Abstract
Clinical toxoplasmosis is commonly reported in the cat, with the most consistent findings being ocular, pulmonic, hepatic, neurological, gastrointestinal and muscular abnormalities. Myocarditis, whilst frequently identified at post-mortem examination, has not been identified ante-mortem.
In immunocompromised humans, myocarditis associated with toxoplasmosis is not an uncommon complication. In such cases, lymphocytic myocardial infiltration can lead to depressed myocardial function, which can be associated with congestive heart failure, rhythm disturbances and pericardial effusions. In addition, myocardial failure has been reported in immunocompetent humans associated with active toxoplasmosis [Chandenier J, Jarry G, Nassif D, Douadi Y, Paris L, Thulliez P, Bourges-Petit E, Raccurt C (2000) Congestive heart failure and myocarditis after seroconversion for toxoplasmosis in two immunocompetent patients. European Journal of Clinical Microbiological Infectious Disease 19, 375–379]. Here we describe a cat with echocardiographic changes consistent with infiltrative or inflammatory disease, and elevated IgG and IgM titres to Toxoplasma gondii. There was resolution of these myocardial changes once the toxoplasmosis was treated.
An 11-year-old, female neutered, domestic longhair cat presented with a 1 week history of lethargy, inappetence and dyspnoea. The cat was fed a commercial diet and had free access to the outside, although she rarely adventured beyond the confines of the garden. She was reported to hunt occasionally, catching predominantly mice. There was no history of fighting. She was not vaccinated but was regularly treated for ectoparasites. The referring veterinary surgeon had examined her 2 days before her referral and reported a grade III/VI systolic murmur for which she was receiving enalapril, (Enacard; Merial), 2.5 mg once daily. She was not receiving any other medications.
On physical examination the cat was found to be mentally depressed and reluctant to move. Her body condition score was 3/9, she weighed 4.6 kg and her coat was in poor condition. Her mucous membranes were pink and capillary refill time was less than 2 s. Her teeth had a small amount of tartar, but no signs of ongoing dental disease. The cat was weak, particularly on her hindlimbs. Her respiratory rate was 56 breaths per min and she was mildly dyspnoic. Her heart rate was 160 beats per min. A grade III/VI systolic murmur was audible; this was loudest over the left heart base. The pulse quality was poor bilaterally, although both femoral pulses were present. There were no pulse deficits. Her temperature was 37.0 °C and abdominal palpation was unremarkable; there was no hepatomegaly or ascites evident. There was no physical evidence of trauma. All peripheral lymph nodes were within normal limits and there was no palpable thyroid goitre. A retinal examination revealed no abnormalities. Systolic blood pressure was measured using a Doppler technique (Parks; Model 811-B). This was found to be decreased at 86 mmHg, (reference range 120–180 mmHg) (Sparkes et al 1999). When hair was clipped from over the right jugular vein, it was noted to be neither distended nor pulsatile. After venipuncture excessive bruising was noted and therefore, clotting times were added to the blood profile (the results of which are shown in Table 1).
Table 1.
