Abstract
The objectives of this study were to compare assay methods for plasma creatinine (Pl-creat) in cats and to describe the disposition of creatinine and iohexol in 12 healthy and moderately azotaemic cats. Exogenous creatinine and iohexol were injected simultaneously by intravenous bolus, and repeated blood samples were taken to determine the pharmacokinetic parameters of each marker. Pl-creat was assayed by high-performance liquid chromatography (HPLC), Jaffé and enzymatic methods. The enzymatic method was shown to be more reliable than the Jaffé method. Two stereoisomers, exo- and endo-iohexol were identified. The plasma clearance of creatinine (2.3±0.66 ml/min/kg) was significantly higher (P<0.001) than that of exo-iohexol (1.7±0.40 ml/min/kg). The volume of distribution (447±97 ml/kg) and elimination half-life (181±77 min) of creatinine were also higher (P<0.001) than those of exo- and endo-iohexol. The estimated daily endogenous production of creatinine was 65±23 mg/kg. None of the pharmacokinetic parameters was changed by the azotaemic status of the animals.
Plasma urea and creatinine concentrations (Pl-creat) are the routine markers used to evaluate renal function. Pl-creat is generally preferred to plasma urea as the concentration of this latter appears to be much more affected by extrarenal factors. However, it is currently admitted that Pl-creat only provides a rough indication of renal function as at least 70–75% of this has to be lost before values above the upper limit of the reference interval can be observed (Finco 1995, Braun et al 2003). The upper limit of the reference interval for Pl-creat is generally reported to be higher in cats than in dogs. However, to our knowledge, no rationally defined reference interval for creatinine has been published in the veterinary literature. The diagnosis of renal dysfunction in moderately azotaemic cats is a challenge in veterinary medicine. This is also true for dogs and results from the fact that Pl-creat is a hybrid parameter which depends not only on its clearance (ie, glomerular filtration rate, GFR) but also on its production by skeletal muscle and distribution in the body. It was shown in dogs with a 66%-decrease in GFR that Pl-creat only increased two-fold (the upper limit of the reference interval) because the endogenous production of creatinine was also decreased (Watson et al 2002). Thus, an understanding of creatinine disposition may be helpful in improving the clinical interpretation of Pl-creat. Paradoxically, no comparison of the different assay methods for Pl-creat (ie, Jaffé, enzymatic and high-performance liquid chromatography (HPLC)) has been carried out in cats. The objectives of this study were to (i) compare the assay methods for Pl-creat in cats, (ii) describe the disposition of creatinine in healthy and moderately azotaemic cats, and (iii) compare, in the same animals, the plasma clearance (Cl) of creatinine (which directly affects Pl-creat) with the Cl of iohexol (which has been proposed for GFR assessment in cats).
Materials and methods
Cats
Twelve client-owned adult cats (five neutered females and seven neutered males), aged 3–13 years old, and weighing 3.0–5.8 kg were selected for this prospective study. The breeds were European Shorthair (n=9), Persian (n=2) and Siamese (n=1). No concomitant treatment was given before or on the day of testing. The cats were divided into two groups: non-azotaemic (Pl-creat<180 μmol/l) and moderately azotaemic cats (180 μmol/l<Pl-creat<270 μmol/l) using the enzymatic method for plasma creatinine assay. Blood samples (3 ml) were taken just before GFR testing to obtain a complete plasma panel: sodium, potassium, chloride, calcium, phosphates, total proteins, urea, creatinine, glucose, cholesterol, alkaline aminotransferase (ALP) aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT), γ-glutamyltransferase (GGT) and lactate dehydrogenase (LDH). Each cat was weighed just before the test and after taking the last blood sample, and was observed for general health and hydration during this period. After testing, the clinical outcome of the animals was monitored for up to 4 years.
Iohexol and creatinine kinetics
The procedure was performed without premedication. Each cat was fasted overnight (at least 12 h) before the experimental procedure, and no food was given during the trial. Water was given ad libitum. The cats were hospitalised for the day just 1–2 h before administration of the test articles. An intravenous catheter was placed in the right cephalic vein. Iohexol (Omnipaque 300; Nycomed, Paris, France) (64.7 mg/kg, ie, 30 mg iodine/kg) was first injected, then creatinine (40 mg/kg of dry creatinine powder (Creatinine anhydrous; Sigma Chemical Co, St Louis, MO) mixed into 2.5 ml of sterile distilled water). After administration of the two test articles, 3 ml of NaCl 0.9% were injected to rinse the catheter. The total duration of these administrations was less than 1 min. One millilitre of blood was directly sampled from the jugular vein after 5, 15, and 30 min, and 1, 2, 3, 6, and 8 h. The blood was placed in a heparinised venoject tube and centrifuged (1000 g, 10 min, 4°C). Two aliquots (0.2 ml) of plasma were stored at −20°C until assayed.
