Abstract
To determine the effectiveness of dietary lysine supplementation in cats with enzootic upper respiratory disease (URD), 50 cats were fed a ration containing 11 or 51 g lysine/kg diet for 52 days. Food intake, body weight, clinical signs, plasma amino acid concentrations and presence of Chlamydophila felis or feline herpesvirus (FHV)-1 DNA within the conjunctival fornix were assessed. Food and lysine intake of both dietary groups decreased between days 17 and 22, coinciding with peak disease and viral presence. Mean disease score for cats fed the supplemented ration (0.94) was higher than for those fed the basal diet (0.21); however, this could be attributed to a small subset of male cats which demonstrated fighting behavior that may have contributed to stress within that cage. FHV-1 DNA was detected on 12 occasions in six cats receiving the supplemented diet and on one occasion in one cat fed the basal diet. C felis DNA was never detected. Mean plasma arginine concentration was lower and plasma lysine concentration was higher in supplemented cats. Mean plasma arginine concentration declined throughout the study in both dietary groups. Data from the present study raise important questions but do not permit a definitive conclusion regarding the efficacy of dietary lysine supplementation in cats with enzootic URD.
The amino acid l-lysine limits in vitro replication of many viruses including feline herpesvirus type 1 (FHV-1) (Pearson et al 1952, Tankersley 1964, Griffith et al 1981, Maggs et al 2000). The antiviral mechanism is unknown, however coincident arginine restriction has proved important in both in vitro (Tankersley 1964, Griffith et al 1981, Maggs et al 2000) and in vivo (Griffith et al 1978, Thein and Hurt 1984, Griffith et al 1987) studies. Cats are exquisitely sensitive to arginine deficiency (Morris and Rogers 1978, Baker 1991). Therefore, application of in vitro data has been cautious and limited to bolus administration of lysine to experimentally-infected cats (Stiles et al 2002, Maggs et al 2003). Once-daily oral administration of 400 mg lysine to 14 cats latently infected with FHV-1 was associated with a significant reduction in basal viral shedding compared with placebo-treated cats (Maggs et al 2003), and twice-daily oral administration of 500 mg lysine to eight cats beginning 6 h prior to and continuing for 3 weeks following primary inoculation with FHV-1 reduced severity of conjunctivitis relative to placebo-treated cats (Stiles et al 2002).
Twice-daily administration of lysine in a tablet or capsule form to individual cats in multi-cat environments, particularly feline shelters, may be impractical, stimulate further viral reactivation through stress (Gaskell and Povey 1977) or facilitate transfer of infectious organisms among cats by operators. Bolus administration via twice-daily application of a known quantity of lysine on to the cat's usual diet may be more practical but is still time consuming and would rely upon complete ingestion twice daily to exert the same bolus effect as that produced by individual tablets or capsules. Dietary lysine supplementation of cats may provide a means of nutritional control of viral shedding. Recently, we established that 8.6% lysine supplementation of the diet did not affect apparent palatability, food intake, or reduce plasma arginine concentration in adult cats (Fascetti et al 2004). Mean plasma lysine of these cats was increased to concentrations similar to that achieved with bolus administration (Maggs et al 2003).
Combined, data from these studies indicate that lysine supplementation decreases in vitro replication of FHV-1, bolus administration of lysine safely reduces basal viral shedding in latently infected cats and disease severity in acutely infected cats, and dietary lysine supplementation of cats appears safe. However, studies to date (Stiles et al 2002, Maggs et al 2003) have assessed small populations of specific pathogen-free (SPF) or random-source cats infected with only two strains of only one of the organisms known to cause upper respiratory disease (URD) in cats. Therefore, they may not truly reflect circumstances in larger, clinically important populations, especially multi-cat environments where control of enzootic URD is extremely challenging due to variable vaccination history, intercurrent disease, physiological stresses, and high turnover of cats of diverse genetic composition and with varied exposure to infectious diseases. The current study was designed to assess the safety and effects of dietary lysine supplementation in a colony of cats with enzootic URD. Specifically, it was designed to assess whether dietary administration of lysine significantly altered plasma lysine or arginine concentrations, severity of clinical disease, or ocular detection rates of Chlamydophila felis or FHV-1.
Materials and methods
Study population and diet
Cats in this study were selected from a colony that had recently experienced an epizootic of URD similar to a prior occurrence reported in 1994 (Hickman et al 1994). Viral and chlamydial vaccines were not used in this colony. All experimental protocols were approved by the Animal Care and Use Administrative Advisory Committee of the University of California, Davis. Following completion of this study, the colony was depopulated and returned to an SPF facility.
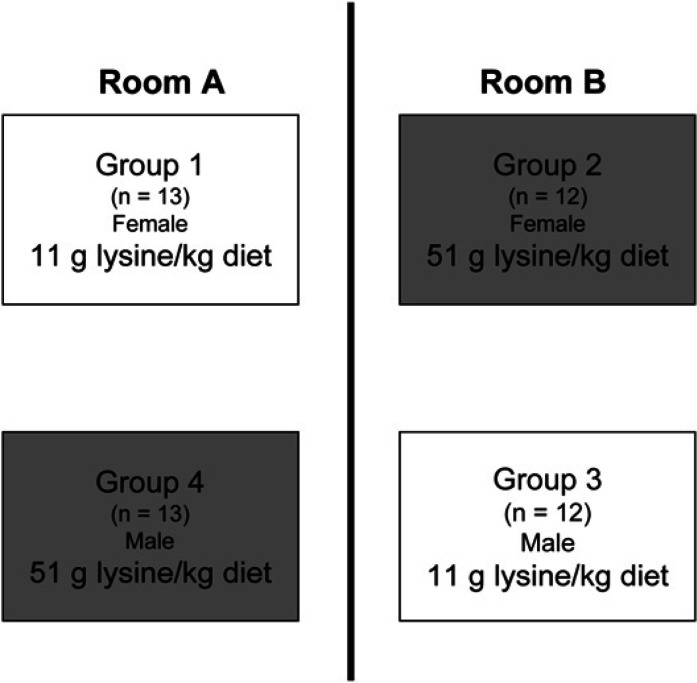
A total of 50 sexually intact domestic shorthair cats (25 males and 25 females) of mixed ages were selected from this colony and on day 0 were stratified according to age (less than or greater than 1 year) and FHV-1 and feline calicivirus (FCV) serological status (seropositive or seronegative) into four groups differing only by gender and lysine content of the diet fed. Cats were then moved into group cages each containing 12 or 13 individuals (Fig 1). Groups of cats were housed in 2.5 m3 wire cages separated by a maximum of 65 cm such that transfer of organisms by sneezed macrodroplets was possible within one room (Wardley and Povey 1977). This experimental design was selected to mimic housing conditions that exist in shelters or other multiple cat environments where rehousing has been demonstrated to cause reactivation and shedding of FHV-1 and recrudescent clinical signs in varying proportions of individuals (Gaskell and Povey 1973, 1977, Maggs et al 2003). Individual history of ocular or URD was not considered and the person assigning cats to each of the four groups was not aware of their subsequent treatments. Room temperature (21±2°C) and light:dark cycle (14:10 hours) were controlled. Other than ration fed, husbandry conditions were identical for cats in all four cages.
