotinin (Trasylol; Bayer), at 0.05 ml/ml of blood. After collection, samples were kept on ice for 15–30 min until centrifugation for 8 min at 1500×g. After separation, each plasma sample was split, placed into a 500 μl vials, and stored at −70°C until assayed for glucose and insulin.
To maintain red blood cell mass, any red cells remaining in EDTA tubes after plasma was removed, were washed and autotransfused as previously described (Appleton et al 2001b).
Glucose concentration was measured in plasma using a YSI glucose analyser (YSI 2300 Stat Plus; Yellow Springs Instrument Company). Insulin concentration was measured using a commercially available kit validated for the detection of feline insulin (Phadeseph Insulin Radioimmunoassay; Pharmacia and Diagnostics AB) (Lutz and Rand 1993).
Statistical analysis
Criteria used for evaluating the simpler measures of insulin sensitivity included strength of association with the minimal model-derived insulin sensitivity index in cats with varying body weights, consistency of association in glucose intolerant cats, simplicity of calculations, and ease of data collection.
Statistical analysis was performed using a statistical software package (Sigmastat version 2.0 for windows; SPSS Inc). Pearson correlation coefficients were used to examine the association between the minimal model-derived insulin sensitivity index and each simplified insulin sensitivity index. Indices of insulin sensitivity and insulin concentrations were tested for normality with the Kolmogorov–Smirnov test. Indices that were not normally distributed were logarithmically transformed to give a normal distribution before statistical analyses. The Spearman rank order correlation coefficient was determined if data remained non-Gaussian after log transformation. Correlations were calculated for the entire group of 32 cats and also separately for the subgroup of seven glucose intolerant obese cats.
A P value of <0.05 was considered significant. All data are expressed as mean±1 standard deviation (SD), followed by the range in parentheses.
Reference ranges were established using 25 ideal-weight cats by calculation of tolerance intervals containing 95% of the population with a probability of 0.9, for normally distributed data (Lumsden and Mullen 1978). Tolerance intervals from data for 25 ideal-weight cats were calculated as mean values±2.49SD (Lumsden and Mullen 1978). Data were analysed for normality by the use of the Kolmogorov–Smirnov test of normality. When not normally distributed, data were logarithmically transformed before calculating tolerance intervals (Lumsden and Mullen 1978). Data were tested for outliers by use of the Grubbs T-statistic, with suspected values rejected when T was greater than its critical value of 3.01 for n=25 cats (P<0.01) (Grubbs 1969).
Results
Mean values for the minimal model-derived insulin sensitivity index were 3.38±1.85×10−4/min/μU/ml in 25 ideal-weight cats, 2.11±1.61×10−4/min/μU/ml for the group of 32 cats with varying body weights and glucose tolerance levels, and 1.26±0.55×10−4/min/μU/ml for the subgroup of seven glucose intolerant obese cats. Tolerance intervals for all simpler measures of insulin sensitivity derived from 25 ideal-weight cats, and mean values for the total group of 32 cats and the subgroup of 7 overweight and obese glucose intolerant cats are summarised in Table 3.
The parameter that most strongly correlated with the minimal model-derived insulin sensitivity index in the group of 32 cats was basal insulin concentration (P<0.001, r=−0.59 Fig 1, Table 4). The means of two basal insulin concentrations and their respective insulin to glucose ratios had a significant inverse relationship with the insulin sensitivity index. However, the relationship did not improve the correlation found with a single value (Table 4). Total area under the insulin curve, 60- and 120-min insulin concentrations after a glucose injection, HOMA, QUICKI, basal insulin to glucose ratios and area under the insulin to glucose curves were also correlated (r values −0.35 to −0.53) with the minimal model-derived insulin sensitivity index ((Fig 2, Table 4). In ideal-weight cats (n =25), no simplified measure of insulin sensitivity was significantly associated with the minimal model-derived insulin sensitivity index (Figs 3 and 4).
Fig 1.