Assays
Three different methods (enzymatic, Jaffé, and HPLC) were used to assay plasma creatinine concentration. An analyser (Ektachem 700 XR, Kodak, Johnson and Johnson Clinical Diagnostic Europe, Illkirch Graffenstaden, France) was used for the enzymatic method. The limit of quantitation was 4 μmol/l and the within- and between-day coefficients of variation were less than 1%.
For the determination of plasma creatinine by modified Jaffé method, 50 μl of plasma were placed in a 96-well plate with 50 μl of 8.8 mM picric acid mixed with 50 μl of 0.4 M NaOH. Calibration standards (5–200 μg/ml) and quality controls were treated in duplicate under the same conditions. The red picrate formed was subjected to spectrophotometric determination and assayed with a microplate reader (μQuant, Bio-Tek Instruments, Winooski, USA) at 492 nm after incubation for 1 h at room temperature. The quantitation limit of the assay was 44 μmol/l and within- and between-day precisions were less than 10%.
Plasma iohexol and creatinine concentrations were simultaneously determined by HPLC with a reverse phase column (Nucléosil C18, 5 μm), according to Laroute et al (1999). Briefly, the mobile phase was a mixture of methanol and phosphate buffer (90:10 v/v). The detection wavelength was at 254 nm. Plasma samples (50 μl) were deproteinised with acetonitrile. The limits of quantification in plasma for creatinine, endo-iohexol and exo-iohexol were 44 μmol/l, 0.8 μg/ml and 4.2 μg/ml, respectively. The coefficients of variation for repeatability and reproducibility were less than 10%.
Pharmacokinetic analysis
The plasma data for iohexol and creatinine were subjected to non-compartmental analysis using WinNonlin (Version 4.0.1, Pharsight, Mountain View, CA), as previously described in dogs (Laroute et al 1999, Watson et al 2002). The area under the curve (AUC) was determined using the trapezoidal rule with extrapolation to infinity. Cl was calculated by dividing the amount of tracer administered by the AUC (Cl=Dose/AUC). The steady state volume of distribution (Vss) and mean residence time (MRT) were determined with the usual pharmacokinetic equations (Gibaldi and Perrier 1982). The daily endogenous production of creatinine was determined by multiplying the Cl of creatinine by the area under the basal creatinine concentration curve over 24 h (Watson et al 2002).
Statistical analysis
Results are given as mean±SD. The GFR method and cat effects were tested using a general linear model (Systat version 8.0, SPSS Inc, Chicago, IL). Comparisons of non-azotaemic and azotaemic cats were based on the Student's t-test. A P value below 0.05 was considered significant. A Bland–Altman plot was used to compare the HPLC assay with either the enzymatic or Jaffé method. The differences between the two methods were plotted against the averages of the two techniques (Bland and Altman 1986).
Results
The clinical findings and outcome for each cat are presented in Table 1. Eight cats (cats 1, 3, 5, 6, 7, 8, 9, and 11) were clinically healthy. Six cats (cats 1, 3, 5, 6, 7 and 11) were considered non-azotaemic (Pl-creat: 144±27 μmol/l). There were no differences in age and body weight between the azotaemic and non-azotaemic cats (5.3±3.4 and 8.3±3.3 years, and 4.5±1.0 and 5.0±0.5 kg, respectively). The Pl-creat in the six azotaemic animals (cats 2, 4, 8, 9, 10, and 12) was 212±28 μmol/l (range: 188–263 μmol/l). Other abnormal plasma biochemistry findings were only observed in azotaemic cats. These were abnormally high plasma urea (>10.4 mmol/l) in cat 2, hypernatraemia (>157 mmol/l) in cats 8 and 10, hyperchloraemia (>128 mmol/l) in cats 8, 9, and 10, and hypercalcaemia (>2.9 mmol/l) in cat 10. The only significant difference (P<0.05) in plasma variables between azotaemic and non-azotaemic cats was observed for plasma phosphate (1.1±0.3 and 1.5±0.1 mmol/l, respectively). Only cat 2 died of chronic kidney disease (CKD) during the 4-year period. Urine was obtained by cystocentesis from two cats. Both cats have adequate urine concentrating ability and were not proteinuric. One cat was non-azotaemic (urine specific gravity, USG: 1.055), the other was azotaemic (USG: 1.042).
Table 1.
