Abstract
Medical records (2005–2009) of a feline-only practice were searched for cats with degenerative joint disease (DJD) treated using meloxicam. DJD was diagnosed by the presence of at least two of the following: (i) altered mobility (observed by the owner), (ii) abnormal physical findings, (iii) characteristic radiographic changes. The primary study cohort consisted of cats older than 7 years that had received meloxicam for variable intervals in excess of 6 months, and for which complete records were available. These cats were subdivided according to whether detectable chronic kidney disease (CKD) was present (‘renal group’), or not (‘non-renal group’), and, for the ‘renal group’, according to the cat's IRIS category. Serum biochemistry, urinalysis (including urine specific gravity [USG]), body mass and condition score were monitored regularly. Progression of CKD in the ‘renal group’ and ‘non-renal group’ of cats was compared to two groups of age- and IRIS-matched control cats not receiving meloxicam (from the same clinic, over the same time period). The study was thus a case—control design, with two study groups. Thirty-eight cats with DJD receiving long-term meloxicam therapy met the inclusion criteria. Of these, 22 cats had stable CKD at the start of treatment (stage 1, eight cats; stage 2, 13 cats; stage 3, one cat). No cats initially had an elevated urinary protein to creatinine ratio. The remaining 16 cats initially had normal renal analytes and adequately concentrated urine. The median age of the ‘renal’ and ‘non-renal’ meloxicam groups was 15.5 and 13.4 years, respectively. The median treatment duration was 467 days in the ‘renal group’ and 327 days in the ‘non-renal group’. After titration (to the lowest effective dose), the median maintenance dose was 0.02 mg/kg/day in both groups (range 0.015–0.033 mg/kg/day). There was no difference in sequential serum creatinine concentration or USG measurements between the ‘non-renal group’ treated with meloxicam compared to control cats not treated with meloxicam. There was less progression of renal disease in the ‘renal group’ treated with meloxicam compared to the age- and IRIS-matched cats with CKD not given meloxicam. These results suggest that a long-term maintenance dose of 0.02 mg/kg of meloxicam can be safely administered to cats older than 7 years even if they have CKD, provided their overall clinical status is stable. Long-term meloxicam therapy may slow the progression of renal disease in some cats suffering from both CKD and DJD. Prospective studies are required to confirm these findings.
Degenerative joint disease (DJD) encompasses osteoarthritis (OA) of appendicular joints and intervertebral disc degeneration, leading to spondylosis deformans (SD). These conditions affect many cats, especially older cats. 1 Some 17–22% of cats are affected to a varying extent.2–4 Recent work from Lascelles's group suggests these figures are likely an underestimate, and that 91% of a carefully selected group of cats representing ages stratified from 6 months to 20 years have radiographic evidence of DJD in at least one joint. 5 SD refers to the development of bony spurs on the vertebral endplates, adjacent to the intervertebral disc. Radiographic features of SD were reported in 15% of cats from a general hospital population, 3 while evidence of SD at necropsy was detected in 67% of cats with a median age of 10.5 years. 6 SD may or may not be associated with clinically-appreciable back pain. There is a significant association between age and prevalence of DJD disease; a retrospective study of cats ≥12 years (mean age 15.2 years) found 90% had radiographic signs of DJD, predominantly OA. 2
Although the radiological prevalence of these disorders in the cat is unambiguous, characterisation and recognition of clinical features associated with them is problematic. Cats with DJD present with a wide range of signs namely difficulty in jumping or a reduction in the height of the jump, lameness or stiffness. Behavioural and lifestyle changes such as altered demeanour, reduced grooming, resentment of handling and inappropriate or painful voiding may be evident also.5,7–9 These conditions impact substantially on a cat's lifestyle and often require long-term treatment to reduce suffering and improve quality of life. 10 Meloxicamis currently the only non-steroidal anti-inflammatory drug (NSAID) licensed for long-term therapy in cats.
Chronic kidney disease (CKD) is very common in cats and impaired kidney function is listed as a contraindication or warning on all NSAID data sheets. In one retrospective study, 53% of cats affected with CKD were older than 7 years while in another survey 15% of cats ≥15 years had impaired renal function.11,12 The prevalence of feline CKD is considered to increase with age,12,13 with most cases lacking a specific aetiology and survival time is variable.13–15 As chronic painful musculoskeletal conditions and CKD are both common in aged cats, the conditions often co-exist.
The objective of this retrospective study was to investigate the long-term use (≥6 months) of meloxicam to treat painful DJD in aged cats, and particularly to ascertain the effects of this treatment on renal function in cats with and without pre-existing overt CKD.
Materials and methods
The computerised database (Ciderhouse Veterinary Software, 63 Monkhouse Drive Endeavour Hills Vic 3802, Australia) of a feline-only practice in inner Melbourne was searched over a 4-year period for cats which had been given long-term meloxicam (Metacam oral suspension 1.5 or 0.5 mg/ml, Boehringer Ingelheim) as therapy for DJD. A presumptive diagnosis of DJD (OA or SD) was made based upon the presence of any two of the following: (i) owner-noted mobility changes (particularly ability or willingness to jump), (ii) physical findings (pain/discomfort, crepitus, restricted range of motion, joint effusion) or (iii) characteristic radiological signs. 2 Changes in jumping behaviour, such as a reluctance to jump or reduced height of jump, were considered key indicators of musculoskeletal pain. A diagnosis was confirmed if there was a beneficial response to a short trial of meloxicam (7–14 days therapy).