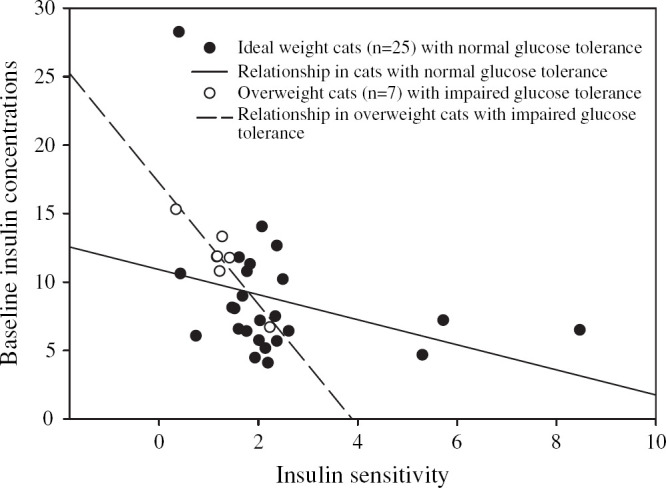
Regression between the minimal model-derived insulin sensitivity index and the corresponding baseline plasma insulin concentrations in 32 mixed-weight cats (r=−0.59, P<0.001), including seven overweight cats with impaired glucose intolerance (r=−0.93, P=0.002). Fewer dots are shown in the figure than were used in the analysis because some values were almost identical for different cats and thus only appear as a single dot instead of two.
Fig 2.
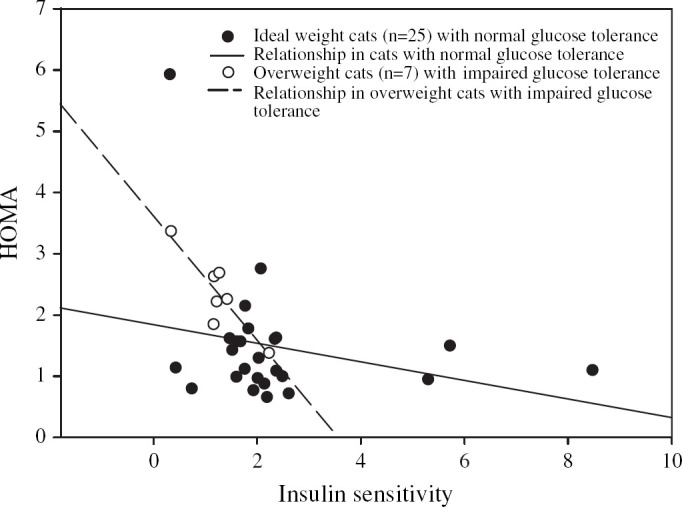
Regression between the minimal model-derived insulin sensitivity index and the corresponding HOMA values in 32 mixed-weight cats (r=−0.47, P=0.007), including seven overweight cats with impaired glucose tolerance (r=−0.88, P=0.009).
Fig 3.
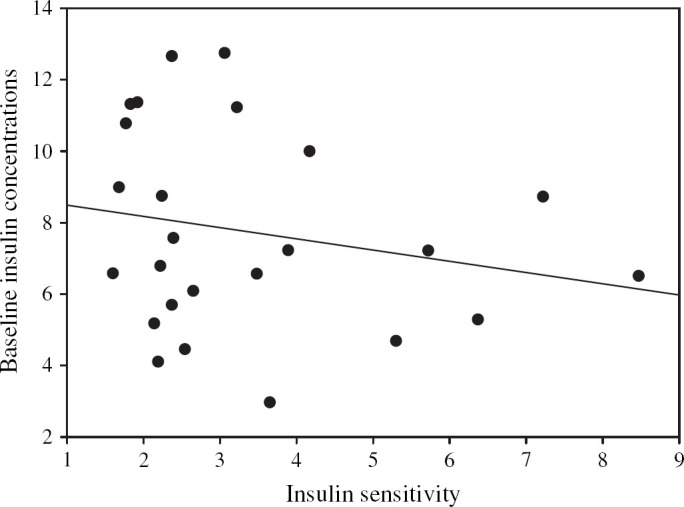
Regression of the minimal model-derived insulin sensitivity and the corresponding baseline plasma insulin concentrations in 25 ideal weight cats. The straight line represents the line of best fit between insulin sensitivity and baseline insulin concentrations (r=−0.23).
Fig 4.
Regression of the minimal model-derived insulin sensitivity and the corresponding HOMA values in 25 ideal weight cats. The straight line represents the line of best fit between insulin sensitivity and HOMA (r=−0.22).
In the subgroup of glucose intolerant overweight and obese cats, basal insulin concentrations had the strongest correlation with the minimal model-derived insulin sensitivity index (P =0.002, r =−0.93), and the association was stronger than in the mixed-weight cats (Fig 1). HOMA, the basal insulin to glucose ratio, and QUICKI were also strongly correlated with the minimal model-derived insulin sensitivity index in glucose intolerant obese cats (Fig 2, Table 4). The area under the insulin to glucose curve and 60 and 120 min insulin concentrations after a glucose injection were not correlated (Table 4).