Fig 1.
Cats were assigned to one of four cages and one of two dietary groups according to gender and lysine content of diet fed.
Prior to the beginning of the study, all cats were fed the same commercially prepared, dry expanded diet. The proximate composition (g/kg) of this diet was crude protein (334), crude fat (67), and crude fiber (34). Following rehousing, the 25 cats from groups 1 (13 females) and 3 (12 males) received a basal ration. The cats from groups 2 (12 females) and 4 (13 males) received the same basal ration supplemented with lysine (Fig 1). Both diets were commercially prepared, experimental, dry expanded diets. Other than amino acid content, the composition of both diets was almost identical (Table 1). Only one batch of each diet was produced and used throughout the entire study. Amino acid content of seven samples of each of the two diets selected from seven different bags of each diet was assessed using an automated amino acid analyzer (Biochrom 30; Biochrom Ltd) (Spitze et al 2003). Cats had free access to water and their respective diet throughout the 52-day study. Food was provided daily in an elongated feeding trough (approximately 1 m×13 cm) that permitted all cats to feed simultaneously if desired. Until completion of data analysis, all investigators other than the single author responsible for diet production (MKW) were masked as to diet fed to each population.
Table 1.
Proximate composition of the experimental diets as reported by the manufacturer
| Basal diet a | Supplemented diet b | |
|---|---|---|
| Crude protein (g/kg diet) | 324 | 351 |
| Crude fat (g/kg diet) | 152 | 146 |
| Crude fiber (g/kg diet) | 13 | 11 |
| Energy density (kcal DE/kg diet) | 4260 | 4190 |
| Lysine (g/kg diet) | 13 | 61 |
| Arginine (g/kg diet) | 17 | 14 |
The basal diet was composed of the following ingredients (g/kg diet): corn gluten meal (223.7); rice, brewers milled (267.3); whole yellow corn (203.4); poultry by-product meal (174.3); fish meal (58.1); wheat flour (46.5); potassium chloride (11.6); mineral mix, containing zinc sulfate, ferrous sulfate, manganese sulfate, copper sulfate, calcium iodate and sodium selenite; remaining mineral mix ingredients and amounts proprietary, Nestlé Purina PetCare Research (2.3); calcium carbonate (1.7); sodium chloride (5.8); choline chloride (2.5); taurine (1.2); vitamin mix, containing retinyl acetate, cholecalciferol, dl-α-tochopheral acetate, thiamine mononitrate, riboflavin, nicotinic acid, calcium d-pantothenate, pyridoxine hydrochloride, cyanocobalamine, d(+)biotin, and menadione; remaining vitamin mix ingredients and amounts proprietary, Nestlé Purina PetCare Research (0.9); vitamin E (0.6); and dl-methionine (0.1).
The lysine-supplemented diet was composed of the following ingredients (g/kg diet): corn gluten meal (225.1); rice, brewers milled (222.3); whole yellow corn (204.7); poultry by-product meal (157.9); fish meal (23.4); wheat flour (46.8); potassium acetate (19.9); sodium acetate anhydrous (16.4); l-lysine (73.1); mineral mix, containing zinc sulfate, ferrous sulfate, manganese sulfate, copper sulfate, calcium iodate and sodium selenite; remaining mineral mix ingredients and amounts proprietary, Nestlé Purina PetCare Research (2.3); calcium carbonate (2.9); choline chloride (2.4); taurine (1.2); vitamin mix, containing retinyl acetate, cholecalciferol, dl-α-tochopheral acetate, thiamine mononitrate, riboflavin, nicotinic acid, calcium d-pantothenate, pyridoxine hydrochloride, cyanocobalamine, d(+)biotin, and menadione; remaining vitamin mix ingredients and amounts proprietary, Nestlé Purina PetCare Research (0.9); vitamin E (0.6); and dl-methionine (0.1).
From days 1 to 49, group daily food intakes were calculated for each cage by difference in mass of food offered daily and that remaining in the group feeders before fresh food was offered the following day. Objective food intake measurements were inadvertently omitted on days 50–52 but subjectively food intake in all cages was stable at this time. Group lysine and arginine intake were calculated for each cage by multiplying group food intake by the analyzed dietary lysine and arginine composition, respectively. Cats were weighed weekly throughout the study.
Clinical and microbiological assessments
Cats were observed daily for general alertness and demeanor and obvious social interactions within all four groups. Clinical signs of respiratory or ocular disease were graded twice weekly for 52 days by trained observers according to a previously published (Maggs et al 2003) semi-quantitative scoring system (Table 2). Scores for each clinical sign were added for each cat to give a total individual disease score and for all cats within a group to give a total group disease score.
Table 2.
Scoring system used to monitor cats for clinical evidence of FHV-1-associated disease
| Conjunctivitis |
| 0=None |
| 1=Mild conjunctival hyperemia |
| 2=Moderate to severe conjunctival hyperemia |
| 3=Moderate to severe conjunctival hyperemia and chemosis |
| Blepharospasm |
| 0=None |
| 1=Eye <25% closed |
| 2=Eye 25–50% closed |
| 3=Eye 50–75% closed |
| 4=Eye completely closed |
| Ocular discharge |
| 0=None |
| 1=Minor serous discharge |
| 2=Moderate mucoid discharge |
| 3=Marked mucopurulent discharge |
| Sneezing |
| 0=None |
| 1=Observed |
| Nasal discharge |
| 0=None |
| 1=Minor serous discharge |
| 2=Moderate mucoid discharge |
| 3=Marked mucopurulent discharge |
To aid in initial stratification of individuals to treatment groups and to assess each subject's serologic response during the study period, serum collected by jugular venepuncture within 5 months before the study start date (baseline) and at the termination of the study (day 52) was assessed for the presence of antibodies to FHV-1 and FCV using recombinant antigen-based Enzyme-linked immunosorbent assays (ELISAs) (Lappin et al 2002). Based on a comparison to standard curves made from a range of positive and negative control calibrators assayed on each ELISA plate, all samples were assigned an estimated reciprocal titer of negative (when the mean of the sample absorbance wells was less than the negative cutoff calibrator), 64, 128, 256, 512, 1024, or 2048 for FHV-1 and for FCV.
