Abstract
To investigate heart rate and its variability, a telemetry device was affixed to 16 healthy, young cats. Prior to inclusion in the study, cats were subject to echocardiographic examination. The heart rate (HR) when cats were restrained for echocardiography (HRr) was calculated from 4–5 consecutive RR intervals obtained from a simultaneously recorded electrocardiogram. Electrocardiographic data were then acquired by telemetry in a quiet room in the veterinary hospital (VTH) and later, in the owner's home (home). The ambulatory data were digitally sampled and the RR interval tachogram from a 4 min epoch subject to Fast Fourier Transform to yield measures of heart rate variability (HRV). Sinus arrhythmia was often observed in resting cats. Heart rates (bpm) expressed as mean (±SD) were: HRr: 187 (±25), HRVTH: 150 (±23), HRhome: 132 (±19); each of these rates was significantly different from the others. Significant differences in profiles of HRV suggested that sympathetic tone was higher (and parasympathetic tone lower) when cats were in the hospital.
Heart rate is one of the vital signs and therefore a cornerstone of the clinical examination. It has prognostic importance (Atkins et al 1992) and is sometimes used in the evaluation of response to therapy. However, when veterinary patients are examined, an environmental effect may confound interpretation of clinical cardiovascular variables. This may be particularly relevant to clinical and experimental investigations of cats; indeed, an environmental effect on blood pressure and heart rate has been demonstrated in this species (Belew et al 1999). Due to this environmental effect, heart rate obtained in the veterinary clinic may not adequately represent the resting state.
In healthy individuals, heart rate varies according to physiologic influences and of these influences, the autonomic nervous system is most important (Task Force of ESC and NASPE 1996, Pumprla et al 2002). Quantification of heart rate variability (HRV) has been used to characterize sympathovagal balance and in so doing, assess autonomic function (Bigger et al 1992, Nolan et al 1998, Fauchier et al 1999, Calvert and Wall 2001, Spier and Meurs 2004). Measures of HRV in the frequency domain are obtained by spectral analysis of heart rate data. Spectral analysis is an approach that serves to distinguish and quantify the component frequencies that comprise complex waveforms. In the case of HRV studies, the complex waveform is a graphic description of the temporal variation in heart rate or heart period. An algorithm called Fast Fourier Transform is the method of spectral analysis most commonly used in the evaluation of HRV (Task Force of ESC and NASPE 1996); resultant measures of spectral power provide insights regarding the relative contributions of sympathetic and vagal activity. Briefly, the high frequency component of heart rate variability is the result of parasympathetic influence (Akselrod et al 1981). The low frequency component results from interaction of the sympathetic and parasympathetic nervous systems and is influenced by the baroreceptor system (Task Force of ESC and NASPE 1996, Pumprla et al 2002). The significance of the very low and ultra low frequency components is less well defined; the renin–angiotensin–aldosterone system likely contributes to this component of variability (Task Force of ESC and NASPE 1996, Pumprla et al 2002). Measures of HRV in the frequency domain do not directly quantify autonomic discharge but are believed to be indices of sympathovagal balance. Altered sympathovagal balance characterized by decreased HRV has been recognized in people and dogs with various disorders (Bigger et al 1992, Fauchier et al 1999, Calvert and Wall 2001). The finding of reduced heart rate variability is prognostically important in people who have suffered myocardial infarction and in those with heart failure (Bigger et al 1992, Nolan et al 1998).
Little information is available regarding heart rate and its variability in pet cats (Hamlin 1989, Ware 1999). Accordingly, an electrocardiographic telemetry device was affixed to 16 healthy cats with the objective of quantifying heart rate and measures of HRV in both the veterinary hospital and home environment. Heart rates were obtained before telemetry when cats were restrained, then by telemetry when cats were unrestrained but confined in the veterinary hospital and later, when they were free to roam the pet-owner's home. Measures of HRV were obtained from ambulatory electrocardiographic recordings made in the veterinary hospital and in the pet-owner's home. It was hypothesized that heart rates obtained under disparate conditions would differ and that dissimilar environments would be associated with differing profiles of HRV.
Materials and methods
Sixteen apparently healthy young adult cats were recruited from pet-owning veterinary students and house officers in the veterinary teaching hospital (VTH). All cats were between 1 and 6 years of age. Ten of the cats were male; none of the cats were sexually intact. They weighed between 3.4 and 8.2 kg; the average body weight was 4.8 kg. The cats were believed to be healthy based on a history obtained from the pet-owner, physical findings and echocardiographic examination.