Discussion
The most important finding in this study was that basal plasma insulin concentration measured after food was withheld for 12 h was the parameter that best fulfilled our criteria for a simple measure of insulin sensitivity in cats with varying body weights and body conditions, and glucose tolerance levels. Basal insulin concentrations were also the easiest and the least expensive of the parameters to obtain, as they did not require measurement of blood glucose concentration, an intravenous glucose injection, or a mathematical calculation, while all other parameters required at least one of these. Measurement of basal plasma insulin concentration is a much simpler and less costly alternative for a veterinarian in practice than measurement of insulin sensitivity using a ‘gold standard’ method such as the minimal model used in this study. The minimal model is a well-accepted alternative method for quantifying insulin sensitivity in humans, and has been shown to correlate well with the glucose clamp over a wide range of insulin sensitivities (Saad et al 1994). The minimal model has also been utilized in normal, obese and diabetic cats (Petrus et al 1998, Feldhahn et al 1999a, Appleton et al 2001a). However, the minimal model estimation of insulin sensitivity requires a 180-min intravenous glucose tolerance test with 31 blood samples and a sophisticated computer program to calculate the insulin sensitivity index.
The reason that the simpler measures were not significantly associated with the minimal model estimate of insulin sensitivity in ideal-weight cats may be the result of several factors. Firstly, the group size was smaller than the mixed-weight cats, secondly, insulin concentrations in general have greater variability and larger standard error of the mean than parameters such as glucose concentration, and thirdly, the coefficient of variation (CV) for insulin sensitivity derived from the minimal model technique is very large in cats. A CV of 35±6% for insulin sensitivity was reported when measured four times over 7 days in healthy cats (Feldhahn et al 1999b).
The strength of the r values in mixed-weight cats suggests that the simpler methods may be more useful for population studies than in individual cats. However, this may be more a consequence of the coefficient of variation of the minimal model-derived insulin sensitivity index, than limitations with the simpler measures. Ideally, this study should be repeated using the more accepted but more difficult to perform ‘gold standard’, the euglycemic clamp.
We calculated the reference ranges for the simplified measures of insulin sensitivity based on tolerance intervals of data from 25 ideal-weight cats. However, the respective upper or lower limit of the reference range corresponding to the lower limit of normal insulin sensitivity, appears to have limited usefulness for identifying healthy cats at risk of developing impaired glucose tolerance as a result of underlying low insulin sensitivity. This is because the values for the lower limit of insulin sensitivity in the reference ranges are similar to the observed range in overweight and glucose intolerant cats known to have reduced insulin sensitivity from intrinsically low insulin sensitivity compounded by substantial weight gain. We have previously shown that cats with insulin concentrations or insulin sensitivity index values above or below the population median, respectively, have two to three times greater risk of developing impaired glucose tolerance with weight gain (Appleton et al 2001a). Therefore, it is recommended that preventative programs should be instituted for cats with basal insulin concentrations or HOMA values above the median of the reference range, rather than only for cats with values outside the reference range.
All of the parameters estimating insulin sensitivity required that food be withheld prior to the test, so the results were not influenced by the increase in glucose or insulin concentrations following eating. We withheld food for 12 h, but newer data show that the post-prandial period in the cat extends up to 24 h if a high carbohydrate meal is consumed (Bouchard and Sunvold 2000). It is, therefore, recommended that food be withheld for 24 h before measuring baseline insulin concentration to reduce the variability associated with meals of different composition.
It is important to realize that prediction of insulin sensitivity based on insulin concentrations alone has certain significant limitations. Firstly, its accuracy is likely to be reduced in the event of stress hyperglycemia, which can be pronounced in cats. Stress hyperglycemia may increase blood glucose and decrease plasma insulin concentrations if struggling occurs before blood sampling (Kinnaird et al 1998).