Haematology, biochemistry and biochemistry/serology results
| (a) Haematology results | ||||
|---|---|---|---|---|
| Haematology | Units | Reference range (adult feline) | Result | |
| Day 1 | Day 28 | |||
| Red blood bell count | ×1012/l | 5.50–10.00 | 9.04 | 7.46 |
| Packed cell volume | l/l | 0.24–0.45 | 0.38 | 0.32 |
| Haemoglobin | g/dl | 8.00–14.00 | 12.30 | 10.20 |
| Mean cell volume | fl | 39.00–55.00 | 41.00 | 43.00 |
| Mean cell haemoglobin concentration | % | 30.00–36.00 | 32.80 | 32.1 |
| White blood cell count | ×109/l | 7.00–20.00 | 49.90 | 9.80 |
| Neutrophil count (segmented) | ×109/l | 2.50–12.80 | 45.91 | 7.84 |
| Neutrophil count (band) | ×109/l | 0.00–0.00 | 0.00 | 0.00 |
| Lymphocyte count | ×109/l | 1.50–7.00 | 1.50 | 1.67 |
| Monocyte count | ×109/l | 0.07–0.85 | 2.50 | 0.30 |
| Eosinophil count | ×109/l | 0.00–1.00 | 0.00 | 0.00 |
| Basophil count | ×109/l | 0.00–0.20 | 0.00 | 0.00 |
| Prothrombin time | s | 7.0–12.0 | 9.3 | – |
| Activated partial thromboplastin time | s | 10.0–20.0 | 16.8 | – |
| (b) Biochemistry results | ||||
| Biochemistry | Units | Reference range (adult feline) | Result | |
| Day 1 | Day 28 | |||
| Alanine transferase | IU/l | 6.00–83.00 | 61.00 | 116.00 |
| Alkaline phosphatase | IU/l | 10.00–100.00 | 10.00 | 30.00 |
| Creatinine kinase | IU/l | 50.00–200.00 | 644.00 | 238.00 |
| Calcium | mmol/l | 2.10–2.90 | 2.01 | 2.44 |
| Cholesterol | mmol/l | 2.00–3.40 | 3.70 | 4.05 |
| Creatinine | μmol/l | 40.00–177.00 | 165.00 | 125.00 |
| Glucose | mmol/l | 3.30–5.00 | 10.30 | 6.30 |
| Phosphate | mmol/l | 1.40–2.50 | 2.07 | 1.69 |
| Potassium | mmol/l | 4.00–5.00 | 4.50 | 5.40 |
| Total protein | g/l | 69.00–79.00 | 57.80 | 59.80 |
| Albumin | g/l | 28.00–39.00 | 19.40 | 23.40 |
| Globulin | g/l | 23.00–50.00 | 38.40 | 36.40 |
| Sodium | mmol/l | 145.00–156.00 | 136.00 | 150.00 |
| Urea | mmol/l | 2.80–9.80 | 14.00 | 9.70 |
| Bile acids | μmol/l | 0.00–7.00 | 7.10 | 21.8 |
| Total T4 | nmol/l | 13.00–48.00 | 15.00 | 24.40 |
| (c) Biochemistry/serology results | |||||
| Biochemistry/serology | Units | Reference range (adult feline) | Result | ||
| Day 1 | Day 28 | Day 64 | |||
| FeLV | +/− | −ve | – | – | |
| FIV | +/− | −ve | – | – | |
| Fructosamine | mmol/l | 2.19–3.47 | 1.30 | – | – |
| Blood culture | +/− | −ve | – | – | |
| Toxoplasma IgG | – | +ve@1/100 | +ve@1/400 | +ve@1/800 | |
| Toxoplasma IgM | – | +ve@1/200 | +ve@1/100 | −ve@1/12.5 | |
Lateral and dorsoventral thoracic radiographs were taken. On the lateral view, there was a mild increase in interstitial pattern within the area of the right middle lung lobe. In the absence of consistent findings on the dorsoventral view, it was felt that this change represented hypostatic congestion. Generally, there was a very mild increase in bronchointerstitial markings. In addition, an airgun pellet was situated at the level of the fifth rib and adjacent to the heart in the left hemithorax. However, absence of local skeletal and pulmonary changes suggested that this was not a recent injury. The cardiac silhouette was generally enlarged, having a globular appearance. Pulmonary vasculature was within normal limits (Fig 1).
Fig 1.
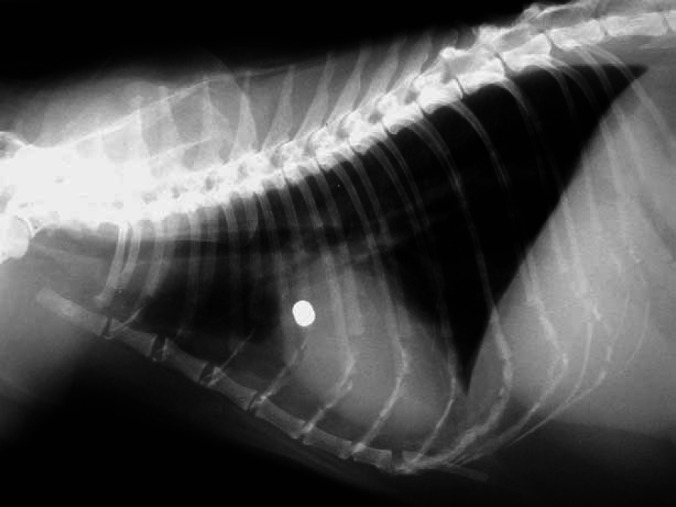
Lateral thoracic radiograph: an airgun pellet can be seen at the level of the fifth rib. A generalised mild increase in the bronchointerstitial markings can be appreciated. In addition, the cardiac silhouette is enlarged, giving the heart a globoid appearance.