Basal creatinine concentration and clinical characteristics of the 12 cats
| Cat | Gender, age (year), BW (kg) | Basal creatinine (μmol/l) (azotaemic status) | Clinical condition/signs | Outcome (after 4 years) |
|---|---|---|---|---|
| 1 | NF, 4, 4.9 | 97 (NAZ) | Healthy | Healthy |
| 2 | NM, 9, 5.7 | 263 (AZ) | Weight loss, mild polyuria/polydipsia | Died from chronic renal failure 2.5 years later |
| 3 | NM, 12, 5.4 | 139 (NAZ) | Healthy | Fibrosarcoma 1 year later, hyperthyroidism 4 years later |
| 4 | NF, 8, 4.1 | 192 (AZ) | Chronic stomatitis | Chronic stomatitis |
| 5 | NM, 3, 4.7 | 163 (NAZ) | Healthy | Died from accident 1.5 years later |
| 6 | NM, 3, 4.8 | 131 (NAZ) | Healthy | Healthy |
| 7 | NF, 6, 4.6 | 159 (NAZ) | Healthy | Lost to follow-up |
| 8 | NF, 10, 4.4 | 188 (AZ) | Healthy | Healthy |
| 9 | NF, 3, 5.8 | 194 (AZ) | Healthy | Healthy |
| 10 | NM, 7, 3.0 | 221 (AZ) | Intermittent pollakiuria, struvite uroliths | Urethral obstruction 1 year later, stable 4 years later |
| 11 | NM, 4, 5.8 | 172 (NAZ) | Healthy | Healthy |
| 12 | NM, 13, 4.4 | 211 (AZ) | Digital basaloid tumour (surgical resection) | Relapse 5 months later and euthanasia |
N=neutered, M=male, F=female, BW=body weight, AZ=azotaemic, NAZ=non-azotaemic.
Creatinine concentration was assayed by enzymatic method.
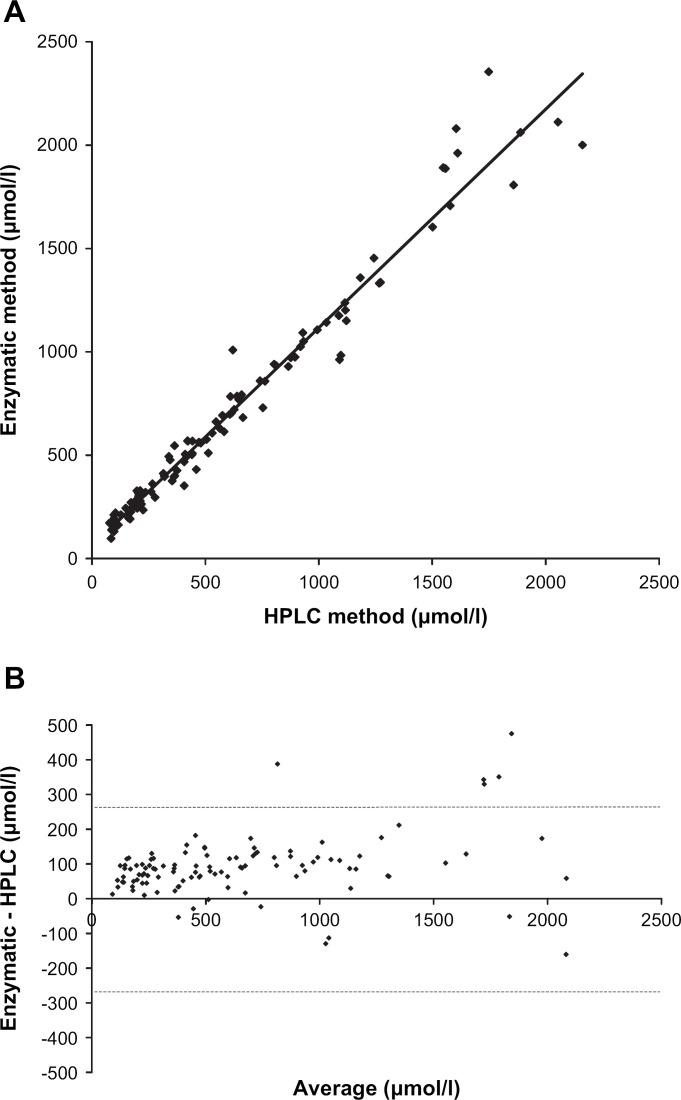
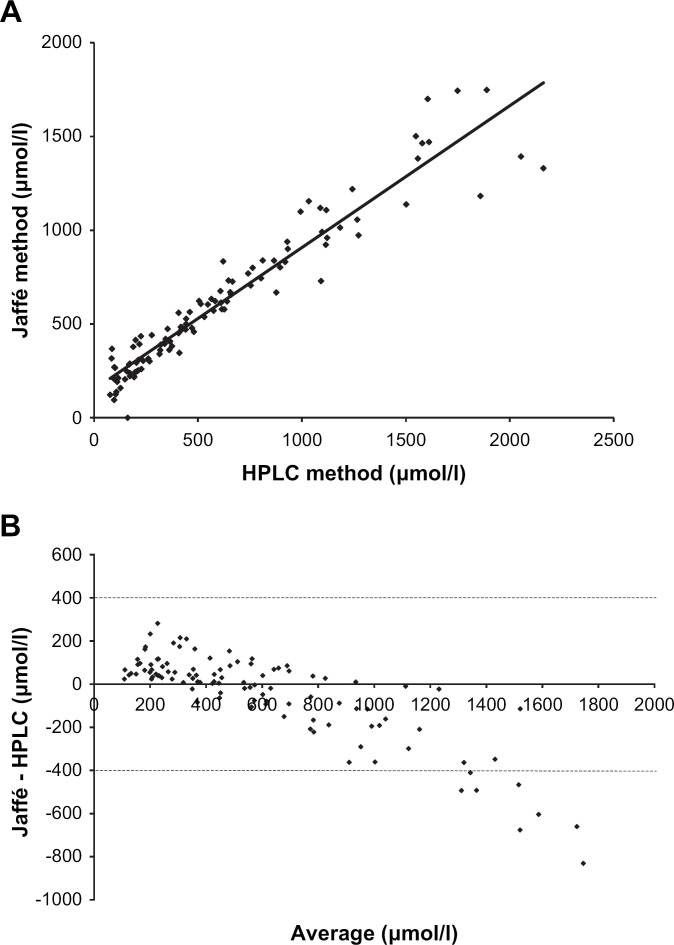
The GFR testing procedure was perfectly well tolerated in all animals and no adverse effect was noticed, or reported by the owner after the test. Body weight was significantly reduced (P<0.05) in nine out of 12 cats at the end of the testing procedure, but the maximal observed decrease was only 3.2% (ie, about 150 g) of the initial body weight. Plasma creatinine was assayed by three different methods (HPLC, Jaffé, and enzymatic) in all samples from all cats (ie, 108 different plasma samples with creatinine ranging from 97 to 2355 μmol/l (enzymatic method)). The plots of plasma creatinine assayed by either enzymatic or Jaffé method vs HPLC method