A second important limitation is that prediction of insulin sensitivity based on insulin concentrations alone may be inaccurate in some cats because β cell failure may develop after prolonged insulin resistance, leading to decreased insulin secretion. It is generally accepted that increased insulin secretion serves as a mechanism to compensate for insulin resistance. Eventually, however, the pancreatic β cells are unable to continue producing increased amounts of insulin. At this stage, insulin secretion begins to progressively decline and glucose intolerance ensues. Therefore, as insulin sensitivity is a measure of the efficiency of insulin action on glucose disposal, it follows that any measure or index of insulin sensitivity should ideally involve information on both insulin and glucose concentrations. Interestingly, even though β cell failure is postulated to be present once glucose intolerance ensues, in our overweight and obese diabetic cats, measurement of baseline plasma insulin concentration still had a high r value (r=−0.93). In these cats, baseline glucose remained in the reference range. It is likely that once β cell failure is such that baseline blood glucose increases, use of baseline insulin concentrations alone to estimate insulin sensitivity would be inaccurate, and a parameter that uses both glucose and insulin concentrations should be substituted. None of the simplified measures of insulin sensitivity would be expected to give valid results in diabetic cats with little or no endogenous insulin secretion. The advantage of the minimal model technique that we used as the ‘gold standard’ is that insulin can be injected at 20 min to allow calculation of insulin sensitivity, even in diabetic animals with no endogenous insulin secretion.
Three simpler methods that used both baseline insulin and glucose concentrations, were strongly correlated with the insulin sensitivity index from the minimal model. These indices were the homeostatic model assessment or HOMA, the quantitative insulin sensitivity check index or QUICKI and the ratio of basal insulin to basal glucose concentrations (Table 4). Of these, HOMA was the most strongly associated measure. With HOMA, an index of insulin sensitivity is determined from the product of the basal insulin and glucose concentrations divided by a constant, thus HOMA becomes larger with decreased insulin sensitivity. HOMA is technically simple and inexpensive in comparison with either the clamp or minimal model approach. It is highly correlated with the minimal model-derived insulin sensitivity index in human patients with varying degrees of glucose tolerance (Fukushima et al 1999, Hermans et al 1999) and with the euglycemic clamp derived values in diabetic patients (Matthews et al 1985, Katsuki et al 2001). In our study, HOMA was correlated with the minimal model estimation of insulin sensitivity (P=0.007, r=−0.47) in cats with varying body weights and insulin sensitivity levels, such as would likely be found in the general population. This correlation was determined from all 32 cats, which included the subset of seven overweight and obese cats with impaired glucose tolerance. The association substantially strengthened (P=0.009, r=−0.88) in the subset of overweight glucose intolerant cats which as a group, had lower insulin sensitivity and higher insulin concentrations than the combined group of 32 mixed-weight cats (Tables 3 and 4).
The strong relationship found between HOMA and the minimal model-derived insulin sensitivity index, particularly in overweight glucose intolerant cats, is consistent with the observation that normal glucose concentration is maintained by adjustment of insulin concentrations to match the degree of insulin sensitivity. In chronically insulin resistant cats, as the β cells eventually begin to fail, insulin secretion falls and glucose concentrations begin to rise. In these cats, HOMA would provide a more accurate assessment of insulin sensitivity than basal insulin concentrations on their own. HOMA may therefore prove more reliable in assessing insulin sensitivity across a broader spectrum of β cell function. It would be expected to be more useful than baseline insulin concentrations in cats with hyperglycemia, but needs further studies in these cats to document a good association with insulin sensitivity as measured by a ‘gold standard’.
QUICKI has previously been shown to correlate with both the minimal model and the glucose clamp in lean, obese and diabetic human patients (Katz et al 2000). Like HOMA, QUICKI can be applied to normoglycemic or hyperglycemic individuals. However, QUICKI was not strongly correlated with the minimal model-derived insulin sensitivity index in mixed-weight cats in our study (P=0.02, r=0.42). Although the association strengthened in the subgroup of overweight and obese glucose intolerant cats (P=0.01, r=0.87), its significance level was lower than that of HOMA. Our findings are similar to those in a previous study in human beings where the correlation between QUICKI and the insulin sensitivity index strengthened as insulin sensitivity was reduced (Katz et al 2000).
In human beings, the ratio of fasting insulin to glucose calculated from a single fasted plasma sample correlated well with the insulin sensitivity index derived from the minimal model technique (Caro 1991). That is, the higher the fasting plasma insulin concentration for a given plasma glucose, the more insulin resistant an individual is. The same result was evident in our cats. Again, this relationship strengthened in overweight and obese glucose intolerant cats, but its significance level was still lower than that of HOMA and QUICKI (Table 4).