To be included in the primary study cohort, cats had to: (1) be older than 7 years; (2) receive meloxicam continuously for longer than 6 months; (3) have complete medical notes with serial serum biochemistry analysis, urinalysis, body mass and condition scores recorded. Inclusion criteria specifically targeted cats treated continuously for greater than 6 months, as data was already available concerning the safety and efficacy of meloxicam given for shorter periods. 7 No cats were excluded from either the treatment or control groups (see below) because of pre-existing or emerging renal conditions. Data collection, inclusion and exclusion of patients and identification of control cats were carried out by the first author (RG). Patients were treated by either of the first two authors (RG, AL).
Age, breed, gender, co-morbidities, concurrent medications, the date therapy commenced, treatment duration and daily dose(s) of meloxicam were recorded. The principal study cohort (n = 38 cats) was further subdivided (see Table 1) on the basis of whether CKD was evident (‘renal group’ — group A; n = 22), or not (‘non-renal group’ — group B; n = 16). The presence or absence of pre-treatment renal disease was determined in cats considering physical findings, sonographic examination, the serum or plasma creatinine concentration, urine protein:creatinine (UPC) and urine specific gravity (USG). Serum creatinine concentration and USG were considered in the assessment of CKD staging according to established International renal interest society (IRIS) recommendations. 16
Table 1.
Study design with explanation of the different groups within the study.
| Study group | Older than 7 years | Number of cats | CKD | IRIS category | DJD | Meloxicam therapy |
|---|---|---|---|---|---|---|
|
| ||||||
| A | Yes | 22 | Yes | 8 IRIS 1 | Yes | Yes |
| 13 IRIS 2 | ||||||
| 1 IRIS 3 | ||||||
| B | Yes | 16 | No * | N/A † | Yes | Yes |
| C ‡ | Yes | 22 | Yes | 8 IRIS 1 | Variable ‖ | No |
| 13 IRIS 2 | ||||||
| 1 IRIS 3 | ||||||
| D § | Yes | 16 | No * | N/A ‡ | Variable ‖ | No |
N/A = not applicable.
No CKD does not mean that subclinical renal disease was not present; only that creatinine concentration was within the RI, USG was >1.038 and kidneys were normal on palpation and using sonography.
These cats may have had renal disease, and indeed it was likely many did, but it was subclinical if present.
Group C is the control for group A.
Group D is the control for group B.
A proportion of these cats had DJD but were not treated with meloxicam due to insufficient severity of DJD, concurrent medication, or lack of owner consent.
Stage 1: inadequate urine concentrating ability in the absence of azotaemia. Creatinine< 140 μmol/l; USG< 1.038.
Stage 2: mild clinical signs associated with CKD. Creatinine 140–250 μmol/l; USG < 1.030.
Stage 3: many clinical signs present. Creatinine 251–440 μmol/l; USG < 1.020.
An age- and renal status (IRIS)-matched control group of 38 cats was chosen at random from 97 cats treated in the same clinic and meeting similar inclusion criteria as the primary study cohort (ie, age, complete records, sequential monitoring, etc) except they had not been treated with meloxicam. This group of control cats was further subdivided according to whether they had chronic kidney disease (‘renal control group’ — group C; n = 22), or not (‘non-renal control group’ — group D; n = 16) (Table 2). Nine cats in the control groups had DJD but were precluded from receiving meloxicam due to either the lack of severity of DJD(one cat), concurrent medications (two cats), or due to co-morbidities and/or owner-related concerns (six cats).
Table 2.
Comparison of the groups at enrolment.
| Group | (A) | (B) | (C) | (D) |
|---|---|---|---|---|
|
| ||||
| Number of cats | 22 | 16 | 22 | 16 |
| Gender (male/female) | 9/13 | 7/9 | 11/11 | 9/7 |
| Median age (years) | 15.5 | 13.4 | 14.3 | 12.3 |
| Median treatment dose (mg/kg/day) | 0.02 | 0.02 | Nil | Nil |
| IRIS stage 1 (number of cats) | 8 | N/A | 8 | N/A |
| IRIS stage 2 (number of cats) | 13 | N/A | 13 | N/A |
| IRIS stage 3 (number of cats) | 1 | N/A | 1 | N/A |
| Median creatinine concentration | 160 | 130 | 160 | 130 |
| [25th—75th percentile] (μmol/l) | [140–200] | [120–140] | [130–190] | [115–145] |
| Median USG | 1.023 | 1.050 | 1.025 | 1.050 |
| Median bodyweight (kg) | 4.9 | 5.1 | 4.2 | 4.6 |
| Median BCS | 5.5 | 6 | 4 | 5 |
N/A = not applicable.
Progression of renal disease in the ‘renal group’ and ‘non-renal group’ of cats receiving meloxicam was compared to their respective control groups from the same clinic over the same time frame (ie, A vs C; B vs D). Thus, the experimental design was in effect a case—control study, but with two distinct arms. Table 3 shows the comparison of concomitant treatments between the meloxicam-treated renal group (A) and meloxicam-treated non-renal group (B); and the untreated renal control group (C) and untreated non-renal control group (D).
Table 3.