Each cat was manually restrained in right and then left lateral recumbency for echocardiographic examination. The echocardiographic study and a simultaneous electrocardiogram were recorded on VHS videotape for later determination of heart rate. After echocardiographic examination, adhesive electrodes were affixed to areas of the trunk that optimized the electrocardiographic signal. The transmitter of a radiotelemetry device (Escort 2000, Medical Data Electronics, Arleta, California, 91331) was bandaged to the cat that was then confined within a cage located in a quiet ward of the teaching hospital. Traffic through the ward was restricted during the time of electrocardiographic recording. After 3–6 h, the cat was discharged to the care of the owner and the telemetry device was set to record in the pet-owner's home. At home, the cat was allowed to move unrestricted within the house. However, access to areas of the house that interrupted telemetry was prevented.
Heart rate
Using the electronic calipers of the echocardiograph, heart rate during manual restraint for echocardiographic examination (HRr) was calculated from the average of 4–5 consecutive RR intervals of a simultaneously recorded electrocardiogram. The electrocardiogram that provided HRr was recorded during acquisition of M-mode images obtained by two-dimensional echocardiographic guidance. These images typically were obtained during the first 10 min of the echocardiographic examination and always when the subject was in right lateral recumbency. Heart rates during ambulatory recording in the VTH (HRVTH) and home environment (HRhome) were obtained from digitized electrocardiographic data as described below.
Ambulatory electrocardiographic recordings
Electrocardiographic recordings obtained by telemetry were digitized at a rate of 1 kHz using a commercially available device (MP100, Biopac Systems, Santa Barbara, CA, 93117) and dedicated physiologic software (AcqKnowledge, Biopac Systems, Santa Barbara, CA, 93117). The resulting data were stored using a portable laptop computer. Electrocardiographic data (3–6 h) were recorded first in the veterinary teaching hospital and then, at the pet-owner's home. The subjects were unobserved much of the time during ambulatory recording and there was no attempt to record respiratory rates or the subject's activity during the recording.
Heart rate variability
The digitized electrocardiographic data were manually scanned and a single 4-min epoch was chosen from each of the hospital and home recordings. The 4-min epoch was chosen arbitrarily based on apparent stationarity, low heart rate and RR interval variability. Heart rate, or more specifically heart period, variability in the frequency domain was analyzed for each 4-min epoch. Specifically, the data were subject first to filtering using a finite impulse response band-pass filter that eliminated frequencies below 15 Hz and above 65 Hz. Then, using the rate detection feature of the software, the RR interval and instantaneous heart rate time-series were constructed. From these, the mean heart rates (HR) recorded in the home or hospital environments (HRhome, HRVTH) were obtained.
Using the RR interval time-series, an RR interval tachogram was constructed. The data were demeaned, detrended and padded with zeros. The tachogram was subject to Fast Fourier Transform using a Hanning window. The units of the resulting spectra are arbitrary; in accordance with the published literature, the ordinate of the display was expressed as ms2/Hz. The integral, or power spectral density, in ms2 was calculated for the high (0.15–0.7 Hz) and low frequency (0.04–0.15 Hz) bands and in this report, respectively, abbreviated as HFhome, HFVTH, LFhome, and LFVTH. The ratio of low to high frequency spectral power (LF/HFhome, LF/HFVTH) was calculated and additionally, high and low frequency power were normalized (nHFhome, nHFVTH, nLFhome, and nLFVTH) to total power minus the power in the very low frequency (<0.04 Hz) band and then multiplied by 100%. The measures of HRV are generally reported according to published recommendations for heart rate variability analysis in people (Task Force of ESC and NASPE 1996). This investigation was approved by the Animal Care Committee of the University of Saskatchewan.
Statistical analysis
A repeated measures analysis of variance with one within-subject effect was used to test for differences between environments. When a significant effect of environment on HR was detected, the Tukey HSD test was used for post hoc evaluation of differences between environments. Calculations were performed using the MIXED procedure of the SAS System. Standardized residual plots were used to assess model adequacy. LF/HF, HF and LF were log-transformed to stabilize variances. Unless otherwise noted, results are presented as mean (±SD) for variables for which raw data were analyzed and geometric mean (lower, upper limits of range of raw data) for variables for which data were log-transformed before analysis. P<0.05 was considered significant.
Results
Electrocardiographic data from 16 cats were acquired and analyzed. The quantity of data discarded due to motion-related artifact was not quantified. Sinus arrhythmia was commonly identified during manual scanning of ambulatory electrocardiographic recordings. This study was not designed to quantify the frequency of ectopic complexes. However, ventricular ectopy was identified in recordings from six of the 16 cats. In five of these cats, ventricular ectopy consisted of three or fewer ventricular complexes. A couplet was recorded from one of the five cats. In one cat, 3–5 complexes of an ectopic ventricular rhythm were recorded many times in the VTH but not in the home. The rate of this ventricular rhythm was about 100 bpm and it was believed to represent an accelerated idioventricular rhythm. Heart rates and HRV data from all subjects were obtained only from periods of uninterrupted sinus rhythm.