Beginning on day 2 and twice weekly thereafter, samples were collected for microbiological analysis following clinical observations and topical application of 0.5% proparacaine hydrochloride ophthalmic solution (Akorn Inc). Both ventral conjunctival fornices of each cat were swabbed vigorously using a single dry sterile Dacron polyester fiber-tipped swab (Fisherbrand, Sterile Swabs; Fisher Scientific). Each swab then was placed into sterile phosphate buffered saline and stored at −20°C for DNA extraction and assessment using polymerase chain reaction (PCR). Prior to PCR analysis, each sample was thawed and vortexed for a total of 1.5 min. Following removal of the swab using forceps treated between samples with 70% ethanol solution, each sample was centrifuged for 20 min at 20,800 g. The supernatant was discarded and the pellet was resuspended in 50 μl of TE buffer (10 mM Tris, 1 mM ethylenediaminetetraacetic acid (EDTA); pH 8.0). A 25-μl aliquot was used in each PCR reaction.
Presence of FHV-1 DNA was detected using a PCR assay, targeting a 322 base pair region, of the thymidine kinase gene of this organism performed without hot start (Weigler et al 1997). Positive controls (DNA extracted from FHV-1 strain 727) (Nasisse et al 1989) and negative controls (DNAse-, RNAse-free water) were included for each PCR reaction. Following thermocycling, 10 μl of reaction mixture underwent electrophoresis in ethidium bromide-stained 1.5% agarose gels and PCR products were identified by visual examination and were digitally photographed (Kodak EDAS 290, Eastman Kodak Company).
A representative subset of 62 swabs also was tested for presence of C felis DNA. Swabs were selected so that they represented all dates, all four groups, a wide range (1–9) of total individual clinical scores, and cats in which FHV-1 DNA had or had not been detected. The presence of C felis DNA was assessed using a PCR protocol that targets a 1094 base pair region of the ompA gene of the organism (Sykes et al 1997). Negative extraction and thermocycling controls (DNAse-, RNAse-free water) and positive controls (DNA extracted from a feline chlamydial vaccine; Eclipse 4, Schering-Plough Animal Health Corp.) were included with each run.
Plasma amino acid assessments
Within 5 months of the study beginning (baseline), and at 11:00 hours on days 17 and 52 of the dietary trial, blood was collected into heparinized syringes from all cats by jugular venepuncture for plasma amino acid analysis (Fascetti et al 2004). Plasma was obtained by centrifugation of the heparinized blood sample at 10,000 g for 15 min, immediately deproteinized with an equal volume of 0.24 mol/l 5-sulfosalicylic acid, and centrifuged again at 10,000 g for 15 min at 4°C. All samples were stored at −80°C until analysis using an automated amino acid analyzer (Fascetti et al 2004).
Statistical analyses
Comparison of baseline age, body weight, and plasma amino acid concentration between the two dietary groups was performed by using Student's t-test or the Mann–Whitney Rank sum test. Comparison of baseline age among the four groups was performed by using the Kruskal–Wallis one-way analysis of variance on ranks. For the FHV-1 and FCV ELISA results, the proportion of cats seronegative at baseline, as well as the proportions of cats that underwent seroconversion (from negative to an estimated titer ≥64 for either virus) or a four-fold or greater increase in estimated titer during the course of the study, were compared between the two dietary groups by using Fisher's exact test. Proportions of cats that were FHV-1 seropositive at baseline were compared among the four groups using χ2 analysis. Analyzed amino acid content of the basal and supplemented diets was compared by using Student's t-test or the Mann–Whitney Rank sum test. Comparisons of the effects of time, dietary group, and the interactions of these factors on total individual clinical scores and on plasma amino acid concentrations were assessed using two-way repeated measures analysis of variance. When significant interactions were observed, post-hoc Student's t-tests conditional on day were performed to assess differences between dietary groups. Pairwise contrasts of total disease scores among the four groups were performed using a Mann–Whitney test. A two-tailed P-value less than 0.05 was considered significant for all analyses.
Results
Study population and diet
At the start of the study, no significant difference in median age, body weight, or proportion of cats seronegative for FHV-1 or FCV was detected between cats receiving the lysine-supplemented diet (groups 2 and 4) and those receiving the basal diet (groups 1 and 3; Table 3). Likewise, no significant difference in median age (P=0.216) or proportion of cats seronegative for FHV-1 (P=0.216) was detected among the four groups. However, all four cats seropositive with respect to FCV in the supplemented dietary group were in group 2.
Table 3.
Baseline characteristics of the two diets (as analyzed) and cats receiving them
| Basal diet | Supplemented diet | P-value | |
|---|---|---|---|
| Mean (±SD) dietary lysine content (g/kg diet) | 11 (±0.5) | 51 (±0.3) | <0.001 |
| Mean (±SD) dietary arginine content (g/kg diet) | 13 (±0.6) | 12 (±0.5) | 0.008 |
| Median (range) cat age (months) | 15 (5–132) | 15 (5–117) | 0.78 |
| Median (range) cat body weight (kg) | 4.0 (1.7–6.5) | 3.9 (2.0–7.1) | 0.92 |
| Number FHV-1 seronegative cats | 12 | 12 | 0.78 |
| Number FCV seronegative cats | 25 | 21 | 0.11 |
| Median (range) plasma lysine concentration (nmol/ml) | 85 (44–311) | 80 (43–144) | 0.33 |
| Mean (range) plasma arginine concentration (nmol/ml) | 103 (54–172) | 99 (50–151) | 0.61 |
FHV=feline herpesvirus, FCV=feline calicivirus.
The mean (±SD) analyzed lysine content of the supplemented diet (51±0.3 g/kg diet) was significantly greater (P<0.001) than that of the basal diet (11±0.5 g/kg diet; Table 3). The mean (±SD) analyzed arginine content of the supplemented diet (12±0.5 g/kg diet) was significantly (P=0.008) less than that for the basal diet (13±0.6 g/kg diet; Table 3). Statistically significant differences in analyzed amino acid content of the basal and supplemented diets also were detected for taurine, asparagine, alanine, methionine, and histidine. These differences were likely due to slight variations in the quantity of the ingredients supplying amino acids to these two diets. Quantities of all amino acids in both diets met or exceeded recommendations for cats from the National Research Council (National Research Council 1986).