Concurrent medications administered to cats receiving meloxicam.
| Concurrent medication | Number of cats (group A) | Number of cats (group B) | Number of cats (group C) | Number of cats (group D) |
|---|---|---|---|---|
|
| ||||
| Glucosamines * | 9 | 8 | 5 | 1 |
| Pentosan polysulphate * | 9 | 8 | 3 | 1 |
| Neomercazole/I131 † | 4/2 | 5/1 | 4/0 | 6/0 |
| Amlodipine ‡ | 8 | 3 | 10 | 0 |
| Tramadol | 2 | 1 | 0 | 0 |
| Cisapride | 2 | 0 | 2 | 0 |
| Insulin glargine | 2 | 0 | 0 | 1 |
| Phenobarbitone | 1 | 0 | 1 | 0 |
| Cobalamine | 1 | 0 | 0 | 0 |
| Benazepril | 1 ‖ | 0 | 1 § | 3 § |
| Famciclovir | 0 | 0 | 1 | 0 |
| Prednisolone | 0 | 0 | 2 | 5 |
| Potassium gluconate | 0 | 0 | 2 | 0 |
| No concurrent medication | 3 | 1 | 5 | 3 |
| Chlorambucil | 0 | 0 | 0 | 2 |
| Frusemide | 0 | 0 | 0 | 2 |
Treatment for DJD.
Treatment for hyperthyroidism.
Treatment for hypertension. One cat in group A had untreated hypertension.
For renal hypertension or congestive heart failure.
Benazepril treated cat for heart failure from a cardiomyopathy.
The majority of serum biochemistry determinations was performed at a private commercial laboratory (Idexx Locked Bag 15, Mt Waverley, VIC 3149 — NATA accredited 10166) whose feline reference interval (RI) for creatinine concentration was 80–200 μmol/l. The remainder were measured using an in-house Idexx dry chemistry analyser, using an ion specific electrode for electrolyte determinations. The creatinine RI for the Idexx machine was said to be 71–212 μmol/l. In-house USG was determined using a Reichert Vet360 refractometer, which is temperature compensated and provides a separate scale calibrated for cat urine. Urine sediment examination and reagent strip determinations were carried out in-house. Routine aerobic urine culture and susceptibility testing and UPC determinations were done at the external laboratory. Blood pressure was measured routinely using a Parkes Doppler device and an appropriately sized cuff. 17
The clinic protocol for management of all cats with CKD included: (i) use of canned and dry ‘prescription’ kidney diets, (Hills K/D canned and dry; Royal Canin Renal support diet sachets and dry formula), (ii) the use of aluminium hydroxide as an enteric phosphate binder (Alu-Tab; 3M Pharmaceuticals, 30–60 mg/kg q 12 h), if the cats had IRIS stage 1 or 2 and serum phosphate levels of greater than 1.6 μmol/l or IRIS stage 3 and serum phosphate levels greater than 1.8 μmol/l, (iii) treatment of serious periodontal disease (extractions, scaling, polishing and antimicrobials), (iv) control of hypertension (using amlodipine), and (v) eradication of urinary tract infections (UTIs), as required. All patients had initial urinary protein estimations by both reagent strips (in-house) and UPC determinations (at the laboratory). Once concurrent disease conditions (hypertension, UTI, periodontal disease) were well managed, the patient was re-assessed as part of a regular senior health screening programme and to decide whether it was considered sufficiently ‘stable’ to commence meloxicam therapy.
The majority of cats in the current study had treatment initiated prior to the release of the feline specific meloxicam formulation, and therefore were treated with doses comparable to those used by Clarke and Bennett and Gunew et al,7,18 generally with titration down to the minimal effective dose. Owners were educated to give meloxicam ideally with or after a meal, and not to use the medication if the cat seemed unwell, became inappetent or developed vomiting or diarrhoea. In this manner, owners were educated to not use the medication if the patient was potentially dehydrated. The need for regular monitoring and veterinary check-ups was emphasised. Meloxicam therapy was started using a median initial dose of 0.05 mg/kg (range 0.01–0.2 mg/kg). If a loading dose was used on the first day of treatment (0.1–0.2 mg/kg) then the dose was reduced to the licensed maintenance dose 24 h later. At a pre-determined time, usually 7–14 days after starting therapy, owners were contacted for a report on their cat's mobility and well-being. Some patients, especially those with CKD, were re-assessed in the clinic at this time, using serum creatinine, USG, bodyweight and body condition score (BCS) to assess renal status. Improvements in mobility typically relied upon the owner's assessment of frequency, ease and willingness of their cat to jump. Owners often also perceived that their cat was ‘happier’, livelier and more interactive. Based on this feedback and other pertinent considerations, regular rechecks were scheduled every 3 or 6 months. Unfortunately, because of the retrospective nature of the study, although serial laboratory determinations were available, they were invariably not at set time points in relation to the commencement of meloxicam therapy.
Each client was advised as to potential benefits of using lower than the registered dosage of meloxicam. Based on risk:benefit perception and response to initial dose rates, the dose was titrated down to the lowest effective dose, as assessed by the owner, over several weeks. The usual suggested next dose was 50% of the starting dose, based on lean bodyweight. If the owners noted a continued positive effect, they further reduced the dose to 0.1 mg of meloxicam suspension per cat (corresponding to two drops per cat of the 1.5 mg/ml oral solution or the 2 kg dose on the calibrated dosing syringe of the licensed product for cats).