Heart rate data obtained in the three different environments are displayed graphically in Fig 1. The circumstances of recording had a significant effect on heart rate (P<0.001): HRr [187 (±25) bpm] was significantly greater than HRVTH [150 (±23) bpm], (P<0.0001), HRVTH was significantly greater than HRhome [132 (±19) bpm], P<0.0019) and HRr was significantly greater than HRhome (P<0.0001). The mean difference [upper, lower limits of 95% confidence interval] between HRr and HRVTH was 37 [27, 47] bpm, the mean difference between HRVTH and HRhome was 19 [9, 28] bpm and the mean difference between HRr and HRhome was 56 [46, 66] bpm.
Fig 1.
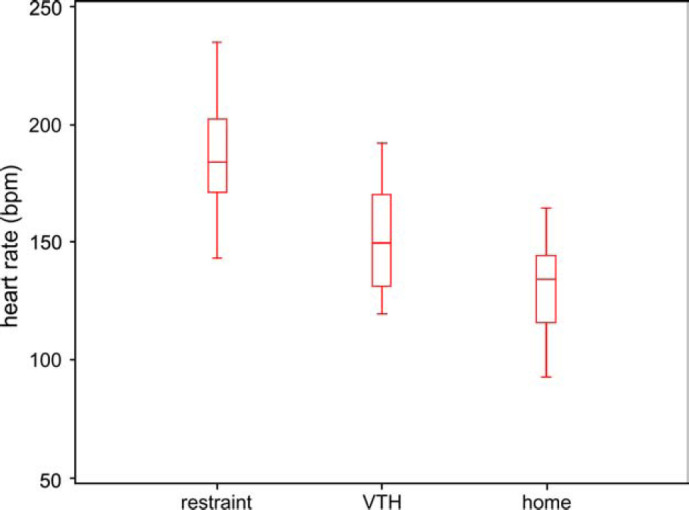
Box and whiskers plots of heart rate data from 16 healthy cats recorded during manual restraint and during ambulatory electrocardiography. Heart rate was obtained first during manual restraint (Restraint) for echocardiography and then from ambulatory electrocardiographic recordings obtained by telemetry in the veterinary teaching hospital (VTH) and then in the subjects's home environment (Home). Heart rate is expressed in beats per minute (bpm). The box represent the interquartile range (IQR) and the line within, the median. The whiskers reflect the most extreme values that are less than 1.5 IQR beyond the upper or lower quartiles respectively.
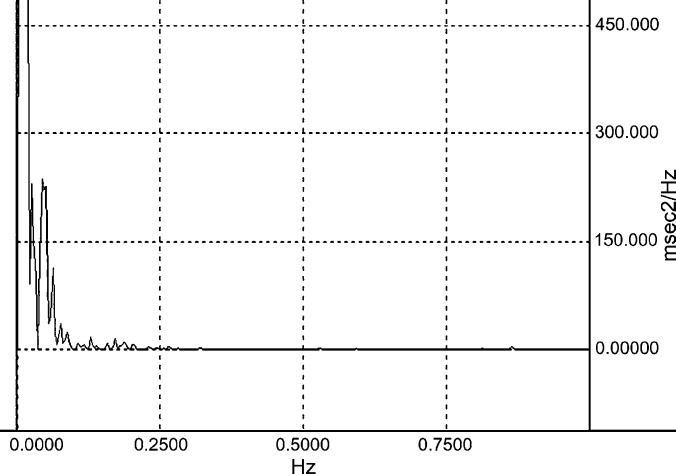
Visual inspection of the heart period spectra often revealed a prominent high frequency peak in home recordings. This high frequency peak was generally attenuated in spectra derived from recordings obtained in the VTH (Figs 2 and 3. High frequency power and normalized high frequency power were significantly greater in the home environment than in the VTH. Normalized low frequency power and the ratio of low frequency to high frequency power were significantly greater in the VTH than in the home environment (Table 1).
Fig 2.