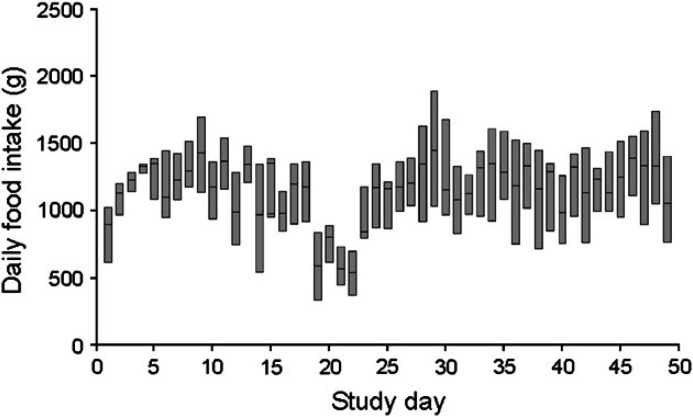
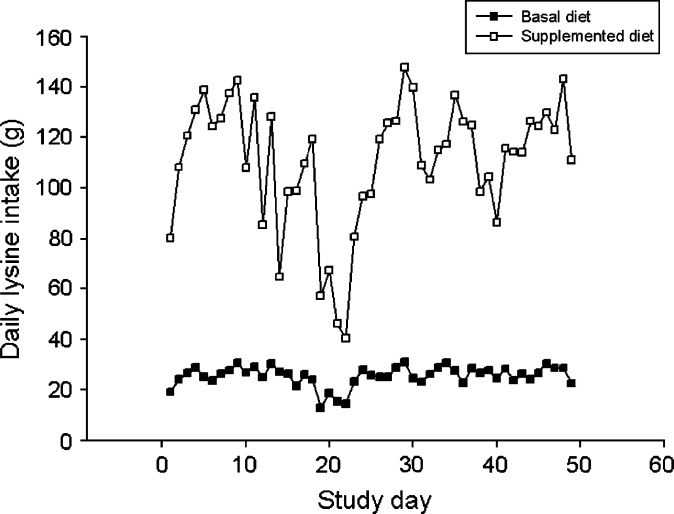
No adverse responses, such as reduced food intake, vomiting or diarrhea were observed when the study diets were introduced. All cats were group-housed, so individual food intake could not be recorded and statistical analysis of cage or dietary group food intake data was not possible. However, trends in food intake were evident in all four groups (Fig 2). In all four groups, food intake increased during days 5–9 and then decreased through day 22. A rapid decrease in food intake was noticed in all four groups between days 17 and 22. This period coincided with peak clinical disease scores (see later). Despite these notable changes in food intake over time, median body weight did not differ significantly between dietary groups at any time (P=0.824–0.986) and did not vary greatly throughout the study. Group lysine intake was derived from food intake data and also could not be subjected to statistical analysis. However, during the period of rapid reduction in food intake, lysine intake in those cats fed the supplemented diet decreased from 119.4 (day 18) to 40.4 g/dietary group/day (day 22). By contrast, lysine intake in cats fed the basal ration decreased from 24.1 (day 18) to 14.6 g/dietary group/day (day 22; Fig 3). Thus, there was a 5.0-fold difference in group lysine intake between dietary groups on day 18 but only a 2.8-fold difference on day 22. This represented a 79 g/dietary group/day reduction in lysine intake over this 3-day period for cats fed the supplemented diet compared with a 9.5 g/dietary group/day reduction in lysine intake over the same period in those cats receiving the basal diet. Because dietary arginine content did not differ between the two diets as greatly as lysine content did, reduction in arginine intake during this period of reduced food intake was less marked than reduction in lysine intake. For cats receiving the basal diet, arginine intake decreased by 11 g/dietary group/day and for those receiving the supplemented diet it decreased by 19 g/dietary group/day.
Fig 2.
Daily food intake for all cats. Line represents median group food intake per cage; boxes represent 5th and 95th percentiles.
Fig 3.
Daily lysine intake for cats fed a basal ration (▪) containing 11 g lysine/kg diet or a supplemented ration (□) containing 51 g lysine/kg diet. Data represent total daily lysine intake for each dietary group.
Clinical and microbiological assessments
Following assignment to group cages, notable differences were observed in social interactions among cats in one group cage relative to all others. Male cats fed the lysine-supplemented diet (group 4; Fig 1) displayed overt aggression toward each other with frequent posturing, fighting, and at times sexual mounting. This behavior persisted in this group throughout the study period, but was not noted among cats in the other three groups. Two cats were removed from group 4 because of lethargy, inappetence, weight loss, and dehydration; one each on days 15 and 16. From day 16, these two cats were housed together, treated with subcutaneous fluid replacement and returned to the group cage on day 31. One cat was removed from group 3 on day 16 as a result of a wound presumed to be inflicted by another cat and was housed alone for the duration of the study. These three cats continued to be fed diets appropriate for the dietary group from which they were removed. Microbiological, serological, and clinical monitoring of these cats was continued and clinical scores, microbial presence, food intakes, and plasma amino acid concentration data for all three cats were included in their respective group data.
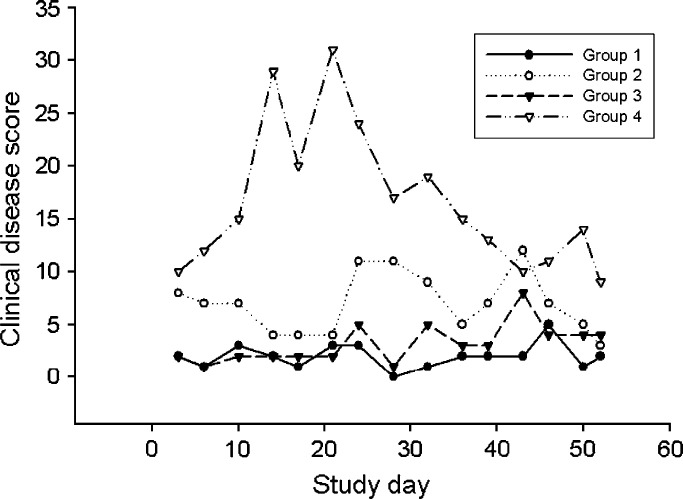
Clinical signs of ocular and URD were seen in 15/25 (60%) cats fed the basal diet and in 22/25 (88%) cats fed the supplemented diet but varied markedly among the four groups (Fig 4). The time of peak total group disease scores in male cats fed the supplemented diet (group 4; Fig 4) corresponded to the period of marked reduction in food (Fig 2) and lysine (Fig 3) intake. Due to the marked difference in behavior and severity of clinical signs seen in cats in group 4, dietary effect on clinical disease first was evaluated separately for each gender. Considered over the whole study period, mean total individual disease score did not significantly differ (P=0.082) between female cats fed the basal ration (0.15) and those fed the supplemented ration (0.58). However, mean total individual disease score over the whole study period for male cats fed the supplemented ration (1.28) was significantly higher (P=0.026) than for those fed the basal diet (0.27). A significant dietary group by time interaction could be demonstrated for male cats (P=0.024) but not for female cats (P=0.27). When data were analyzed without consideration of gender, mean total individual disease score over the whole study period for all cats fed the supplemented diet (0.94) was significantly higher (P=0.0048) than for those fed the basal diet (0.21). Neither a significant difference over time (P=0.11) nor a significant interaction between dietary group and time (P=0.088) was demonstrated.