are shown in Figs. 1A and 2A. The Bland–Altman comparisons of these methods of creatinine assay are shown in Figs. 1B and 2B. The enzymatic method was shown to slightly overestimate the creatinine concentrations assessed by HPLC method over the range of observed values. The Jaffé method, compared to the HPLC method, overestimated low creatinine concentrations and inversely underestimated the high creatinine concentrations.
Fig 1.
(A) Plasma creatinine concentrations assayed by enzymatic method plotted against the plasma creatinine concentrations assayed by the HPLC method. The linear regression equation was y=1.057x+60.40 (r2=0.97). The plasma creatinine values were observed in 12 cats following intravenous bolus administration of exogenous creatinine at a dose level of 40 mg/kg. (B) Bland–Altman plot of the differences between plasma creatinine assayed using the enzymatic and the HPLC methods against the average value for each plasma creatinine concentration. The plasma creatinine values were observed in 12 cats following intravenous bolus administration of exogenous creatinine at a dose level of 40 mg/kg. The dotted lines represent the mean difference plus and minus 1.96 times the standard deviation of the differences.
Fig 2.
(A) Plasma creatinine concentrations assayed by Jaffé method plotted against the plasma creatinine concentrations assayed by the HPLC method. The linear regression equation was y=0.756x+151.43 (r2=0.92). The plasma creatinine values were observed in 12 cats following intravenous bolus administration of exogenous creatinine at a dose level of 40 mg/kg. (B) Bland–Altman plot of the differences between plasma creatinine assayed using the Jaffé and the HPLC methods against the average value for each plasma creatinine concentration. The plasma creatinine values were observed in 12 cats following intravenous bolus administration of exogenous creatinine at a dose level of 40 mg/kg. The dotted lines represent the mean difference plus and minus 1.96 times the standard deviation of the differences.