Statistical analysis
The age of the cats in each treatment group, treatment duration and maintenance dose of meloxicam were calculated using a commercial software package (SAS System; SAS Institute, Cary, North Carolina, USA, version 8.2). Values were expressed as medians, interquartile ranges (IQR) and total ranges. Renal analytes in groups A and C, and B and D, were compared. Statistical analysis was carried out using a time adjusted area-under-the-curve (AUC) changes from base-line time 0 until the last recorded value (n). 19
t0: time point of first measurement (baseline).
tn: time point of last measurement.
Ci: difference of analyte concentration at time point i = 0,…, n to baseline.
The Wilcoxon rank-sum-test was used to make comparisons between the groups. With this nonparametric test, the distribution of the adjusted AUC of two groups was compared regarding the location. Under the null hypothesis, it is assumed that there is no location shift in the distributions of the two treatment groups. P-values lower than the two-sided significance level of 0.05 resulted in the null hypothesis being rejected.
For the analysis of the difference between the initial and final BCS in each group the one-sample t-test was used.
Results
From a client database of 3016 cats, 214 cats were prescribed at least one bottle of meloxicam oral suspension. Of these 214, 38 cats met the inclusion criteria for the meloxicam-treated groups (A and B). Of these 38 cats, 22 had CKD diagnosed prior to treatment, while CKD was not evident in the remaining 16 cats. No cats with stable CKD had elevated UPC ratios. Table 2 provides a comparison of the treatment groups at enrolment, Table 3 shows the range of drugs administered to cats within the study cohort, while Table 4 shows comparison of concurrent medical conditions at enrolment. Table 5 shows the comparison of the groups at the end of the study period.
Table 4.
Concurrent medical conditions at enrolment.
| Concurrent disease | Group A CKD | Group B | Group C CKD | Group D |
|---|---|---|---|---|
|
| ||||
| No other medical conditions | 5 | 8 | 6 | 5 |
| Hyperthyroidism | 6 | 6 | 4 | 6 |
| Hypertension | 9 | 3 | 10 | 0 |
| Diabetes | 2 | 0 | 0 | 1 |
| FIV | 1 | 1 | 1 | 1 |
| Neoplasia | 1 | 1 | 0 | 1 |
| Epilepsy | 1 | 0 | 1 | 0 |
| Megacolon/severe constipation | 2 | 0 | 0 | 0 |
| UTI | 1 | 0 | 0 | 0 |
| Glaucoma | 1 | 0 | 0 | 0 |
| Hypokalaemia | 0 | 0 | 2 | 0 |
| Chronic feline herpesvirus-1 rhinitis | 0 | 0 | 1 | 0 |
| Systemic lupus erythematosus | 0 | 0 | 0 | 1 |
| Inflammatory bowel disease | 0 | 0 | 1 | 2 |
| Chronic bronchitis | 0 | 0 | 0 | 1 |
| Congestive heart failure | 1 | 0 | 0 | 2 |
Table 5.
Comparison of the groups at the end of the study.
| Group | (A) | (B) | (C) | (D) |
|---|---|---|---|---|
|
| ||||
| Number of cats | 22 | 16 | 22 | 16 |
| Gender of cats (male/female) | 9/13 | 7/9 | 11/11 | 9/7 |
| Median treatment dose (mg/kg/day) | 0.02 | 0.02 | Nil | Nil |
| IRIS stage 1 (number of cats) | 3 | 2 | 2 | 1 |
| IRIS stage 2 (number of cats) | 16 | 2 | 11 | 6 |
| IRIS stage 3 (number of cats) | 2 | 0 | 6 | 1 |
| IRIS stage 4 (number of cats) | 1 | 0 | 3 | 2 |
| Median creatinine concentration [25th—75th percentile] (μmol/l) | 170 [150–220] | 150 [110–170] | 240 [160–360] | 155 [150–190] |
| Median USG | 1.018 | 1.045 | 1.015 | 1.029 |
| Median bodyweight (kg) | 4.7 | 5.0 | 3.8 | 4.6 |
| Median BCS | 5 | 5.5 | 3 | 4 |
| Median duration of therapy/follow-up (days) | 467 | 327 | 475 | 648 |
There was no statistically significant difference in baseline in bodyweight or USG between group A and C cats, or between group B and D cats, as might be expected for carefully matched control groups. The median BCS of group A cats was, however, significantly greater than the BCS for group C cats (5.4 vs 4.0; P = 0.005 Wilcoxon-rank-sum-test); likewise, BCS for group B cats was higher than group D cats (6 vs 5; P = 0.03). Although the median age of the group A cats (with DJD and CKD; 15.5 years) was greater than the median age (13.4 years) of group B cats (with DJD but not CKD), the difference was not significant (P = 0.09). The median treatment duration was 467 days for group A cats, compared to 327 days for group B cats. After dose titration to the lowest effective dose, the median maintenance dose for meloxicam was 0.02 mg/kg daily for both treatment groups (A and B), with a range of 0.015 and 0.033.