Fig 4.
Total group disease scores for female (groups 1 and 2; circles) or male (groups 3 and 4; triangles) cats fed a basal ration containing 11 g lysine/kg diet (groups 1 and 3; filled symbols) or a supplemented ration containing 51 g lysine/kg diet (groups 2 and 4; open symbols).
FHV-1 DNA was detected in the conjunctival fornix of seven cats on 13 occasions. In cats fed the basal ration, FHV-1 DNA was detected in one sample collected on day 52 from a cat in group 1. In cats fed the supplemented ration, FHV-1 DNA was detected in 12 samples collected on six different days from six cats in group 4. This represented a significant difference in viral presence between dietary groups (P=0.005). Viral DNA was detected in cats from group 4 on days 14 through 32; spanning the period of peak clinical signs in these cats (Fig 4). C felis DNA was not detected in the conjunctival fornix of any cat at any time examined.
None of the cats in this study had a four-fold or greater increase in estimated FHV-1 serum antibody titer between baseline and final samples. However, seven cats (one cat fed the basal diet and six cats fed the supplemented diet) were negative for FHV-1 antibodies in the baseline sample but positive for FHV-1 antibodies in the final sample (Table 4). For cats that became seropositive for FHV-1 antibodies over the course of the study, reciprocal titers in the final sample varied from 64 to 512 and FHV-1 DNA was detected in the conjunctival fornix of five of these seven cats on at least one occasion. Of the cats fed the basal diet, none had detectable FCV antibodies in the baseline sample and two cats had detectable FCV antibodies (reciprocal titers of 64) in the final sample. The total clinical scores for these two cats were two and five, respectively. Of the cats fed the supplemented diet, four cats had detectable FCV antibodies in both the baseline sample (reciprocal titers of 64) and final sample (reciprocal titers of 64, 128, 128, and 128, respectively). An additional three cats fed the supplemented diet but negative for FCV antibodies in the baseline sample had detectable FCV antibodies in the final sample (reciprocal titers of 64). No cat in either dietary group experienced a four-fold or greater increase in serum FCV antibody titer. Of these seven FCV seropositive cats, four were in group 2 and had total clinical scores of 0, 0, 24, and 40. The remaining three were in group 4 and had total clinical scores of 1, 25, and 61. To assess potential influence of FCV co-infection on severity of clinical signs, the nine cats with positive terminal FCV titers were excluded from the analysis and mean total clinical scores compared among the four groups. Total median group clinical score in group 4 (12) was still greatest when compared to group 1 (0), group 2 (3), or group 3 (1). Using these revised values, median total disease score in group 4 was significantly greater than that in groups 1 and 3 (P<0.05) but no significant difference was detected between median total disease scores for groups 4 and 2. Of the seven cats in which FHV-1 was detected, two had serological evidence of exposure to FCV; one cat from group 1 and one cat from group 4.
Table 4.
Clinical scores and FHV-1 PCR assay results in cats negative for FHV-1 antibodies in the baseline sample but positive for FHV-1 antibodies in the final sample (day 52)
| Final FHV-1 titer | Peak clinical score | Total clinical score | PCR result | |
|---|---|---|---|---|
| Basal ration | ||||
| Cat 1 | 64 | 3 | 3 | Negative |
| Supplemented ration | ||||
| Cat 2 | 64 | 6 | 57 | Positive |
| Cat 3 | 128 | 4 | 25 | Negative |
| Cat 4 | 256 | 9 | 25 | Positive |
| Cat 5 | 256 | 2 | 12 | Positive |
| Cat 6 | 512 | 7 | 13 | Positive |
| Cat 7 | 512 | 4 | 11 | Positive |
The basal ration contained 11 g lysine/kg diet and the supplemented ration containing 51 g lysine/kg diet. FHV-1=feline herpesvirus type 1.
Plasma amino acid assessments
Baseline median plasma lysine concentration of cats receiving the supplemented diet (80 nmol/ml) was not significantly different (P=0.332) from that of cats receiving the basal diet (85 nmol/ml; Table 3 and Fig 5A). Likewise, baseline mean plasma arginine concentration of cats receiving the supplemented diet (99 nmol/ml) was not significantly different (P=0.623) from that of cats receiving the basal diet (103 nmol/ml; Table 3 and Fig 5B). These baseline data are similar to published plasma lysine and arginine concentrations for cats (Stiles et al 2002, Fascetti et al 2004). No significant differences in baseline plasma concentrations of any other amino acid tested were detected between the two dietary treatment groups (P=0.110–0.996).