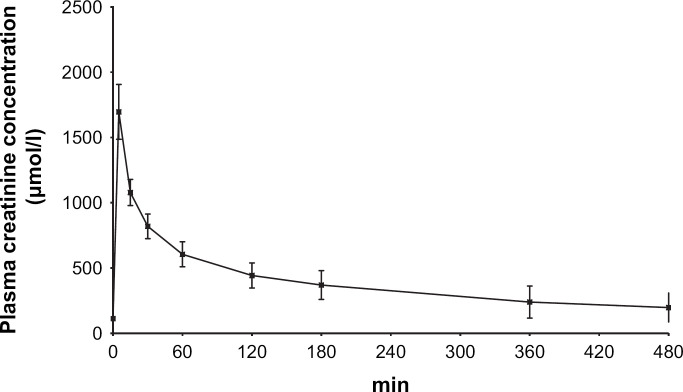
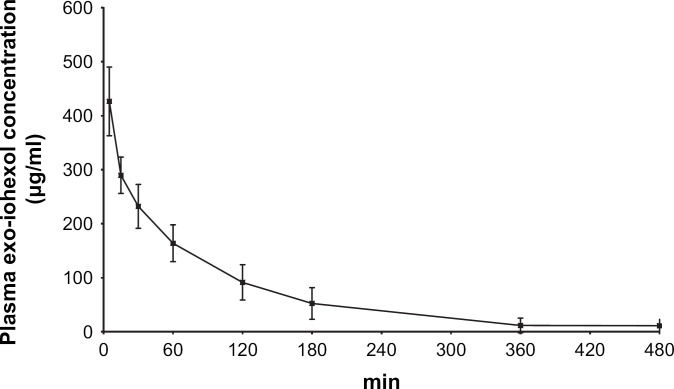
The plasma concentration vs time profiles for creatinine and exo-iohexol are presented in Figs 3 and 4. The corresponding pharmacokinetic parameters in non-azotaemic and azotaemic cats are given in Tables 2 and 3.
Fig 3.
Plasma creatinine concentration vs time profile (mean±SD) after intravenous bolus administration of exogenous creatinine at a dose level of 40 mg/kg in 12 cats.
Fig 4.
Plasma exo-iohexol concentration vs time profile (mean±SD) after intravenous bolus administration of iohexol at a dose level of 64.7 mg/kg in 12 cats.
Table 2.
Pharmacokinetic parameters (expressed as mean±SD) of creatinine (assayed using the enzymatic method) in 12 cats after intravenous bolus of creatinine at a dose level of 40 mg/kg
| Cl (ml/min/kg) | Vss (ml/kg) | MRT (min) | t1/2 (min) | |
|---|---|---|---|---|
| Whole population (n=12) | 2.3±0.66 | 447±97 | 222±106 | 181±77 |
| Non-azotaemic cats (n=6) | 2.3±0.73 | 475±105 | 226±94 | 184±68 |
| Azotaemic cats (n=6) | 2.2±0.64 | 420±89 | 217±126 | 177±92 |
Cl=plasma clearance, Vss=steady state volume of distribution, MRT=mean residence time, t1/2=elimination half-life.
Table 3.
Pharmacokinetic parameters (expressed as mean±SD) of exo-iohexol in 12 cats after intravenous bolus of iohexol at a dose level of 64.7 mg/kg
| Cl (ml/min/kg) | Vss (ml/kg) | MRT (min) | t1/2 (min) | |
|---|---|---|---|---|
| Whole population (n=12) | 1.7±0.40 | 148±22 | 96±32 | 74±23 |
| Non-azotaemic cats (n=6) | 1.8±0.32 | 153±26 | 86±14 | 66±10 |
| Azotaemic cats (n=6) | 1.5±0.45 | 144±18 | 106±43 | 82±30 |
Cl=plasma clearance, Vss=steady state volume of distribution, MRT=mean residence time, t1/2=elimination half-life.
For iohexol, two stereoisomers, exo-iohexol and endo-iohexol, were identified in the iohexol formulation and in the plasma after injection. Most of this (84%) was exo-iohexol. At 8 h, the plasma exo-iohexol concentrations were below the level of quantitation in eight out of 12 cats. For exo- and endo-iohexol, the percentage of AUC, which was extrapolated to infinity for calculation of the total AUC, was less than 11% in all cats. The Cls of exo- and endo-iohexol differed slightly but significantly from each other (1.7±0.4 vs 1.4±0.4 ml/min/kg; P<0.05).
The plasma creatinine concentrations (enzymatic assay) at 5 min and 8 h were 1910±244 and 263±86 μmol/l, respectively. The percentage of the AUC that was extrapolated was less than 17% for creatinine in all but two cats. The extrapolated portion in cats 2 and 6 was 35% and 27%, respectively. At 8 h, the plasma creatinine had returned to the pre-dosing level in only one cat. Similar assessments of plasma creatinine clearance were obtained by enzymatic (2.3±0.66 ml/min/kg) and HPLC (2.4±0.67 ml/min/kg) methods. The observed maximum difference in one cat was 25% but less than 15% in 10 cats. A significantly higher plasma creatinine clearance was obtained with the Jaffé assay (3.5±1.53 ml/min/kg, P<0.01), with differences up to 132% in one cat and exceeding 20% in nine cats.