Insulin resistance is genetically determined in human beings, but worsened by obesity and physical inactivity (Reaven 1988, Porte 1991). Approximately 20% of non-obese people have similar levels of insulin resistance as obese subjects (Reaven 1988). It has been speculated that these normal-weight insulin resistant individuals may represent a genetically ‘pre-diabetic’ state, and in combination with life-style factors such as diet, physical inactivity and obesity, they are predisposed to developing type 2 diabetes (Caro 1991). A previous study in cats identified a subset of cats that had low underlying insulin sensitivity even before weight gain (Appleton et al 2001a). These cats were predisposed to develop impaired glucose tolerance and their insulin sensitivity decreased further with weight gain. Cats with low underlying sensitivity even when ideal-weight, are the cats that need to be identified early so preventative measures such as maintenance of optimal body weight and modification of other dietary and life-style factors can be enforced. Fortunately, all the simplified measures of insulin sensitivity were most strongly correlated with the minimal model-derived insulin sensitivity index in overweight and obese cats that had reduced glucose tolerance and relatively low insulin sensitivity.
As a practical guide, we propose that insulin and glucose concentrations should be measured in cats after withholding food for 24 h. If blood glucose concentrations are increased, or if insulin concentrations are normal or decreased, HOMA should be calculated as it provides important additional data and the calculation is simple. If basal insulin concentrations or HOMA are increased, preventative intervention programs designed to reduce the demand on pancreatic β cells for insulin secretion should be instigated. These could include dietary modifications such as providing diets with a low glycemic load, for example, those with carbohydrate sourced from a blend of sorghum and corn instead of rice (Sunvold and Bouchard 1998b, Appleton et al 2004), and substituting protein for carbohydrate (Farrow et al 2002, Frank et al 2002). Strict adherence to maintenance of ideal body weight and increasing daily physical activity should be initiated. Physical activity increases insulin sensitivity independently of body weight in human beings (Regensteiner et al 1991, Henriksson 1995), and a recent study in overweight cats found that 10 min of active play daily was as effective as calorie restriction in reducing body weight (Giles et al 2003).
Conclusion
In conclusion, determination of increased basal plasma insulin concentrations after withholding food, gives an indication of reduced insulin sensitivity in individual cats across varying body weights and degrees of glucose tolerance. However, because prolonged insulin resistance leads to pancreatic β cell failure and decreased insulin secretion, estimating insulin sensitivity based on insulin concentrations alone may not give accurate data in all cats, especially hyperglycemic cats. Thus, basal insulin concentrations, if determined in conjunction with HOMA which uses both insulin and glucose concentrations, may be useful as screening tests in clinical practice, or as markers of insulin sensitivity in larger epidemiological or population screening studies. Identification of these simpler indices of insulin sensitivity may enable assessment of insulin sensitivity in a clinical setting to become more routine. If reduced insulin sensitivity can be identified in clinically healthy or overweight cats, it may prove easier to enlist owners of these cats to instigate life-style changes in their cats. Changes that should be implemented to prevent or retard the onset of glucose intolerance and type 2 diabetes include maintenance of ideal body weight, increased physical activity, and dietary modifications to decrease glycemic load such as substituting protein for carbohydrate and using a carbohydrate source with a low glycemic index.