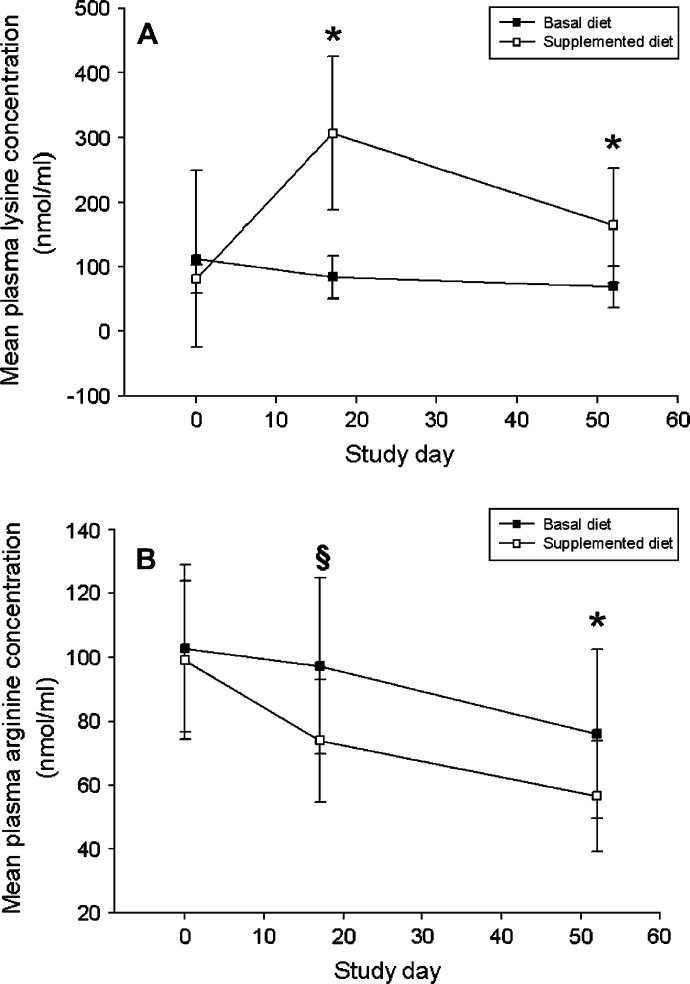
Fig 5.
(A) Mean (±SD) plasma lysine concentration for cats fed a basal ration (▪) containing 11 g lysine/kg diet or a supplemented ration (□) containing 51 g lysine/kg diet. *P<0.0001. (B) Mean (±SD) plasma arginine concentration for cats fed a basal ration (▪) containing 11 g lysine/kg diet or a supplemented ration (□) containing 51 g lysine/kg diet. §P=0.0011; *P=0.0030.
Over the study period, a significant interaction between the effects of time and dietary group upon plasma lysine concentration was detected regardless of gender (P<0.0001). On day 17, mean plasma lysine concentration in cats receiving the supplemented diet (307 nmol/ml) was significantly higher (P<0.0001) than it was in cats receiving the basal diet (84 nmol/ml). On day 52, mean plasma lysine concentration in cats receiving the supplemented diet (164 nmol/ml) was again significantly higher (P<0.0001) than it was in cats receiving the basal diet (69 nmol/ml; Fig 5A). A significant interaction between the effects of time and dietary group upon plasma arginine concentration was not detected (P=0.069). Mean plasma arginine concentration declined over the course of the study in male cats from both dietary treatment groups and in females fed the supplemented diet; it rose transiently from day 0 to day 17 in females receiving the basal diet, although this rise was not significant (P=0.34). On day 17, mean plasma arginine concentration in all cats receiving the supplemented diet (74 nmol/ml) was lower (P=0.0011) than it was in all cats receiving the basal diet (97 nmol/ml; Fig 5B). This difference held for males alone (P=0.0073) and females alone (P=0.053). On day 52, mean plasma arginine concentration in all cats receiving the supplemented diet (57 nmol/ml) was again significantly lower (P=0.0030) than it was in all cats receiving the basal diet (76 nmol/ml). However, at this time, there was no significant difference in mean plasma arginine concentration between female cats receiving the supplemented diet (59 nmol/ml) and the basal diet (66 nmol/ml) (P=0.35). By contrast, on day 52 mean plasma arginine concentration in male cats receiving the supplemented diet (54 nmol/ml) was significantly lower (P=0.0030) than it was in male cats receiving the basal diet (87 nmol/ml).
Discussion
This study was designed to assess the effects of constant dietary lysine supplementation over an extended period in a diverse group of cats with enzootic URD. As such, it differed from previous studies (Stiles et al 2002, Maggs et al 2003) in a number of ways. Earlier studies have examined the effect of bolus administration of lysine in small groups of SPF or random-source cats of narrow age, gender and genetic composition, experimentally infected with a single strain of only one of the organisms known to produce URD (FHV-1). Therefore, this study introduced a large number of variables intended to mimic clinical applications of this amino acid in multi-cat environments. The apparent lack of effect of this diet to limit signs of URD or presence of FHV-1 in the conjunctival fornix, despite significant elevations in plasma lysine concentration of supplemented animals must be assessed against this study design.
Mean total individual disease score over the whole study period was significantly higher for male cats fed the supplemented ration (1.28) than for those fed the basal diet (0.27). However, this difference in total individual disease score would be clinically manifest as only one of mild conjunctival hyperemia, mild serous nasal discharge, mild serous ocular discharge, mild blepharospasm, or sneezing, and therefore may not be clinically significant. Additionally, a dietary effect on clinical disease was not noted in female cats and the difference between total clinical scores was reduced to only 0.73 when data for both genders were combined. Regardless, these data are in contrast to results of a previous study (Stiles et al 2002) in which bolus lysine administration reduced clinical evidence of conjunctivitis in cats undergoing primary exposure to FHV-1. There are a number of potential explanations for these results. First, the presence of a second respiratory pathogen may have influenced the results of the present study. C felis DNA was not detected in any cats tested, making this organism unlikely, however, presence of other organisms including Mycoplasma felis and Bordetella bronchiseptica was not assessed. Also, because FCV antibodies were detected in the baseline serum sample of four cats and an additional five cats seroconverted during the study, primary infection with FCV or immune escape of latent FCV may have caused clinical disease in some cats. However, only one of the five cats that seroconverted for FCV antibodies during the study developed significant clinical illness. In addition, when all FCV seropositive cats were excluded from the analysis, group 4 still had the greatest mean total group clinical score. Unfortunately, no attempt was made to identify FCV in the present study. In future studies it would be optimal to prove FCV infection by culture or reverse transcription PCR. To the authors' knowledge, the in vitro or in vivo effects of lysine upon FCV have not been reported.
Second, the natural heterogeneity among and within these groups of cats may have produced a bias in our results not seen in studies with SPF cats. Throughout the study, marked differences in behavior were seen in male cats fed the lysine-supplemented diet. Cats were not stratified into groups according to temperament or history of URD as this was intended to reflect a clinically realistic multi-cat environment. Regardless of the basis for this difference, this group's behavior likely affected results of this study since stress is well known to alter shedding of FHV-1 (Gaskell and Povey 1977). This hypothesis is supported by the FHV-1 detection data generated in this study. Although FHV-1 DNA was detected significantly more frequently in cats receiving the supplemented (12 episodes; six cats) than those receiving the basal diet (one episode; one cat), viral DNA was detected only in male cats receiving the supplemented diet.