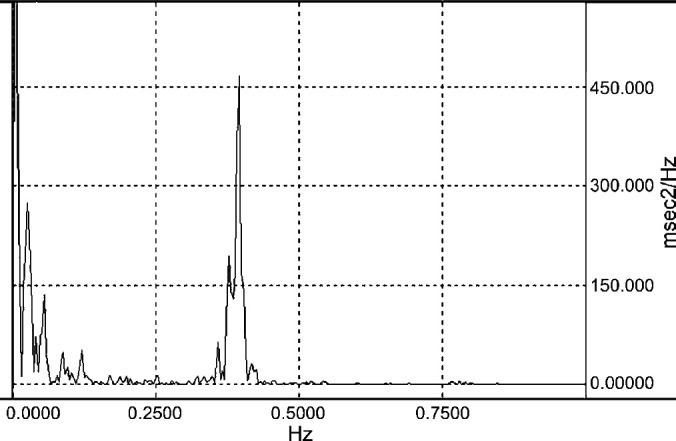
Heart period spectrogram of a healthy 4 years old female-spayed cat derived from ambulatory electrocardiographic data obtained by telemetry while the subject was in the home environment. The spectrogram provides a graphic representation of the frequency content of the RR interval time series. The units of the ordinate are arbitary; here they are expressed as milliseconds2/Herz (msec2/Hz). The abscissa is in Hz. Total RR interval variability is partitioned by frequency so that the amplitude in a given frequency band provides a measures of the relative contribution to the total variability. There is a prominent oscillation of the heart period about a frequency of 0.4 Hz; this is responsible for the peak in the high frequency (0.15–0.7 Hz) band. Though respiratory rate was not measured, the central frequency of this oscillation likely corresponds to a respiratory rate of 24 breaths per minute. Heart rate variability in this high frequency band results from vagal influence and in this case reflects a prominent sinus arrhythmia.
Fig 3.
Heart period spectrogram of the healthy 4 year old female-spayed cat of Figure 2; it was derived from ambulatory electrocardiographic data obtained by telemetry while the subject was confined in a cage in the veterinary teaching hospital. The spectrogram provides a graphic representation of the frequency content of the RR interval time series. The units of the ordinate are arbitrary; here they are expressed as milliseconds2/Herz (msec2/Hz). The abscissa is in Hz. Total RR interval variability is partitioned by frequency so that the amplitude in a given frequency band provides a measure of the relative contribution to the total variability. The prominent oscillation of heart period about 0.4 Hz which was evident in the home recording is absent.
Table 1.
Electrocardiographic data were obtained during manual restraint (Restraint) for echocardiography and then during telemetry in the veterinary teaching hospital (VTH) and in the home (Home) environment
| Variable | Restraint | VTH | Home | P value |
|---|---|---|---|---|
| HR (bpm) | 187 (±25) | 150 (±23) | 132 (±19) | <0.0001 |
| HiF (msec2) | n/r | 1.16 [0.19, 10.2] | 2.19 [0.18, 31.72] | 0.0483 |
| LoF (msec2) | n/r | 1.92 [0.36, 9.89] | 1.37 [0.31, 8.6] | 0.2823 |
| LoF/HiF (msec2) | n/r | 1.65 [0.40, 6.12] | 0.63 [0.16, 4.9] | 0.0007 |
| nHiF | n/r | 39.2 (±0.19) | 60.1 (±0.19) | 0.0007 |
| nLoF | n/r | 60.8 (±0.19) | 39.8 (±0.19) | 0.0007 |
Measures of heart rate variability were not recorded (n/r) during manual restraint. Variables for which raw data were analyzed are expressed as mean (±SD); data that were log transformed for analysis are expressed as geometric mean [lower, upper limits of range of raw data]. Statistical differences determined by repeated measures ANOVA and in the case of heart rate, (HR) post-hoc testing using Tukey HSD. HiF=spectral power in the high frequency band, LoF=spectral power in the low frequency band, nHiF=spectral power in the high frequency band expressed in normalized units, nLoF=spectral power in the low frequency band expressed in normalized units.
Discussion
In this study, heart rates obtained during restraint for echocardiographic examination were compared to heart rates obtained by ambulatory electrocardiography recorded first in the VTH and then in the home. Additionally, measures of heart rate variability obtained in the VTH and in the home were compared. The important findings were as follows: (1) heart rate obtained during restraint was greater than heart rate obtained by ambulatory electrocardiography and heart rate recorded by telemetry in the VTH was greater than heart rate recorded in the pet-owner's home and (2) the HRV profile recorded in the VTH differed from that recorded in the home environment.
Heart rates obtained during restraint by electrocardiography were similar to rates recorded by others (Rogers and Bishop 1971, Robertson et al 1976, Gompf and Tilley 1979, Hamlin 1989). In general, mean heart rates of healthy awake cats have been reported to range between 159 and 206 bpm; the lowest of these mean rates was recorded from cats that were awake but trained (Rogers and Bishop 1971). The mean heart rate obtained from home recordings was 132 bpm. This figure is somewhat lower than a mean heart rate of 157 bpm obtained from 24-h ambulatory electrocardiographic recordings of healthy cats (Ware 1999). This discrepancy is likely explained by differences in methodology. The mean heart rate reported by that investigator was obtained from 24 h of electrocardiographic data and reflects all recorded fluctuations in heart rate. In contrast, the mean heart rate reported here is the average rate during an arbitrarily selected 4-min epoch and the difference is therefore unsurprising. Of more direct relevance, Hamlin (1989) reported a mean heart rate of 118 bpm when healthy cats were examined at home. In that study, heart rates were obtained by precordial palpation for an interval of 15 s.