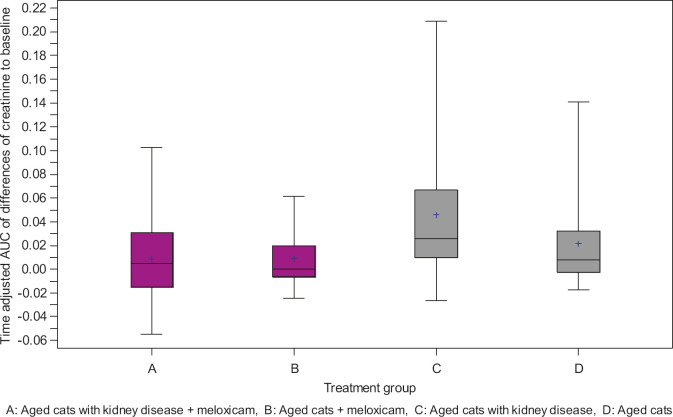
Meloxicam had no detectable deleterious impact on renal function in either of the treated groups. Interestingly, median serum creatinine concentrations increased more slowly over time in cats with CKD treated with meloxicam, (group A; median 160–170 μmol/l,) than in ‘control’ CKD cats (group C; median 160–240 μmol/l) not receiving meloxicam(P = 0.014). There was no statistical difference, however, in sequential serum creatinine concentrations between the ‘non-renal group’ treated with meloxicam (group B; median 130–150 μmol/l) compared to control cats not treated with meloxicam (group D; median 130–155 μmol/l) (P = 0.417). Figure 1 shows box and whisker plots of time adjusted AUC of differences in creatinine to baseline.
Fig 1.
Box and whisker plots of time adjusted AUC of differences in creatinine to baseline. The box encompasses the IQR, the median is the line within the box, while whiskers define the entire range of values.
Therewere no significant differences in urine concentrating ability. Thus, median USG decreased over time in cats with CKD treated with meloxicam (group A; 1.023–1.018) and in ‘control’ CKD cats (group C; 1.025–1.015) not receiving meloxicam (P = 0.632). Similarly, there was no difference in sequential USG measurements between the ‘non-renal group’ treated with meloxicam (1.050–1.045) compared to control cats not treated with meloxicam (1.050–1.029) P = 0.137.
It was presumed that many of the older cats in the non-CKD groups may actually have had subclinical CKD, and indeed many of these cats were classified as having CKD during the course of the study (see Table 5). Therefore, we repeated the analysis by pooling the cats treated with meloxicam (groups A and B; n = 38) and the cats not treated with meloxicam (groups C and D; n = 38). Again, the serum creatinine concentrations in cats given meloxicam increased significantly less (P = 0.019) than in cats not given the drug.
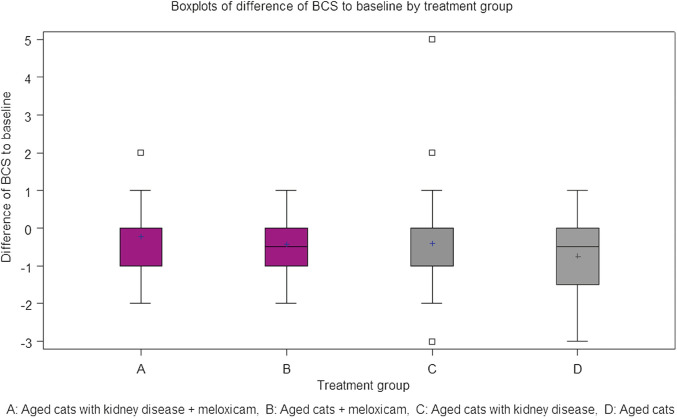
Finally, for all groups, BCS declined over the course of the study (Fig 2), as might be expected for a group of elderly cats with various co-morbidities, including CKD. The decline in BCS appeared similar across the four study groups. A vs group C cats, respectively (0.23; 0.44; P = 0.26). B vs group D cats respectively (0.41; 0.75; P = 0.53). Indeed, groups A and B, if anything, fared better than the corresponding control groups C and D.
Fig 2.
Box and whisker plots of time adjusted AUC of differences in BCS to baseline in the various study cohorts over the course of the trial.
Discussion
The principal aim of this retrospective study was to determine whether meloxicam could be safely given to cats with DJD and concurrent stable CKD, without compromising renal function further. Taken as a whole, the data strongly suggests that meloxicam has no adverse impact on the progression of renal dysfunction, at least when administered cautiously in a carefully selected cohort of CKD patients. It is interesting to note that cats with CKD receiving meloxicam may have actually fared better, on average, than the ‘control’ cats with CKD not receiving meloxicam.
The dose adjustment approach, based on a subjective measure of efficacy, resulted in a very similar maintenance dose (0.02 mg/kg), to 0.05 mg per cat used by Clarke and Bennett, 0.01–0.03 mg/kg in Gunew et al or 0.05 mg/kg every other day advocated recently by Lascelles and Robertson. This is currently an ‘off-label dosage’, as the licensed maintenance dose is 0.05 mg/kg/day; the dose required to control pain in the acutely painful sodium urate model of acute synovitis. 20
Renal function is partially dependent on prostaglandins which regulate vascular tone (and thus intrarenal blood flow) and electrolyte and water homeostasis. The risk of renal damage following NSAID use is considered to be highest in animals that have concurrent hypovolaemia or hypotension, as such patients are dependent on locally produced prostaglandins to maintain renal perfusion. Therefore, an effect of renal cyclooxygenase (COX) inhibition by NSAID is expected under such circumstances. There is no evidence to date that increasing COX-2 selectivity improves the renal safety of a given NSAID. Unfortunately, there is no published information about the distribution of the isoforms of the COX enzyme and their function in the cat kidney. Furthermore, there is no evidence to indicate that well hydrated cats with CKD are more dependent on renal prostaglandins to maintain renal perfusion than normal healthy cats.