Finally, it is possible that plasma lysine concentrations achieved by the supplemented diet were insufficient to reduce signs of URD due to FHV-1. Because all cats in this study were group-housed, individual food intake, and therefore lysine intake, could not be recorded. However, dietary lysine intakes of adult cats eating a commercial dry expanded diet range from approximately 1 to 3 g lysine/day, with kittens eating approximately half this amount (Smalley et al 1985). Using this information, one can estimate that cats receiving a 500 mg bolus of lysine twice daily, while simultaneously eating to meet their energy needs would receive approximately 2–4 g lysine/day, with kittens eating approximately half this amount. In a previous study, there was no significant difference in food intake between cats consuming a diet containing 36 and 61 g lysine/kg diet/day (Fascetti et al 2004). The average±SEM lysine intake for these two dietary treatment groups was 2.8±0.4 and 4.1±0.5 g/day, respectively. One could hypothesize that adult cats eating the supplemented diet in the current study consumed somewhere between these two intakes, with the cats under 1 year of age consuming less. Therefore, the current study diet approximated the amount of lysine a cat receiving a 500 mg bolus twice daily and eating normally might consume.
In the present study, median plasma lysine concentration of cats fed the supplemented diet at a time when clinical signs and viral DNA detection rates were high (day 17) was 314 nmol/ml. This is similar to that achieved in a previous study, in which cats provided lysine as a once-daily oral bolus of 400 mg (309 nmol/ml) demonstrated decreased viral shedding but not a significant reduction in clinical disease scores (Maggs et al 2003). However, it is less than half the plasma lysine concentration reported for cats that demonstrated decreased severity of conjunctivitis when administered a twice-daily oral bolus of 500 mg lysine (669 nmol/ml) (Stiles et al 2002). When comparing plasma lysine concentrations among the three studies, it is important to note that in one study (Maggs et al 2003), plasma samples were obtained 3 h following lysine administration so as to assess approximate peak plasma lysine concentration. In the other study (Stiles et al 2002), time of sampling relative to lysine administration was not noted, and in the present study, cats were fed the lysine-supplemented diet ad libitum. Thus, there was likely wide variation in the elapsed time between lysine intake and plasma amino acid sampling among cats in the study reported here. Moreover, dietary lysine and arginine concentrations and food intake were not reported in the two previous publications, making it difficult to evaluate the contribution of diet in those studies (Stiles et al 2002, Maggs et al 2003). Regardless, it is possible that a critical plasma lysine concentration must be exceeded before clinical effects are detected, as has been demonstrated in humans infected with herpes simplex virus type 1 (Thein and Hurt 1984). If true, this may represent a problem inherent with dietary lysine supplementation for therapy of feline herpetic disease. While bolus administration of lysine can be continued during a period of diminished appetite, we have demonstrated in the present study that voluntary lysine intake decreases at this time in animals receiving dietary lysine supplementation because the time of peak total group disease scores for all four groups (approximately 17–22 days following rehousing) corresponded to a period of marked reduction in food intake.
The present study also permitted assessment of the safety of this lysine-supplemented diet and its effect upon plasma lysine and arginine concentrations in cats fed ad libitum and group-housed as is the case in many multi-cat environments where URD presents serious management and health problems. Previously, it had been demonstrated that as much as 86 g lysine/kg diet may be fed without any recognized adverse effects (Fascetti et al 2004). However, in that study, cats were individually housed and received the test diets for only 14 days. Secondly, only female cats were studied and only six cats were fed a diet containing lysine in quantities approximating those fed in the study reported here. Finally, cats in the previous study (Fascetti et al 2004) had a narrower range of age (2–10 years) and body weight (3–7.5 kg) than cats in the present study. Therefore, data from the present study provide insight into the safety of this lysine-supplemented diet when fed ad libitum for over 7 weeks to group-housed cats of both genders, and diverse age (5 months to 9.75 years) and body weight (2–7.1 kg). Although the arginine content of both diets formulated for this study was close to the National Research Council-recommended minimum requirement of 10 g/kg diet for cats, plasma arginine concentrations declined in both dietary groups throughout the study, and mean plasma arginine concentration was significantly decreased in cats receiving the supplemented diet compared with those receiving the basal diet. Despite this, no clinical evidence of arginine deficiency or change in body weight was noted in any cats from either dietary group. Previous work from our laboratory demonstrated a decrease in plasma arginine concentrations during a 14-day study in which adult cats were fed a diet containing a similar amount of arginine, however this change was not significant (Fascetti et al 2004). It is possible that the longer duration of the present study (52 days) revealed the significance of this change.
Another possible reason for decreased plasma arginine concentrations in cats fed a diet adequate in arginine is increased utilization of arginine in the urea cycle during the formation of urea from ammonia. Although the digestibility of the experimental diets used in this study was not determined, it is possible that some of the protein in the diet may not have been fully digested in the upper gastrointestinal tract and may have passed into the large intestine. This could have induced a change in the resident bacteria at this site with subsequent increased ammonia production. This may have been exacerbated by decreased voluntary food, and therefore arginine intake, by cats in all four groups at the time they demonstrated more severe clinical signs of URD.
The more notable decrease in plasma arginine concentration in cats receiving the supplemented diet and their failure following normalization of food intake to return to levels similar to those of the group receiving the basal diet may be attributed to additional arginine utilization during immune activation in cats experiencing more serious clinical signs (Barbul 1990, Kirk et al 1993). It may also reflect differences in amount of crude protein between the two diets. The additional dietary lysine (and therefore crude protein) in the supplemented diet presumably increased the amount of ammonia requiring metabolism via the urea cycle with associated consumption of additional arginine. A decline in plasma arginine concentration and orotic aciduria has been demonstrated in kittens fed diets with increasing concentrations of crude protein and limiting levels of essential amino acids (Taylor et al 1997). Unfortunately, urine was not collected in this study, so orotate excretion could not be evaluated. These observations make it difficult to draw a definitive conclusion regarding the safety of this diet and support the need for additional research to better understand the long-term impact of reduced plasma arginine concentrations on arginine status in the cats consuming this experimental diet.
Data from the present study raise important questions but do not permit a definitive conclusion regarding the efficacy of dietary lysine supplementation in cats with enzootic URD. Of major concern is the observation that food intake (and therefore lysine intake) decreases at the time when lysine supplementation is desired. Additionally, further assessment is required to examine the effects of lysine upon feline upper respiratory and ocular pathogens other than FHV-1. Finally, results of this study suggest that plasma arginine concentrations should be monitored in cats receiving protracted lysine supplementation. Further assessment of arginine metabolism in cats fed this experimental diet also is warranted.