Echocardiography demonstrated the presence of a moderate amount of pericardial fluid, although there was no evidence of cardiac tamponade. The echogenicity of the myocardium was increased and it had a granular appearance. There was a generalised symmetrical thickening of the myocardium (Table 2), with both the left and right ventricles appearing to be involved. The right ventricular free wall was particularly affected (Fig 2). Colour flow Doppler demonstrated turbulence in both the left and right ventricular outflow tracts. Spectral analysis of the aortic and pulmonary artery flow demonstrated a wide dispersion of flow, with the velocities being increased to 1.69 m/s (reference range: 1.02±0.19 m/s) and 1.89 m/s (reference range: 1.02±0.15 m/s) (Fox 1999), respectively (Table 2). The intra-atrial septum was severely distorted, demonstrating a dramatic increase in echogenicity and having a nodular appearance (Figs 2 and 3). The atrioventricular and semi-lunar valves subjectively appeared normal and there was no valvular incompetence detected. These changes were felt to be consistent with myocardial infiltration or myocarditis.
Table 2.
Echocardiography measurements
| Parameter | Unit | Reference range (adult feline) a | Result | |
| Day 0 | Day 64 | |||
| IVSd | cm | 0.3–0.6 | 0.61 | 0.53 |
| LVDd | cm | 1.0–2.1 | 1.32 | 1.42 |
| LVPWd | cm | 0.2–0.6 | 0.64 | 0.52 |
| IVSs | cm | 0.4–0.9 | 0.86 | 0.64 |
| LVDs | cm | 0.4–1.1 | 0.56 | 0.74 |
| LVPWs | cm | 0.4–1.0 | 0.81 | 0.74 |
| %FS | % | 40–67 | 57.67 | 47.96 |
| Ao | cm | 0.6–1.2 | 0.81 | 0.83 |
| LAD | cm | – | 1.22 | 1.06 |
| PV | m/s | 1.02±15 b | 1.89 | 1.09 |
| AV | m/s | 1.02±19 b | 1.69 | 1.11 |
d=diastole, s=systole, IVS=interventricular septum, LVD=left ventricular diameter, LVPW=left ventricular posterior wall, IVS=interventricular septum, FS=fractional shortening, Ao=aortic diameter end diastole, LAD=left atrial diameter end diastole, PV=velocity of blood flow within the pulmonary artery, AV=velocity of blood flow within the aorta.
Reference range: Sisson et al 1991
Reference range: Fox 1999.
Fig 2.

Right parasternal long axis view at the atrial level. The thickened interatrial septum (IAS) is clearly visible, as is the hypertrophy of the right ventricular free wall (RVFW).
Fig 3.

Left apical four chamber view demonstrating the nodular interatrial septum (IAS), thickening of the right ventricular free wall (RVFW) and a moderate pericardial effusion (PE).
In light of the echocardiographic and haematological findings, paired blood samples were taken 4 h apart and sent for blood culture, an additional sample was sent for detection of IgG and IgM antibodies to Toxoplasma gondii (Immunofluorescent antibody test (IFA), Axiom Laboratories, Devon, UK) and a sample was tested for the presence of feline leukaemia virus antigen and antibodies to feline immunodeficiency virus (Snap-Combo FeLV-FIV, IDEXX Labs Inc). The snap test was negative for both FIV and FeLV.