The Cl of creatinine assessed by HPLC or enzymatic method was significantly higher (P<0.001) than the Cl of exo- and endo-iohexol. The Vss, MRT and elimination half-life (t1/2) of creatinine were also higher (P<0.001) than those of exo- and endo-iohexol, which were similar. None of the pharmacokinetic parameters of creatinine, exo- or endo-iohexol differed according to the azotaemic status of the cats. However, the lowest values of Cl for iohexol and creatinine (0.8 and 1.1 ml/min/kg, respectively) were observed in cat 2 which died of CKD 2.5 years later. The estimated endogenous production of creatinine was 65±23 mg/kg of body weight per day and did not change with azotaemic status.
Discussion
The animals selected in the present study were non-azotaemic and azotaemic cats. However, the degree of azotaemia in the latter was moderate, as the values were close to 180 μmol/l, the upper limit of the reference range provided by our laboratory or others (Elliott and Barber 1998). Renal dysfunction could not be confirmed from the azotaemic status, except for cat 2 which died of CKD. The concomitant abnormal biochemical findings (elevated plasma urea, chloride, calcium, sodium) observed in the azotaemic cats can be present in feline CKD. USG is reduced in cats with spontaneous CKD associated with moderate to severe azotaemia (Elliott and Barber 1998), but not in the early stages of induced renal dysfunction (Ross and Finco 1981). Consequently, cats with moderately elevated plasma creatinine, as shown here, can provide a challenge for the clinician as the origin of this abnormality cannot easily be explained by other concomitant findings. Unfortunately, blood pressure was not measured in these animals although azotaemia is frequently observed in hypertensive cats (Chetboul et al 2003). The subsequent outcome clearly confirmed the GFR results: the only cat which died from CKD during the 4 years was cat 2 which had the lowest plasma iohexol and creatinine clearance values.
This study is the first to compare methods for assaying plasma creatinine in cats. A wide range of plasma creatinine concentrations was generated in vivo by administering exogenous creatinine. The HPLC method was used as a reference method (Blijenberg et al 1994). The results of the present study clearly demonstrate that the Jaffé method is unsuitable for creatinine assay in cats. Although the Jaffé and HPLC methods were well correlated, the former, as shown by the Bland–Altman plot, overestimates the basal level of creatinine and may give false positive results. As previously mentioned in dogs (Balint and Visy 1965), the Jaffé method not only measures creatinine, but also non-creatinine chromogens. Inversely, for unclear reasons, the Jaffé method underestimates high concentrations of creatinine leading to an overestimation of plasma creatinine clearance. The enzymatic method slightly overestimated the results obtained by HPLC method but did not produce a major bias in the Cl estimation. This method thus provides a reasonable alternative to the HPLC method.
Apart from the analytical issue, it should also be kept in mind that Pl-creat is a hybrid parameter which depends on the production, distribution and elimination of the analyte. All these parameters which potentially affect Pl-creat in cats were investigated by carrying out a creatinine pharmacokinetics study. GFR was estimated over the same period by the simultaneous administration of iohexol.
The procedure was well tolerated and was performed in awake animals, as anaesthetic agents and tranquilisers may affect the disposition of the test articles. The total amount of blood sampled did not exceed 11 ml as only a very small volume of plasma was required for the assay. None of the cats showed any signs of dehydration and the change in body weight at the end of the test, although statistically significant, was negligible. The blood sampling strategy allowed the overall AUC for both iohexol and creatinine to be determined with adequate accuracy, as the extrapolated area of the AUC did not exceed 17% in most cats. Due to the longer half-life of creatinine, the extrapolated portion represented a higher percentage of the AUC in two cats. In previous studies with iohexol, the number of blood samples was lower, varying from three (Becker et al 2000, Miyamoto 2001a, b), to six (Brown et al 1996a), or seven (Haller et al 2003) post-dosing. The last blood sample was taken earlier at 180 min (Haller et al 2003), 195 min (Brown et al 1996a), or 240 min (Becker et al 2000, Miyamoto 2001a) and up to 360 min in azotaemic cats (Miyamoto 2001a, b). Generally, the higher the number of blood samples and the longer the sampling period, the better the accuracy of the AUC.