Acknowledgements
The authors acknowledge the assistance of Ms Debbie Bee for animal care and Dr Zengshou Yu for analytical support. Supported in part by Nestlé Purina PetCare Research and by the Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis.
References
- Baker D.H. Comparative nutrition of cats and dogs, Annual Review of Nutrition 11, 1991, 239–263. [DOI] [PubMed] [Google Scholar]
- Barbul A. Arginine and immune function, Nutrition 6, 1990, 53–58. [PubMed] [Google Scholar]
- Fascetti A.J., Maggs D.J., Kanchuk M.L., Clarke H.E., Rogers Q.R. Excess dietary lysine does not cause lysine–arginine antagonism in adult cats, Journal of Nutrition 134, 2004, 2042S–2045S. [DOI] [PubMed] [Google Scholar]
- Gaskell R.M., Povey R.C. Re-excretion of feline viral rhinotracheitis virus following corticosteroid treatment, Veterinary Record 93, 1973, 204–205. [DOI] [PubMed] [Google Scholar]
- Gaskell R.M., Povey R.C. Experimental induction of feline viral rhinotracheitis virus re-excretion in FVR-recovered cats, Veterinary Record 100, 1977, 128–133. [DOI] [PubMed] [Google Scholar]
- Griffith R.S., DeLong D.C., Nelson J.D. Relation of arginine–lysine antagonism to herpes simplex growth in tissue culture, Chemotherapy 27, 1981, 209–213. [DOI] [PubMed] [Google Scholar]
- Griffith R.S., Norins A.L., Kagan C. A multicentered study of lysine therapy in herpes simplex infection, Dermatologica 156, 1978, 257–267. [DOI] [PubMed] [Google Scholar]
- Griffith R.S., Walsh D.E., Myrmel K.H., Thompson R.W., Behforooz A. Success of l-lysine therapy in frequently recurrent herpes simplex infection. Treatment and prophylaxis, Dermatologica 175, 1987, 183–190. [PubMed] [Google Scholar]
- Hickman M.A., Reubel G.H., Hoffman D.E., Morris J.G., Rogers Q.R., Pedersen N.C. An epizootic of feline herpesvirus, type 1 in a large specific pathogen-free cat colony and attempts to eradicate the infection by identification and culling of carriers, Laboratory Animals 28, 1994, 320–329. [DOI] [PubMed] [Google Scholar]
- Kirk S.J., Hurson M., Regan M.C., Holt D.R., Wasserkrug H.L., Barbul A. Arginine stimulates wound healing and immune function in elderly human beings, Surgery 114, 1993, 155–159. [PubMed] [Google Scholar]
- Lappin M.R., Andrews J., Simpson D., Jensen W.A. Use of serologic tests to predict resistance to feline herpesvirus 1, feline calicivirus, and feline parvovirus infection in cats, Journal of the American Veterinary Medical Association 220, 2002, 38–42. [DOI] [PubMed] [Google Scholar]
- Maggs D.J., Collins B.K., Thorne J.G., Nasisse M.P. Effects of l-lysine and l-arginine on in vitro replication of feline herpesvirus type-1, American Journal of Veterinary Research 61, 2000, 1474–1478. [DOI] [PubMed] [Google Scholar]
- Maggs D.J., Nasisse M.P., Kass P.H. Efficacy of oral supplementation with l-lysine in cats latently infected with feline herpesvirus, American Journal of Veterinary Research 64, 2003, 37–42. [DOI] [PubMed] [Google Scholar]
- Morris J.G., Rogers Q.R. Ammonia intoxication in the near-adult cat as a result of a dietary deficiency of arginine, Science 199, 1978, 431–432. [DOI] [PubMed] [Google Scholar]
- Nasisse M.P., Guy J.S., Davidson M.G., Sussman W.A., Fairley N.M. Experimental ocular herpesvirus infection in the cat. Sites of virus replication, clinical features and effects of corticosteroid administration, Investigative Ophthalmology and Visual Science 30, 1989, 1758–1768. [PubMed] [Google Scholar]
- National Research Council Nutrient Requirements of Cats, 1986, National Academy Press: Washington, DC, pp 10–11. [Google Scholar]
- Pearson H.E., Lagerborg D.L., Winzler R.J. Effects of certain amino acids and related compounds on propagation of mouse encephalomyelitis virus, Proceedings of the Society for Experimental Biology and Medicine 79, 1952, 409–411. [DOI] [PubMed] [Google Scholar]
- Smalley K.A., Rogers Q.R., Morris J.G. The nitrogen requirement of the weanling kitten, British Journal of Nutrition 53, 1985, 501–512. [DOI] [PubMed] [Google Scholar]
- Spitze A.R., Wong D.L., Rogers Q.R., Fascetti A.J. Taurine concentrations in animal feed ingredients; cooking influences taurine content, Journal of Animal Physiology and Animal Nutrition 87, 2003, 251–262. [DOI] [PubMed] [Google Scholar]
- Stiles J., Townsend W.M., Rogers Q.R., Krohne S.G. Effect of oral administration of l-lysine on conjunctivitis caused by feline herpesvirus in cats, American Journal of Veterinary Research 63, 2002, 99–103. [DOI] [PubMed] [Google Scholar]
- Sykes J.E., Studdert V.P., Anderson G., Browning G.F. Comparison of Chlamydia psittaci from cats with upper respiratory tract disease by polymerase chain reaction analysis of the ompA gene, Veterinary Record 140, 1997, 310–313. [DOI] [PubMed] [Google Scholar]
- Tankersley R.W. Amino acid requirements of herpes simplex virus in human cells, Journal of Bacteriology 87, 1964, 609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor T.P., Morris J.G., Kass P.H., Rogers Q.R. Increasing dispensable amino acids in diets of kittens fed essential amino acids at or below their requirement increases the requirement of arginine, Amino Acids 13, 1997, 257–272. [Google Scholar]
- Thein D.J., Hurt W.C. Lysine as a prophylactic agent in the treatment of recurrent herpes simplex labialis, Oral Surgery, Oral Medicine, and Oral Pathology 58, 1984, 659–666. [DOI] [PubMed] [Google Scholar]
- Wardley R.C., Povey R.C. The clinical disease and patterns of excretion associated with three different strains of feline caliciviruses, Research in Veterinary Science 23, 1977, 7–14. [PubMed] [Google Scholar]
- Weigler B.J., Babineau C.A., Sherry B., Nasisse M.P. High sensitivity polymerase chain reaction assay for active and latent feline herpesvirus-1 infections in domestic cats, Veterinary Record 140, 1997, 335–338. [DOI] [PubMed] [Google Scholar]