The potential nephrotoxicity of meloxicam has been investigated in many species. The pharmacokinetics of meloxicam remained unchanged in humans with kidney disease. 21 Radiolabelling demonstrated that the route of excretion of meloxicam in the cat was predominantly faecal, with 21% of meloxicam recovered in urine, primarily as inactive metabolites. 22 Accumulation of metabolites due to renal insufficiency is unlikely to be problematic, because metabolites of meloxicam in the cat are the same as identified in other species,22,23 which have individually been shown to be pharmacologically inactive and specifically to have no direct nephrotoxicity. 23 Meloxicam is a potent inhibitor of prostaglandin synthesis at sites of inflammation, but a weak inhibitor of ‘constitutional’ prostaglandin synthesis in the gut and kidney.24–26 In mandatory safety studies carried out prior to the registration of Metacam oral suspension, daily administration of meloxicam to young healthy cats at one, three and five times recommended maintenance dose given for 3 months was associated with no impact on renal analytes, USG or histology of renal specimens collected at necropsy. 27 Thus, a priori, there is no reason to expect renal function to deteriorate in cats during chronic meloxicam therapy over and above what would be expected due to natural progression of the CKD. At the recommended maintenance dose there were no gastrointestinal changes, however, at three and five times the maintenance dose, given daily for 3 months, there were gastrointestinal changes typical of a NSAID.
This study demonstrated that long-term use of meloxicam caused no deterioration in any routine biochemical measures used to assess renal function in aged cats without detectable CKD (group B). It was of great interest that administration of meloxicam to a carefully selected group of aged cats with stable CKD (group A) appeared to minimise further deterioration in indicators of renal function, compared to a control population of cats with CKD not receiving meloxicam (group C; see Fig 1), ie, median serum creatinine concentrations increased significantly more slowly in group A cats (10 μmol/l) compared to group C cats (80 μmol/l) (P = 0.014). Although this finding will be of great comfort to the owners of cats with CKD requiring NSAID therapy for DJD, it must be emphasised that there was some selection bias in determining whether cats received meloxicam or not. A concerted effort was made to ensure that meloxicam was only administered to cats with stable CKD, and that bacterial UTI, renal hypertension and periodontal disease were attended to prior to commencing meloxicam. ‘Stable’ kidney disease was established by ruling out pre-renal and post-renal azotaemia and by demonstrating minimal changes in creatinine and bodyweight in the 4–8 weeks prior to instituting meloxicam therapy. 16 In addition patients were required to be in good body condition (BCS ± 5), 28 with attentive owners in order to be considered candidates for meloxicam therapy. The ‘control’ group of cats was matched based on age and IRIS stage, and Table 4 demonstrates that the concurrent medical conditions were also fairly evenly matched. However, due to the retrospective nature of this study and the number of co-morbities present in a population of aged cats, the groups could not be perfectly evenly matched by presence of, or severity of, the concurrent diseases.
The age- and IRIS-matched control group (C) was selected to demonstrate the expected rate of progression of kidney disease in older cats using the clinic's standard kidney disease management protocol. Cats chosen for this control group were aged patients for which serial serum biochemistry and urinalysis data were available. Typically, they had sequential monitoring because of concurrent medical conditions, which may or may not have had a negative impact on the progression of their renal disease.
Although nine cats (eight in group C and one in group D) in the control groups were determined to have DJD they were not treated with meloxicam because of insufficient severity of DJD at the time (one cat, group D), concurrent medication (one benazepril, one prednisolone), or lack of owner consent (six cats). Of the six cats without owner consent to treat, only one cat had concurrent disease considered by the attending clinician to be too unstable to warrant treatment with a NSAID. The inclusion of these cats would have impacted on the median values for renal analytes and BCS over the course of the study, and may have accounted for some of the differences observed between groups A and C. However, the rate of rise in creatinine in group A cats appears qualitatively less than reported in other studies of cats with CKD, for example those looking at the impact of ‘prescription diets’, angiotensin-converting enzyme (ACE) inhibitors or anti-hypertensive agents.13,15
The use of ACE inhibitors and/or diuretics together with NSAIDs has been shown in human medicine to significantly increase the risk of nephrotoxicity. This is known as the ‘double or triple whammy’ effect. 29 ACE inhibitors are recommended for the treatment of proteinuric CKD in cats. However, in this study, none of the cats with CKD had significantly elevated UPC values (≥0.4) at enrolment, consistent with other reports that proteinuric glomerulopathy is rare (or transient) in the cat.30,31 Only one cat in group A received an ACE inhibitor, but this was prescribed for cardiomyopathy diagnosed 2 years prior to starting meloxicam. The results of the present study should not, therefore, be extrapolated to cats with CKD receiving concurrent ACE inhibitor therapy.