The patient was placed on a low rate intravenous infusion of Hartmann's solution, and treated with: cefazolin (Kefzol, Eli Lilly and Co) at 20 mg/kg IV q8hrs, clindamycin (Antirobe; Pharmacia and Upjohn) 75 mg PO q24hrs, and marbofloxacin (Marbocyl: Vetoquinol) 10 mg PO q24hrs. At this time the ACE-inhibitor (enalapril) was withdrawn because the cat was markedly hypotensive.
Over the subsequent 2 days the cats respiratory rate and effort returned to normal (24 bpm). Systolic blood pressure increased to 136 mmHg, appetite returned to normal, heart rate increased to 180 bpm, and the murmur reduced to a grade II/VI systolic murmur, now loudest over the right heart base. At this time the cat was very bright, and moved about comfortably. She was taken off the intravenous fluids. The cefazolin was discontinued and replaced with oral cephalexin (Ceporex; Schering Plough Animal Health) at 50 mg q12hrs, and she was discharged from the hospital.
At the first follow-up appointment, 1 week later, the cat was reported to be continuing to improve. However, 3 weeks after discharge the cat developed diarrhoea. At this time blood culture and serology for toxoplasmosis had been received. The blood cultures were negative. Her IgG and IgM titres for Toxoplasma gondii were positive at 1/100 and 1/200, respectively, suggesting an active infection at the time of sampling. A further blood sample was taken (Table 1), and the echocardiogram was repeated. The pericardial effusion had resolved, and the myocardial thickness was decreased, the intra-atrial septum was not as nodular as it had been previously but it still appeared grossly abnormal. Due to this improvement, and the presence of diarrhoea, the dose of clindamycin was not increased and dosing remained at once daily. The cephalexin and marbofloxacin were withdrawn.
Further follow-up examinations demonstrated a gradual decrease in IgM levels, along with a rising IgG titre and a subsequent improvement in the echocardiographic appearance. After 8 weeks of treatment her heart appeared grossly normal with the exception of a thin hyperechoic line on the intra-atrial septum, which was thought to represent fibrosis. Her systolic murmur had resolved. At this time the IgM was negative at 1/12.5 and the IgG was positive at 1/800. A further 2 weeks of clindamycin was given before withdrawal of therapy. Since that time (10 months), there has been no recurrence of clinical signs.
Myocarditis is an histological diagnosis and, therefore, can only be made at post-mortem examination or with an endomyocardial biopsy. Myocarditis is rarely identified in the cat. There are post-mortem studies that describe a form of restrictive cardiomyopathy, associated with myocarditis, endomyocarditis and fibrosis. However, the aetiology of this condition remains unknown. In addition, myocarditis has been reported in association with vegetative endocarditis and sepsis. Whilst this cat initially presented with clinical symptoms, which may be attributed to sepsis, there were no vegetative valvular lesions identified, and blood cultures were negative. Viral DNA has been isolated from formalised cats' hearts with various forms of cardiomyopathy, however, the relevance of this finding remains undetermined. Recently, Bartonella henselae type I DNA has been isolated from the aortic valve leaflet in a cat with endocarditis (Chomel et al 2003). Unfortunately, there was no serological analysis for Bartonella species in this case, although there were no echocardiographic evidence consistent with endocarditis, this differential cannot be excluded.
The echocardiographic features displayed in this case could equally have represented myocardial infiltration, by either neoplastic cells or inflammatory mediators. In the cat myocardial lymphoma is not uncommon and the distribution of lesions may be similar to those reported in this case. However, complete resolution of a cardiac lymphoma without chemotherapy is unlikely. Furthermore, cardiac infiltration has been reported in hypereosinophilic syndrome in the cat. However, such cases typically have a circulating eosinophilia, which was not present in this case.
Whilst pericardial effusion is not uncommon in cats, it is most often associated with severe cardiac disease (Liu 1970), FIP, neoplasia (Rush et al 1990) or uraemia (Van Vleet and Ferrans 1986). Although, in this case the echocardiographic changes were marked, the cardiac function remained relatively normal, the cat was not ureamic and resolution of the echocardiographic changes without immunomodulatory therapy would make FIP or neoplastic causes unlikely. Aspiration of the pericardial fluid may have been helpful in the diagnosis of a cardiac neoplasm.