References
- Anderson R., Hammon R.F., Savage P.J., Saad M.F., Laws A., Kades W.W., Sands R. Evan, Cefalu W. Exploration of simple insulin sensitivity measures derived from frequently sampled intravenous glucose tolerance (FSIGT) tests, American Journal of Epidemiology 142, 1995, 724–732. [DOI] [PubMed] [Google Scholar]
- Appleton D.J., Rand J.S., Priest J., Sunvold G.D., Vickers J.R. Dietary carbohydrate source affects glucose concentrations, insulin secretion and food intake in overweight cats, Nutrition Research 24, 2004, 447–467. [Google Scholar]
- Appleton D.J., Rand J.S., Sunvold G.D. Insulin sensitivity decreases with obesity, and lean cats with low insulin sensitivity are at greatest risk of glucose intolerance with weight gain, Journal of Feline Medicine and Surgery 3, 2001a, 211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton D.J., Rand J.S., Sunvold G.D., Priest J. Determination of reference values for glucose tolerance, insulin tolerance and insulin sensitivity tests in clinically normal cats, American Journal of Veterinary Research 62, 2001b, 630–636. [DOI] [PubMed] [Google Scholar]
- Beard J.C., Bergman R.N., Ward W.K., Porte D.J. The insulin sensitivity index in nondiabetic man: correlation between clamp-derived and IVGTT-derived values, Diabetes 35, 1986, 362–369. [DOI] [PubMed] [Google Scholar]
- Bergman R.N., Ider Y.Z., Bowden C.R., Cobelli C. Quantitative estimation of insulin sensitivity, American Journal of Physiology 236, 1979, E667–E677. [DOI] [PubMed] [Google Scholar]
- Bouchard G.F., Sunvold G.D. Effect of dietary carbohydrate source on postprandial plasma glucose and insulin concentration in cats. Reinhart G.A., Carey D.P. Recent Advances in Canine and Feline Nutrition, 2000, Orange Frazer Press: Wilmington, Ohio, 91–101. [Google Scholar]
- Caro J.F. Insulin resistance in obese and nonobese man, Journal of Clinical Endocrinology and Metabolism 73, 1991, 691–695. [DOI] [PubMed] [Google Scholar]
- Cederholm J., Wibell L. Insulin release and peripheral insulin sensitivity at the oral glucose tolerance test, Diabetes Research and Clinical Practice 10, 1990, 167–175. [DOI] [PubMed] [Google Scholar]
- DeFronzo R.A., Soman V., Sherwin R.S., Hendler R., Felig P. Insulin binding to monocytes and insulin action in human obesity starvation and refeeding, Journal of Clinical Investigation 62, 1978, 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- DeFronzo R.A., Tobin J.D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance, American Journal of Physiology 237, 1979, E214–E223. [DOI] [PubMed] [Google Scholar]
- Farrow H.A., Rand J.S., Sunvold G.D. The effect of high protein, high fat or high carbohydrate diets on postprandial glucose and insulin concentrations in normal cats, Proceedings from the American College of Veterinary Internal Medicine Forum (ACVIM), 2002, 794.
- Feldhahn J., Rand J.S., Martin G.M. Insulin sensitivity in normal and diabetic cats, Journal of Feline Medicine and Surgery 1, 1999a, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhahn J.R., Rand J.S., Kinnaird E. The effect of interday variation and a short-term stressor on insulin sensitivity in clinically normal cats, Journal of Feline Medicine and Surgery 1, 1999b, 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E. Insulin resistance is central to the burden of diabetes, Diabetes/Metabolism Reviews 13, 1997, 81–86. [DOI] [PubMed] [Google Scholar]
- Ferrannini E., Mari A. How to measure insulin sensitivity, Journal of Hypertension 16, 1998, 895–906. [DOI] [PubMed] [Google Scholar]
- Finegood D.T., Hramiak I.M., Dupre J. A modified protocol for estimation of insulin sensitivity with the minimal model of glucose kinetics in patients with insulin-dependent diabetes, Journal of Clinical Endocrinology and Metabolism 70, 1990, 1538–1549. [DOI] [PubMed] [Google Scholar]
- Frank G., Anderson W., Pazak H., Hodgkins E., Ballam J., Laflamme D. Use of a high-protein diet in the management of feline diabetes mellitus, Supplement to the Compendium on Continuing Education for the Practicing Veterinarian 24, 2002, 10–16. [Google Scholar]
- Fukushima M., Taniguchi A., Sakai M. Homeostasis model assessment as a clinical index of insulin resistance: comparison with the minimal model analysis, Diabetes Care 22, 1999, 1911–1912. [DOI] [PubMed] [Google Scholar]
- Giles R, Gruffydd-Jones T, Sturgess C. (2003). A preliminary investigation into the effect of different strategies for achieving weight loss in cats (Abstract). 46th Annual Congress of The British Small Animal Veterinary Association.
- Grubbs F.E. Procedures for detecting outlying observations in samples, Technometrics 11, 1969, 1–21. [Google Scholar]
- Henriksson J. Influence of exercise on insulin sensitivity, Journal of Cardiovascular Risk 2, 1995, 303–309. [PubMed] [Google Scholar]
- Hermans M.P., Levy J.C., Morris R.J., Turner R.C. Comparison of insulin sensitivity tests across a range of glucose tolerance from normal to diabetes, Diabetologia 42, 1999, 678–687. [DOI] [PubMed] [Google Scholar]
- Katsuki A., Sumida Y., Gabazza E.C., Sea Murashima. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes, Diabetes Care 24, 2001, 362–367. [DOI] [PubMed] [Google Scholar]
- Katz A., Nambi S.S., Mather K., Baron A.D., Follmann D.A., Sullivan G., Quon M.J. Quantitative insulin sensitivity check index: a simple accurate method for assessing insulin sensitivity in humans, Journal of Clinical Endocrinology and Metabolism 85, 2000, 2402–2410. [DOI] [PubMed] [Google Scholar]
- Kinnaird E.R., Rand J.S., Baglioni A.J., Jr., Blackshaw J. Stress hyperglycaemia in cats, American College of Veterinary Internal Medicine 16th Annual Forum, 1998.