In healthy individuals, the cardiac rhythm is not regular. Rather, heart rate oscillates about distinct frequencies. It is accepted that vagal discharge is primarily responsible for HRV in the high frequency band (Task Force of ESC and NASPE 1996, Pumprla et al 2002). In studies of human heart rate variability, the high frequency band extends from 0.15 to 0.4 Hz; the central frequency of this band corresponds to a rate of 15 cycles per min which is close to the respiratory rate of normal individuals. Indeed, HRV in the high frequency band corresponds to the respiratory sinus arrhythmia which is often evident in healthy individuals. For the purpose of this study, the range of the high frequency band was extended from 0.15 Hz to 0.7 Hz because it was anticipated that healthy cats might have higher respiratory rates than people. The central frequency of this modified high frequency band corresponds to a rate of about 25 cycles per min which is within the range of respiratory rates observed in healthy cats. This non-standard frequency range was used for all studies and so any effect on the variables obtained was consistent across subjects and environments. The origin of oscillations in heart rate that occur at frequencies between 0.04 and 0.15 Hz has been debated. Sympathetic activity is important although vagal influence and the baroreceptor system also contribute (Task Force of ESC and NASPE 1996, Pumprla et al 2002).
Spectral power in the high frequency band and, normalized high frequency power was significantly greater in home recordings than in recordings obtained in the VTH. Normalized low frequency power and the ratio of spectral power in the low and high frequency bands were greater in recordings obtained in the VTH. The analysis of HRV does not directly quantify autonomic discharge. However, measures of HRV in the frequency domain are believed to reflect sympathovagal balance. The findings of this study suggest that the relative contribution of sympathetic discharge was greater and the contribution of vagal activity was less in the VTH than in the home environment. This supposition is supported by the lower rate and more prominent sinus arrhythmia in home recordings.
The findings reported herein provide evidence of the confounding effect that environment may have on cardiovascular variables in the cat. The mean difference between heart rate obtained during restraint and heart rate obtained in the home environment was 56 bpm; although this finding relates only to healthy cats, it may still be useful in the management of cats with disease. Further, the reported data attest to the feasibility of obtaining measures of HRV in cats. In people, HRV has been used as means to evaluate sympathovagal balance. Decreased HRV has prognostic importance in patients who have suffered myocardial infarction (Kleiger et al 1987, Bigger et al 1992) and in patients with heart failure, decreased HRV is an independent risk factor for death due to progressive heart failure (Nolan et al 1998). In people, reduced HRV has also been shown to predict sudden cardiac death (Fauchier et al 1999, Bilchick et al 2002, La Rovere et al 2003).
Published veterinary investigations have addressed heart rate variability in dogs (Little and Julu 1995, Haggstrom et al 1996, Minors and O'Grady 1997, Calvert and Wall 2001, Fujii and Wakao 2003, Spier and Meurs 2004), horses (Thayer et al 1997) and cattle (Pomfrett and Austin 1997). Heart rate variability in cats has been evaluated in experimental preparations (Lombardi et al 1990, Montano et al 1992, 2000, Massimini et al 2000,) but to the author's knowledge, this is the first report that relates to heart rate variability in ambulatory pet cats. Myocardial disease, specifically hypertrophic cardiomyopathy, is the most common acquired heart disease in cats. Severely affected cats most often develop congestive heart failure but some die suddenly, presumably because of lethal arrhythmia (Atkins et al 1992, Rush et al 2002). Evaluation of measures of HRV in cats with hypertrophic cardiomyopathy might provide useful information. Although findings have been somewhat inconsistent, there is some evidence that depressed HRV provides prognostic information in people with this disorder (Fei et al 1995, Doven et al 2001, Kawasaki et al 2003).