Why might institution of meloxicamtherapy slow deterioration in renal function in cats with stable CKD?; (1) It could be argued that the effect was indirect, and a consequence of improved mobility and overall ‘quality of life’ subsequent to pain relief. This might result in a better appetite (for prescription diets), increased water consumption and improved mobility leading to an overall increase in caloric intake, better hydration and reduced tissue catabolism. Increased lean body mass in cats receiving meloxicam would, however, tend to increase both skeletal muscle creatinine generation and serum creatinine concentration but this was not our finding. (2) Another possibility is that the beneficial effect of meloxicam is the result of a direct effect on the kidney, with a reduction in the on-going interstitial inflammation and fibrosis that would otherwise cause further deterioration in renal function. Recent studies have shown that COX-2 selective NSAIDs can reduce proteinuria in humans and rats with stable glomerulonephropathy, without reduction of blood pressure.32–34 As proteinuria has been shown to play an important role in the progression of renal disease in the cat and chronic renal injury can be mediated by prostaglandins, NSAIDs may indeed exhibit a renoprotective effect.30,35,36 The hypothesis that chronic inflammation in the renal interstitium of cats with CKD may be reduced bymeloxicamis worthy of further prospective analysis, to confirm and extend the present findings in other settings, and to better characterise underlying phenomena.
Although non-blinded, this retrospective study provides a case—control design and reflects patient management that is practical in a small animal clinic. As the inclusion criteria selected for cats that had been treated for longer than 6 months, it could be argued that the study was biased by including only cats which could tolerate long-term meloxicam and excluding patients whose renal analytes or clinical status were deleteriously affected by meloxicam in the first 6 months of treatment. However, analysis of the 214 cats treated in this clinic with meloxicam for any duration did not identify any cats which fulfilled the other inclusion criteria but had treatment terminated due to a negative impact on CKD.
A further limitation of our study is the absence of long-term survival data. At the time of writing (June 2010) only eight cats in the CKD-meloxicam group (A) had been euthanased, and critically none because of renal failure. It is hoped long-term survival data will be available subsequently in a follow-up publication.
Conclusion
The present work demonstrates that when administered with care, long-term therapy with meloxicam at a median dose of 0.02 mg/kg/day can be administered safely to aged cats with CKD, provided they are clinically stable. The results further suggest that meloxicam may actually slow the progression of renal disease in cats with both DJD and CKD by direct or indirect mechanisms. Clearly more work is needed to confirm and extend these observations. The present data supports the use of meloxicam to treat aged cats with painful musculoskeletal disorders, irrespective of whether or not they have CKD. However, patients should be carefully selected and regular and judicious monitoring of cats treated with meloxicam is of course mandatory.
Conflict of interest
Laura Johnston and Wibke Stansen are employees of Boehringer Ingelheim. Richard Malik is one of an expert panel convened by ISFM to develop consensus guidelines for the long-term use of NSAIDs in cats. Scott Brown has served as a consultant for Boehringer Ingelheim Vetmedica, Inc.
Acknowledgements
Richard Malik is supported by the Valentine Charlton Bequest of the Centre for Veterinary Education of the University of Sydney.
References
- 1.Lascelles BD. Feline degenerative joint disease. Vet Surg 2010; 39: 2–13. [DOI] [PubMed] [Google Scholar]
- 2.Hardie EM, Roe SC, Martin FR. Radiographic evidence of degenerative joint disease in geriatric cats: 100 cases (1994–1997). J Am Vet Med Assoc 2002; 220: 628–32. [DOI] [PubMed] [Google Scholar]
- 3.Clarke SP, Mellor D, Clements DN, et al. Prevalence of radiographic signs of degenerative joint disease in a hospital population of cats. Vet Rec 2005; 157: 793–9. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey DR. Osteoarthritis in cats: a retrospective radiological study. J Small Anim Pract 2005; 46: 425–9. [DOI] [PubMed] [Google Scholar]
- 5.Lascelles BD, Robertson SA. DJD-associated pain in cats: what can we do to promote patient comfort? J Feline Med Surg 2010; 12: 200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Read RM, Smith RN. A comparison of spondylosis deformans in the English and Swedish cat and in the English dog. J Small Anim Pract 1968; 9: 159–66. [DOI] [PubMed] [Google Scholar]
- 7.Gunew MN, Menrath VH, Marshall RD. Long-term safety, efficacy and palatability of oral meloxicam at 0.01–0.03 mg/kg for treatment of osteoarthritic pain in cats. J Feline Med Surg 2008; 10: 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett D, Morton C. A study of owner observed behavioural and lifestyle changes in cats with musculoskeletal disease before and after analgesic therapy. J Feline Med Surg 2009; 11: 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson SA, Lascelles BD. Long-term pain in cats: how much do we know about this important welfare issue? J Feline Med Surg 2010; 12: 188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sparkes AH, Heiene R, Lascelles BD, et al. ISFM and AAFP consensus guidelines: long-term use of NSAIDs in cats. J Feline Med Surg 2010; 12: 521–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dibartola SP, Rutgers HC, Zack PM, Tarr MJ. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984). J Am Vet Med Assoc 1987; 190: 1196–202. [PubMed] [Google Scholar]
- 12.Lulich JP, Osborne CA, O'Brien TD, Polzin DJ. Feline renal failure: questions, answers, questions. Compend Contin Educ Pract Vet 1992; 14: 127–52. [Google Scholar]
- 13.Elliott J, Barber PJ. Feline chronic renal failure: clinical findings in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998; 39: 78–85. [DOI] [PubMed] [Google Scholar]
- 14.Lefebvre HP, Toutain PL. Angiotensin-converting enzyme inhibitors in the therapy of renal diseases. J Vet Pharmacol Ther 2004; 27: 265–81. [DOI] [PubMed] [Google Scholar]
- 15.Ross SJ, Osborne CA, Kirk CA, Lowry SR, Koehler LA, Polzin DJ. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. J Am Vet Med Assoc 2006; 229: 949–57. [DOI] [PubMed] [Google Scholar]
- 16.International Renal Interest Society Staging of CKD 2006; http://www.iris-kidney.com/guidelines/en/staging_ckd.shtml; (accessed Mar 12, 2010).