- Kolterman O.G., Insel J., Saekow M., Olefsky J.M. Mechanisms of insulin resistance in human obesity, Journal of Clinical Investigation 65, 1980, 1272–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso M. How good a marker is insulin level for insulin resistance?, American Journal of Epidemiology 137, 1993, 959–965. [DOI] [PubMed] [Google Scholar]
- Lumsden J.H., Mullen K. On establishing reference values, Canadian Journal of Comparative Medicine 42, 1978, 293–301. [PMC free article] [PubMed] [Google Scholar]
- Lutz T.A., Rand J.S. Comparison of five radioimmunoassay kits for the measurement of feline insulin, Research in Veterinary Science 55, 1993, 64–69. [DOI] [PubMed] [Google Scholar]
- Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Traecher D.F., Turnre R.C. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man, Diabetologia 28, 1985, 412–419. [DOI] [PubMed] [Google Scholar]
- Petrus D.J., Jackson M.W., Kemnitz J.W., Finegood D.T., Panciera D. Assessing insulin sensitivity in the cat: evaluation of the hyperinsulinaemic euglycaemic clamp and the minimal model analysis, Research in Veterinary Science 65, 1998, 179–181. [DOI] [PubMed] [Google Scholar]
- Porte D.J. Beta-cells in type 2 diabetes mellitus, Diabetes 40, 1991, 166–180. [DOI] [PubMed] [Google Scholar]
- Reaven G.M. Banting Lecture 1988. Role of insulin resistance in human disease, Diabetes 37, 1988, 1595–1606. [DOI] [PubMed] [Google Scholar]
- Reaven G.M., Olefsky J.M. Relationship between insulin response during the intravenous glucose tolerance test, rate of fractional glucose removal and the degree of insulin resistance in normal adults, Diabetes 23, 1974, 454–459. [DOI] [PubMed] [Google Scholar]
- Regensteiner J.G., Mayer E.J., Shetterly S.M., Eckel R.H., Haskell W.L., Marshall J.A., Baxter J., Hamman R.F. Relationship between habitual physical activity and insulin levels among nondiabetic men and women. San Luis Valley Diabetes Study, Diabetes Care 1114, 1991, 1066–1074. [DOI] [PubMed] [Google Scholar]
- Rowland M., Tozer T.N. Assessment of area, Clinical Pharmacokinetics: Concepts and Applications, 2nd edn, 1989, Lea and Febiger: Philadelphia, pp. 459–471 [Google Scholar]
- Saad M., Anderson R.L., Laws A., Watanabe R., Kades W., Chen I., Sands R.D., Pei D., Savage P.J., Bergman R.A. A comparison between the minimal model and the glucose clamp in the assessment of insulin sensitivity across the spectrum of glucose tolerance, Diabetes 43, 1994, 1114–1121. [DOI] [PubMed] [Google Scholar]
- Sheen A.J. From obesity to type 2 diabetes, Acta Clinica Belgica 47 (Suppl 14), 1992, 30–36. [DOI] [PubMed] [Google Scholar]
- Sunvold G.D., Bouchard G.F. Assessment of obesity and associated metabolic disorders. Reinhart G.A., Carey D.P. 1st edn, Recent Advances in Canine and Feline Nutrition Vol 2, 1998a, Orange Frazer Press: Wilmington, OH, 135–148. [Google Scholar]
- Sunvold G.D., Bouchard G.F. The glycaemic response to dietary starch. Reinhart G.A., Carey D.P. 1st edn, Recent Advances in Canine and Feline Nutrition Vol 2, 1998b, Orange Frazer Press: Wilmington, OH, 123–131. [Google Scholar]
- Welch S., Gebhart S.S.P., Bergman R.N., Phillips L.S. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects, Journal of Clinical Endocrinology and Metabolism 71, 1990, 1508–1518. [DOI] [PubMed] [Google Scholar]