Toxoplasmosis has been reported to cause myocarditis in man, but the majority of reports are limited to immunocompromised individuals (Al-Kassab et al 1995), typically following transplantation (Mayes et al 1995, Gallino et al 1996, Hermanns et al 2001). Similar reports are rare in the cat. However, one study has demonstrated that of 100 clinically affected cats presented for post-mortem examination, 11% had evidence of a pericardial effusion and microscopic examination of 59 hearts revealed 62.7% to have histological evidence of myocarditis (Dubey and Carpenter 1993). Similar studies in experimentally infected kittens revealed myocardial lesions in 100% of cases (Dubey et al 1996).
In this case the clinical signs at initial presentation were most likely attributable to disseminated toxoplasmosis. The patient did not have any signs of congestive heart failure on either echocardiography or radiography. The blood tests demonstrated a neutrophilia, lymphocytosis and monocytosis, all of which have been reported in toxoplasmosis (Dubey and Lappin 1998). In addition, hypoalbuminaemia is typical in the acute phase of infection, as is an elevated creatinine kinase (CK), where there is myositis. The initial weakness and bruising may have been caused by myositis and vasculitis associated with the infection. Whether in this case the elevated CK was due to skeletal or cardiac myositis cannot be determined, although measurement of cardiac troponin I (cTnI) may have helped in this differentiation. The elevations in alanine aminotransferase (ALT) and bile acids probably represent hepatic involvement, although why these changes were not present at the initial evaluation is unknown. Unfortunately, abdominal ultrasound and radiography were not performed in this case, so other organ involvement cannot be confirmed or discounted. However, a further blood sample obtained after treatment, demonstrated that both the ALT and bile acids had returned to the normal range. The body temperature and blood pressure were subnormal, her heart rate was relatively low and she was demonstrating signs typical of systemic shock, as is classically seen in cats. However, whether this was the result of sepsis induced vasodilation, or diminished right ventricular filling (the left ventricular load appearing normal on echocardiography), could not be determined.
The improvement on antibiotic therapy was rapid, with a vast improvement in demeanour being noticed within the first 24 h. In view of the cardiac lesions, a judicious rate of crystalloid infusion was administered. Initially, a triple antibiotic regimen was introduced. Once the serology results confirmed an active toxoplasmosis the other antibiotics were withdrawn. At this time the dose of clindamycin was reassessed as the prescribed dose was less than is advised for the treatment of toxoplasmosis (Lappin et al 1989). However, at this time the cat had diarrhoea and in light of her significant clinical improvement, we elected to continue treatment at the same dose. Once the other antibiotics were withdrawn the diarrhoea resolved spontaneously with no further adverse effects. While the role of other infectious organisms, which may have responded to these antibiotics, cannot be ruled out, the negative blood cultures make this unlikely.
The nodular appearance of the intra-atrial septum and nodular hypertrophy of the right ventricular free wall in particular, along with a marked circulating neutrophilia, led us to the assumption that these changes were the result of an infiltrative or inflammatory assault. As discussed previously toxoplasmosis can lead to myocarditis and pericardial effusion. In this case there was strong serological evidence of an active infection with Toxoplasma gondii and the resolution of cardiac changes reflected in the decreasing IgM titres suggested that the changes were secondary to the ongoing infection (Lappin 1996). Whilst a tissue biopsy is needed to confirm this diagnosis, the exclusion of other differentials and the clinical course of disease support this presumptive diagnosis. To our knowledge this is the first ante-mortem report of myocarditis most likely to be associated with toxoplasmosis in the cat.
Acknowledgements
The authors wish to thank all members of the University of Edinburgh Small Animal Hospital who helped in the management of this case. KES was supported by Petplan Charitable Trust, DGM is supported by Nestlé Purina Petcare.