Sinus arrhythmia was encountered commonly in recordings obtained by ambulatory electrocardiography. It has been suggested that the finding of sinus arrhythmia in feline patients is associated with heart disease (Edwards 1987) and respiratory tract disease (Miller et al 1999). There is one report of sinus arrhythmia in a cat that was associated with a central nervous system disease (Rishniw and Bruskiewicz 1996). While it is possible that sinus arrhythmia in hospitalized cats is abnormal, the current findings and those of Ware suggest that sinus arrhythmia is unusual in the hospital setting because vagal tone is diminished in unfamiliar environments. Ventricular ectopic complexes were recorded from six of the 16 enrolled cats. This study was not designed to quantify ventricular ectopy and the total duration of recordings was not standardized. However, five of the cats had fewer than three ventricular ectopic complexes which suggests that ventricular ectopy in echocardiographically normal cats is infrequent. Interestingly, one of the cats developed periods of accelerated idioventricular rhythm during confinement in the VTH but not while in the home environment. These findings are similar to those reported by others (Fox et al 1998, Ware 1999) who have detected infrequent ventricular ectopy by ambulatory electrocardiographic monitoring in healthy cats.
This study has some limitations. It would have been better to randomize the order in which the different circumstances of recording were imposed so that the effect of environment could be distinguished from an effect of time. Possibly, the cats became more accustomed to wearing the device and this could explain differences in heart rate and HRV. Further, the method used did not take into account the possibility of a circadian effect on measures of HRV; an effect which has recently been reported in studies of HRV in healthy dogs (Matsunaga et al 2001). The methods used were primarily chosen based on practicality. The cats were provided by students and house officers who were working in the VTH. It was most convenient for the owners of the cats to present them for preliminary echocardiographic study during the day and then, return the cat to the home environment after completion of the working day. Chemical validation of the results of HRV analysis was not part of this study. The administration of atropine and perhaps a β-blocker might have helped to confirm the autonomic basis of the spectral characteristics that were recorded. However, the patterns were similar to those described in people and in dogs. Due to budgetary limitations, the cats were not subject to extensive evaluation to confirm that they were normal. Echocardiographic examination was used to exclude cardiovascular disease. Because it is known that diabetes mellitus (Pagani 2000) and hyperthyroidism (Burggraaf et al 2001) alter indices of HRV in affected people, only outwardly healthy cats that were younger than 6 years of age were studied. Both of these endocrinopathies are unusual in young cats (Panciera et al 1990, Scarlett 1994) and in the absence of clinical signs it seems unlikely that the results were confounded by inclusion of subjects with subclinical endocrinopathy. However, it does mean that the results of this study apply only to young, outwardly healthy cats.
In this investigation, HR of restrained cats exceeded HR obtained from ambulatory subjects. Further, HR recorded in the hospital was greater than HR obtained from subjects that were in their home environment. Significant differences in measures of HRV obtained in the VTH and the home environment were detected. Together these findings suggest differences in sympathovagal balance such that sympathetic tone is higher, and vagal tone lower, when cats are in a hospital environment. Heart rate and rhythm recorded in unfamiliar circumstances may inadequately represent the true resting state and this is relevant to both clinical practice and clinical investigations.
Acknowledgements
The author gratefully acknowledges the students and house officers of the WCVM who provided cats for use in this study, as well as Dr Daniel L Ward who provided invaluable assistance in the form of statistical analysis and consultation.
This study was performed at the Western College of Veterinary Medicine (WCVM), University of Saskatchewan, Saskatoon, SK, Canada when the author was affiliated with the Department of Veterinary Internal Medicine, WCVM.
The work was supported by a grant from the President's Natural Sciences and Engineering Research Council Fund and a WCVM Companion Animal Health Fund Grant.