- 17.Sparkes AH, Caney SM, King MC, Gruffydd-Jones TJ. Inter- and intraindividual variation in Doppler ultrasonic indirect blood pressure measurements in healthy cats. J Vet Intern Med 1999; 13: 314–8. [DOI] [PubMed] [Google Scholar]
- 18.Clarke SP, Bennett D. Feline osteoarthritis: a prospective study of 28 cases. J Small Anim Pract 2006; 47: 439–45. [DOI] [PubMed] [Google Scholar]
- 19.Gibaldi M, Perrier D. Pharmacokinetics. 2nd edn. New York: Marcel Dekker, 1982. [Google Scholar]
- 20.Carroll G, Narbe R, Kerwin S, Taylor L, Peterson K, Hartsfield S. Dose range-finding study for the efficacy of meloxicam in cats administered prior to sodium urate-induced arthritis. Vet Anaesth Analg 2010; 38: 394–406. [DOI] [PubMed] [Google Scholar]
- 21.Boulton-Jones JM, Geddes CG, Heinzel G, Türck D, Nehmiz G, Bevis PJ. Meloxicam pharmacokinetics in renal impairment. Br J Clin Pharmacol 1997; 43: 35–40. [DOI] [PubMed] [Google Scholar]
- 22.Grudé P, Guittard J, Garcia C, Daoulas I, Thoulon F, Ebner T. Excretion mass balance evaluation, metabolic profile analysis and metabolite identification in plasma and excreta after oral administration of [14C]-Meloxicam to the male cat. J Vet Pharmacol Ther 2010; 33: 396–407. [DOI] [PubMed] [Google Scholar]
- 23.Engelhardt G, Trummlitz G. Biological activity of the main metabolites of meloxicam. Drugs Exp Clin Res 1990; 16: 53–6. [PubMed] [Google Scholar]
- 24.Engelhardt G, Homma D, Schlegel K, Utzmann R, Schnitzler C. Anti-inflammatory, analgesic, antipyretic and related properties of meloxicam, a new nonsteroidal anti-inflammatory agent with favourable gastrointestinal tolerance. Inflamm Res 1995; 44: 423–33. [DOI] [PubMed] [Google Scholar]
- 25.Engelhardt G. Pharmacology of meloxicam, a new nonsteroidal anti-inflammatory drug with improved safety profile through preferential inhibition of COX-2. Br J Rheumatol 1996; 35: 4–12. [DOI] [PubMed] [Google Scholar]
- 26.Pairet M, van Ryn J, Schierok H, Mauz A, Trummlitz G, Engelhardt G. Differential inhibition of cyclooxygenases −1 and −2 by meloxicam and its 40-isomer. Inflamm Res 1998; 47: 270–6. [DOI] [PubMed] [Google Scholar]
- 27.Metacam® EPAR Scientific Discussion http://www.ema.europa.eu/vetdocs/PDFs/EPAR/metacam/032397en6.pdf (accessed Mar 12, 2010).
- 28.LaFlamme DP, Kealy RD, Schmidt DA. Estimation of body fat by body condition score [Abstract]. J Vet Intern Med 1994; 8: 154. [Google Scholar]
- 29.Loboz KK, Shenfield GM. Drug combinations and impaired renal function — the ‘triple whammy’. Br J Clin Pharmacol 2005; 59: 239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syme HM, Markwell PJ, Pfeiffer D, Elliott J. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med 2006; 20: 528–35. [DOI] [PubMed] [Google Scholar]
- 31.King JN, Tasker S, Gunn-Moore DA, Strehlau G. BENRIC Study Group. Prognostic factors in cats with chronic kidney disease. J Vet Intern Med 2007; 21: 906–16. [PubMed] [Google Scholar]
- 32.Gonçalves AR, Fujihara CK, Mattar AL, et al. Renal expression of COX-2, ANG II, and AT1 receptor in remnant kidney: strong renoprotection by therapy with losartan and a nonsteroidal anti-inflammatory. Am J Physiol Renal Physiol 2004; 286: 945–54. [DOI] [PubMed] [Google Scholar]
- 33.Vogt L, de Zeeuw D, Woittiez AJ, Navis G. Selective cyclooxygenase-2 (COX-2) inhibition reduces proteinuria in renal patients. Nephrol Dial Transplant 2009; 24: 1182–9. [DOI] [PubMed] [Google Scholar]
- 34.Gluhovschi G, Velciov S, Kaycsa A, et al. The effect on proteinuria and urinary NAG of treatment with meloxicam in chronic glomerular disease patients — a preliminary study. Int J Clin Pharmacol Ther 2009; 47: 444–53. [DOI] [PubMed] [Google Scholar]
- 35.King JN, Gunn-Moore DA, Tasker S, Gleadhill A, Strehlau G. Benazepril in renal insufficiency in Cats Study Group. Tolerability and efficacy of benazepril in cats with chronic kidney disease. J Vet Intern Med 2006; 20: 1054–64. [DOI] [PubMed] [Google Scholar]
- 36.Syme HM. Proteinuria in cats. Prognostic marker or mediator? J Feline Med Surg 2009; 11: 211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]