Plasma iohexol kinetics had already been used to assess GFR in cats (Brown et al 1996a, Meyer-Lindenberg et al 1998, Becker et al 2000, Miyamoto 2001a, b, Haller et al 2003). The Cl of iohexol varied from 1.8±0.56 ml/kg/min (Becker et al 2000) to 3.7 ml/min/kg (Miyamoto 2001a). Only the total iohexol was assessed in these previous studies. In our study, two stereoisomers of iohexol were identified by HPLC as previously described (Jacobsen 1982, Laroute et al 1999), exo-iohexol being the most important one in dogs. The Cl of endo-iohexol was about 20% lower than that of exo-iohexol. The Vss of exo-iohexol was 150 ml/kg, ie, similar to that determined in dogs (about 200 ml/kg) and close to the volume of the extracellular fluid. The MRTs and the half-lives of elimination in the two species were also similar (Laroute et al 1999).
This study is the first to provide a full description of creatinine disposition in cats. Plasma creatinine clearance in cats has, to our knowledge, only been investigated in one previous study (Miyamoto 1998). The Cl in 10 healthy cats was 4.2±1.0 ml/min/kg. The author concluded that the plasma creatinine clearance approach did not provide an accurate indication of GFR because the extrapolated portion extending from the last blood sampling time (240 min post-dosing) to infinity was 27–40%. Moreover, the assay was performed with the Jaffé method which overestimates the Cl. In comparison with dogs (Watson et al 2002), the Cl and Vss of creatinine in cats are about 25% lower, but the t1/2 is similar in both species. The Cl of creatinine in the present study was very close to values reported by other authors for GFR measurements based either on urine creatinine clearance (Russo et al 1986, Rogers et al 1991, Brown et al 1996a, b, Deguchi and Akuzawa 1997) or on the Cl of other GFR markers (Russo et al 1986, Rogers et al 1991, Uribe et al 1992, Brown et al 1996b, Meyer-Lindenberg et al 1998, Haller et al 2003). Moreover, glomerular filtration is the sole component of renal creatinine excretion in this species (Finco and Barsanti 1982). However, the 35% discrepancy between plasma creatinine clearance and iohexol clearance remains unexplained. Further investigation is needed in cats to compare plasma exogenous creatinine clearance with reference methods such as urine inulin or creatinine clearance. Nevertheless, the Cl was not affected by azotaemic status for either marker. The daily creatinine production rate in the present study was about 50% higher than in dogs (43±5 mg/kg/day) and showed greater inter-individual variability (35% vs 12%). The higher basal level of creatinine observed in cats compared to dogs results from the lower Cl, smaller Vss and greater production rate. Interestingly, it was observed in our study that a change in production rate could induce an increase in plasma concentration while the Cl of creatinine was unaffected. For example, the plasma creatinine clearances in cats 3 and 10 were similar (2.5 and 2.3 ml/min/kg, respectively) whereas the basal plasma creatinine levels were quite different (139 and 221 μmol/l). The reason for this discrepancy was that the creatinine production rate in cat 10 (83 mg/kg/day) was 46% higher than in cat 1 (57 mg/kg/day). Such differences may explain why moderately azotaemic cats can have normal renal function.
In conclusion, the reason why Pl-creat may be moderately elevated in cats while the GFR remains unaltered can be explained from the creatinine pharmacokinetics. Our results also emphasise the need to refine the reference values for Pl-creat currently used in felines.
Acknowledgement
This study was supported by Novartis Animal Health.
References
- Balint P., Visy M. ‘True creatinine’ and ‘pseudocreatinine’ in blood plasma of the dog, Acta Physiologica Acadaemia Scientifica Hungaria 1965 (28), 1965, 265–272. [Google Scholar]
- Becker T.J., Graves T.K., Kruger J.M., Braselton W.E., Nachreiner R.F. Effects of methimazole on renal function in cats with hyperthyroidism, Journal of the American Journal of Animal Hospital Association 2000 (36), 2000, 215–223. [DOI] [PubMed] [Google Scholar]
- Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement, Lancet i, 1986, 307–310. [PubMed] [Google Scholar]
- Blijenberg B.G., Brouwer H.J., Kuller T.J., Leeneman R., van Leeuuwen C.J.M. Improvement in creatinine methodology: a critical assessment, European Journal of Clinical Chemistry and Clinical Biochemistry 32, 1994, 529–537. [DOI] [PubMed] [Google Scholar]
- Braun J.P., Lefebvre H.P., Watson A.D. Creatinine in the dog: a review, Veterinary Clinical Pathology 32, 2003, 162–179. [DOI] [PubMed] [Google Scholar]