References
- Akselrod S., Gordon D., Ubel F.A., Shannon D.C., Berger A.C., Cohen R.J. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control, Science 213, 1981, 220–222. [DOI] [PubMed] [Google Scholar]
- Atkins C.E., Gallo A.M., Kurzman I.D., Cowen P. Risk factors, clinical signs, and survival in cats with a clinical diagnosis of idiopathic hypertrophic cardiomyopathy: 74 cases (1985–1989), Journal of the American Veterinary Medical Association 201, 1992, 613–618. [PubMed] [Google Scholar]
- Belew A.M., Barlett T., Brown S.A. Evaluation of the white-coat effect in cats, Journal of Veterinary Internal Medicine 13, 1999, 134–142. [DOI] [PubMed] [Google Scholar]
- Bigger J.T., Jr., Fleiss J.L., Steinman R.C., Rolnitzky L.M., Kleiger R.E., Rottman J.N. Frequency domain measures of heart period variability and mortality after myocardial infarction, Circulation 85, 1992, 164–171. [DOI] [PubMed] [Google Scholar]
- Bilchick K.C., Fetics B., Djoukeng R., Fisher S. Gross, Fletcher R.D., Singh S.N., Nevo E., Berger R.D. Prognostic value of heart rate variability in chronic congestive heart failure (veterans affairs' survival trial of antiarrhythmic therapy in congestive heart failure)*1, American Journal of Cardiology 90, 2002, 24–28. [DOI] [PubMed] [Google Scholar]
- Burggraaf J., Tulen J.H., Lalezari S., Schoemaker R.C., De Meyer P.H., Meinders A.E., Cohen A.F., Pijl H. Sympathovagal imbalance in hyperthyroidism, American Journal of Physiological and Endocrinological Metabolism 281, 2001, E190–E195. [DOI] [PubMed] [Google Scholar]
- Calvert C.A., Wall M. Effect of severity of myocardial failure on heart rate variability in Doberman pinschers with and without echocardiographic evidence of dilated cardiomyopathy, Journal of the American Veterinary Medical Association 219, 2001, 1084–1088. [DOI] [PubMed] [Google Scholar]
- Doven O., Sayin T., Guldal M., Karaoguz R., Oral D. Heart rate variability in hypertrophic obstructive cardiomyopathy: association with functional classification and left ventricular outflow gradients, International Journal of Cardiology 77, 2001, 281–286. [DOI] [PubMed] [Google Scholar]
- Edwards N.J. The arrhythmias, Bolton's Handbook of Canine and Feline Electrocardiography, 1987, WB Saunders Company: Philadelphia, pp. 60–151 [Google Scholar]
- Task Force of ESC and NASPE Heart rate variability: standards of measurement, physiological interpretation, and clinical use, Circulation 93, 1996, 1043–1065. [PubMed] [Google Scholar]
- Fauchier L., Babuty D., Cosnay P., Fauchier J.P. Prognostic value of heart rate variability for sudden death and major arrhythmic events in patients with idiopathic dilated cardiomyopathy, Journal of the American College of Cardiology 33, 1999, 1203–1207. [DOI] [PubMed] [Google Scholar]
- Fei L., Slade A.K., Prasad K., Malik M., McKenna W.J., Camm A.J. Is there increased sympathetic activity in patients with hypertrophic cardiomyopathy?, Journal of the American College of Cardiology 26, 1995, 472–480. [DOI] [PubMed] [Google Scholar]
- Fujii Y., Wakao Y. Spectral analysis of heart rate variability in dogs with mild mitral regurgitation, American Journal of Veterinary Research 64, 2003, 145–148. [DOI] [PubMed] [Google Scholar]
- Fox P.R., Moise N.S., Price RA, et al. Analysis of continuous ECG (Holter) monitoring in normal cats and cardiomyopathic cats in congestive heart failure (Abstract), Journal of Veterinary Internal Medicine 12, 1998, 199. [Google Scholar]
- Gompf R.E., Tilley L.P. Comparison of lateral and sternal recumbent positions for electrocardiography of the cat, American Journal of Veterinary Research 40, 1979, 1483–1486. [PubMed] [Google Scholar]
- Haggstrom J., Hamlin R.L., Hansson K., Kvart C. Heart rate variability in relation to severity of mitral regurgitation in Cavalier King Charles spaniels, Journal of Small Animal Practice 37, 1996, 69–75. [DOI] [PubMed] [Google Scholar]
- Hamlin R.L. Heart rate of the cat, Journal of the American Animal Hospital Association 25, 1989, 284–286. [Google Scholar]
- Kawasaki T., Azuma A., Sakatani T., Hadase M., Kamitani T., Kawasaki S., Kuribayashi T., Sugihara H. Prognostic value of heart rate variability in patients with hypertrophic cardiomyopathy, Journal of Electrocardiology 36, 2003, 333–338. [DOI] [PubMed] [Google Scholar]
- Kleiger R.E., Miller J.P., Bigger J.T., Jr., Moss A.J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction, American Journal of Cardiology 59, 1987, 256–262. [DOI] [PubMed] [Google Scholar]
- La Rovere M.T., Pinna G.D., Maestri R., Mortara A., Capomolla S., Febo O., Ferrari R., Franchini M., Gnemmi M., Opasich C., Riccardi P.G., Traversi E., Cobelli F. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients, Circulation 107, 2003, 565–570. [DOI] [PubMed] [Google Scholar]