References
- Al-Kassab A.K., Habte-Gabr E., Mueller W.F., Azher Q. Fulminant disseminated toxoplasmosis in an HIV patient, Scandinavian Journal of Infectious Disease 27, 1995, 183–185. [DOI] [PubMed] [Google Scholar]
- Chandenier J., Jarry G., Nassif D., Douadi Y., Paris L., Thulliez P., Bourges-Petit E., Raccurt C. Congestive heart failure and myocarditis after seroconversion for toxoplasmosis in two immunocompetent patients, European Journal of Clinical Microbiological Infectious Disease 19, 2000, 375–379. [DOI] [PubMed] [Google Scholar]
- Chomel B.B., Wey A.C., Kasten R.W., Stacy B.A., Labelle P. Fatal case of endocarditis associated with Bartonella henselae type I infection in a domestic cat, Journal of Clinical Microbiology 41, 2003, 5337–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J., Lappin M. Toxoplasmosis and neosporosis. Greene C. Infectious Diseases of the Dog and Cat, 1998, W.B. Saunders: Philadelphia, 493–510. [Google Scholar]
- Dubey J.P., Carpenter J.L. Histologically confirmed clinical toxoplasmosis in cats: 100 cases (1952–1990), Journal of the American Veterinary Medical Association 203, 1993, 1556–1566. [PubMed] [Google Scholar]
- Dubey J.P., Mattix M.E., Lipscomb T.P. Lesions of neonatally induced toxoplasmosis in cats, Veterinary Pathology 33, 1996, 290–295. [DOI] [PubMed] [Google Scholar]
- Fox P.R. Echocardiographic and Doppler methods. Fox P., Moise N., Sisson D. Textbook of Canine and Feline Cardiology, 1999, W.B. Saunders: Philadelphia, 130–171. [Google Scholar]
- Gallino A., Maggiorini M., Kiowski W., Martin X., Wunderli W., Schneider J., Turina M., Foolath F. Toxoplasmosis in heart transplant recipients, European Journal of Clinical Microbiological Infectious Disease 15, 1996, 389–393. [DOI] [PubMed] [Google Scholar]
- Hermanns B., Brunn A., Schwarz E.R., Sachweh J.S., Seipelt I., Schroder M.J., Vogel U., Schoendube F.A., Buettner R. Fulminant toxoplasmosis in a heart transplant recipient, Pathology Research and Practice 197, 2001, 211–215. [DOI] [PubMed] [Google Scholar]
- Lappin M., Greene C., Winston S., Toll S., Epstein M. Clinical feline toxoplasmosis; serologic diagnosis and therapeutic management of 15 cases, Journal of Veterinary Internal Medicine 3, 1989, 139–143. [DOI] [PubMed] [Google Scholar]
- Lappin M.R. Feline toxoplasmosis: interpretation of diagnostic test results, Seminars in Veterinary Medicine and Surgery (Small Animal) 11, 1996, 154–160. [DOI] [PubMed] [Google Scholar]
- Liu S.-K. Acquired cardiac lesions leading to congestive heart failure in the cat, American Journal of Veterinary Research 31, 1970, 2071–2088. [PubMed] [Google Scholar]
- Mayes J.T., O'Connor B.J., Avery R., Castellani W., Carey W. Transmission of Toxoplasma gondii infection by liver transplantation, Clinical Infectious Diseases 21, 1995, 511–515. [DOI] [PubMed] [Google Scholar]
- Rush J.E., Keene B.W., Fox P.R. Pericardial disease in the cat: a retrospective evaluation of 66 cases, Journal of the American Animal Hospital Association 29, 1990, 39–46. [Google Scholar]
- Sisson D.D., Knight D.H., Helinski C. Plasma taurine concentrations and M-mode echocardiographic measurements in healthy cats and cats with dilated cardiomyopathy, Journal of Veterinary Internal Medicine 5, 1991, 232–238. [DOI] [PubMed] [Google Scholar]
- Sparkes A.H., Caney S.M.A., King M.C.A., Gruffydd-Jones T.J. Inter- and intraindividual variation in Doppler ultrasonic indirect blood pressure measurements in healthy cats, Journal of Veterinary Internal Medicine 13, 1999, 314–318. [DOI] [PubMed] [Google Scholar]
- Van Vleet J.F., Ferrans V.J. Myocardial disease of animals, American Journal of Pathology 124, 1986, 98–178. [PMC free article] [PubMed] [Google Scholar]