- Brown S.A., Finco D.R., Boudinot F.D., Wright J., Tarver S.L., Cooper T. Evaluation of a single injection method, using iohexol, for estimating glomerular filtration rate in cats and dogs, American Journal of Veterinary Research 57, 1996a, 105–110. [PubMed] [Google Scholar]
- Brown S.A., Haberman C., Finco D.R. Use of plasma clearance of inulin for estimating glomerular filtration rate in cats, American Journal of Veterinary Research 57, 1996b, 1702–1705. [PubMed] [Google Scholar]
- Chetboul V., Lefebvre H.P., Pinhas C., Clerc B., Boussouf M., Pouchelon J.L. Spontaneous feline hypertension: clinical and echocardiographic abnormalities, and survival rate, Journal of Veterinary Internal Medicine 17, 2003, 89–95. [DOI] [PubMed] [Google Scholar]
- Deguchi E., Akuzawa M. Renal clearance of endogenous creatinine, urea, sodium, and potassium in normal cats and cats with chronic renal failure, Journal of Veterinary Medical Sciences 59, 1997, 509–512. [DOI] [PubMed] [Google Scholar]
- Elliott J., Barber P.J. Feline chronic renal failure: clinical findings in 80 cases diagnosed between 1992 and 1995, Journal of Small Animal Practice 39, 1998, 78–85. [DOI] [PubMed] [Google Scholar]
- Finco D.R. Evaluation of renal functions. Osborne C.A., Finco D.R. Canine and Feline Nephrology and Urology, 1995, Williams & Wilkins: Baltimore, 216–229. [Google Scholar]
- Finco D.R., Barsanti J. Mechanism of urinary excretion of creatinine by the cat, American Journal of Veterinary Research 43, 1982, 2207–2209. [PubMed] [Google Scholar]
- Gibaldi M., Perrier D. Pharmacokinetics, 2nd edn, 1982, Marcel Dekker: New York. [Google Scholar]
- Haller M., Rohner K., Müller W., Reutter F., Binder H., Estelberger W., Arnold P. Single-injection inulin clearance for routine measurement of glomerular filtration rate in cats, Journal of Feline Medicine and Surgery 5, 2003, 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen T. The preclinical development of iohexol (Omnipaque): a new X-ray contrast medium, Farmakotherapi 38, 1982, 45–57. [Google Scholar]
- Laroute V., Lefebvre H.P., Costes G., Toutain P.L. Measurement of glomerular filtration rate and effective renal plasma flow in the conscious beagle dog by single intravenous bolus of iohexol and p-aminohippuric acid, Journal of Pharmacological and Toxicological Methods 41, 1999, 17–25. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Westhoff A., Wohlsein P., Pohlenz J., Nolte I. Die Messung der glomerulären Filtrationrate (GFR) nach Jodkontrastmittelgabe mit dem Renalyzer PRX90 bei gesunden und nierenkranken Katzen, Berliner Münchener Tierarztliche Wochenschrift 111, 1998, 344–351. [PubMed] [Google Scholar]
- Miyamoto K. Evaluation of single-injection method of inulin and creatinine as a renal function test in normal cats, Journal of Veterinary Medical Science 60, 1998, 327–332. [DOI] [PubMed] [Google Scholar]
- Miyamoto K. Clinical application of plasma clearance of iohexol on feline patients, Journal of Feline Medicine and Surgery 3, 2001a, 143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K. Use of plasma clearance of iohexol for estimating glomerular filtration rate in cats, American Journal of Veterinary Research 62, 2001b, 572–575. [DOI] [PubMed] [Google Scholar]
- Rogers K.S., Komkov A., Brown S.A., Lees G.E., Hightower D., Russo E.A. Comparison of four methods of estimating glomerular filtration rate in cats, American Journal of Veterinary Research 52, 1991, 961–964. [PubMed] [Google Scholar]
- Ross L.A., Finco D.R. Relationship of selected clinical renal function tests to glomerular filtration rate and renal blood flow in cats, American Journal of Veterinary Research 42, 1981, 1704–1710. [PubMed] [Google Scholar]
- Russo E.A., Lees G.E., Hightower D. Evaluation of renal function in cats, using quantitative urinalysis, American Journal of Veterinary Research 47, 1986, 1308–1312. [PubMed] [Google Scholar]
- Uribe D., Krawiec D.R., Twardock A.R., Gelberg H.B. Quantitative renal scintigraphic determination of the glomerular filtration rate in cats with normal and abnormal kidney function, using 99mTc-diethylenetriaminepentaacetic acid, American Journal of Veterinary Research 53, 1992, 1101–1107. [PubMed] [Google Scholar]
- Watson A.D.J., Lefebvre H.P., Concordet D., Laroute V., Ferre J.P., Braun J.P., Conchou F., Toutain P.L. Plasma exogenous clearance test in dogs: comparison with other methods and proposed limited sampling strategy, Journal of Veterinary Internal Medicine 16, 2002, 22–33. [DOI] [PubMed] [Google Scholar]