- Little C.J., Julu P.O. Investigation of heart rate variability in a dog with upper respiratory tract obstruction, Journal of Small Animal Practice 36, 1995, 502–506. [DOI] [PubMed] [Google Scholar]
- Lombardi F., Montano N., Finocchiaro M.L., Ruscone T.G., Baselli G., Cerutti S., Malliani A. Spectral analysis of sympathetic discharge in decerebrate cats, Journal of the Autonomic Nervous System 30 suppl, 1990, S97–S99. [DOI] [PubMed]
- Massimini M., Porta A., Mariotti M., Malliani A., Montano N. Heart rate variability is encoded in the spontaneous discharge of thalamic somatosensory neurones in cat, Journal of Physiology (London) 526, 2000, 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga T., Harada T., Mitsui T., Inokuma M., Hashimoto M., Miyauchi M., Murano H., Shibutani Y. Spectral analysis of circadian rhythms in heart rate variability of dogs, American Journal of Veterinary Research 62, 2001, 37–42. [DOI] [PubMed] [Google Scholar]
- Miller M.S., Tilley L., Smith F.W.K.S., Jr., Fox P.R. Electrocardiography. Sisson D., Fox P.R., Moise N.S. Textbook of Canine and Feline Cardiology: Principles and Clinical Practice, 2nd edn, 1999, WB Saunders: Philadelphia, 67–105. [Google Scholar]
- Minors S.L., O'Grady M.R. Heart rate variability in the dog: is it too variable?, Canadian Journal of Veterinary Research 61, 1997, 134–144. [PMC free article] [PubMed] [Google Scholar]
- Montano N., Cogliati C., da Silva V.J.D., Gnecchi-Ruscone T., Massimini M., Porta A., Malliani A. Effects of spinal section and of positive-feedback excitatory reflex on sympathetic and heart rate variability, Hypertension 36, 2000, 1029–1034. [DOI] [PubMed] [Google Scholar]
- Montano N., Lombardi F., Ruscone T. Gnecchi, Contini M., Finocchiaro M.L., Baselli G., Porta A., Cerutti S., Malliani A. Spectral analysis of sympathetic discharge, R–R interval and systolic arterial pressure in decerebrate cats, Journal of the Autonomic Nervous System 40, 1992, 21–31. [DOI] [PubMed] [Google Scholar]
- Nolan J., Batin P.D., Andrews R., Lindsay S.J., Brooksby P., Mullen M., Baig W., Flapan A.D., Cowley A., Prescott R.J., Neilson J.M.M., Fox K.A.A. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart), Circulation 98, 1998, 1510–1516. [DOI] [PubMed] [Google Scholar]
- Pagani M. Heart rate variability and autonomic diabetic neuropathy, Diabetes Nutrition and Metabolism 13, 2000, 341–346. [PubMed] [Google Scholar]
- Panciera D.L., Thomas C.B., Eicker S.W., Atkins C.E. Epizootiologic patterns of diabetes mellitus in cats: 333 cases (1980–1986), Journal of the American Veterinary Medical Association 197, 1990, 1504–1508. [PubMed] [Google Scholar]
- Pomfrett C.J.D., Austin A.R. Bovine spongiform encephalopathy (BSE) disrupts heart rate variability (HRV), Journal of Physiology 501, 1997, 69P. [Google Scholar]
- Pumprla J., Howorka K., Groves D., Chester M., Nolan J. Functional assessment of heart rate variability: physiological basis and practical applications, International Journal of Cardiology 84, 2002, 1–14. [DOI] [PubMed] [Google Scholar]
- Rishniw M., Bruskiewicz K. ECG of the month. Respiratory sinus arrhythmia and wandering pacemaker in a cat, Journal of the American Veterinary Medical Association 208, 1996, 1811–1812. [PubMed] [Google Scholar]
- Robertson B.T., Figg F.A., Ewell W.M. Normal values for the electrocardiogram in the cat, Feline Practice 6 (20), 1976, 22. [Google Scholar]
- Rogers W.A., Bishop S.P. Electrocardiographic parameters of the normal domestic cat: a comparison of standard limb leads and an orthogonal system, Journal of Electrocardiology 4, 1971, 315–321. [DOI] [PubMed] [Google Scholar]
- Rush J.E., Freeman L.M., Fenollosa N.K., Brown D.J. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999), Journal of the American Veterinary Medical Association 220, 2002, 202–207. [DOI] [PubMed] [Google Scholar]
- Scarlett J.M. Epidemiology of thyroid diseases of dogs and cats, Veterinary Clinics of North America: Small Animal Practice 24, 1994, 477–486. [DOI] [PubMed] [Google Scholar]
- Spier A.W., Meurs K.M. Assessment of heart rate variability in Boxers with arrhythmogenic right ventricular cardiomyopathy, Journal of the American Veterinary Medical Association 224, 2004, 534–537. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Hahn A.W., Sollers J.J., van Doornen L., Johnson P.J. Heart rate variability in the horse by ambulatory monitoring, Biomedical Science Instruments 33, 1997, 482–485. [PubMed] [Google Scholar]
- Ware W.A. Twenty-four-hour ambulatory electrocardiography in normal cats, Journal of Veterinary Internal Medicine 13, 1999, 175–180. [DOI] [PubMed] [Google Scholar]