Abstract
Seventeen cats were presented with acute onset of complex partial seizures with orofacial involvement (salivation, facial twitching, lip smacking, chewing, licking or swallowing), motor arrest (motionless starring) and behavioural changes. In 11 cats hippocampal necrosis (HN) was confirmed by histopathology. In a further six cats hippocampal changes were suggested by magnetic resonance imaging. The mean monitoring time of eight cats which were not euthanased in the acute phase of the disease, was 408 days (60–908): four cats are still alive. In all surviving cases, the owners reported a good quality of life. We conclude that an acute cluster of complex partial seizures with orofacial involvement are often associated with HN and that HN is not necessarily a fatal condition. Supportive and antiepileptic therapy can result in remission. The long-term outcome can be good to excellent; therefore, euthanasia should be avoided in the acute phase of the signs.
In humans, the diagnosis of epilepsy is dependent upon a very detailed and accurate history. The ictal clinical signs (semiology) are of great importance, as this is a key feature for classification for epileptic syndromes. 1 The meticulous semiological history is not only important for the diagnosis, but it allows the determination of the region of the brain from which the seizure arises. 2 Earlier publications mentioned a specific partial seizure type in cats, describing as ‘twitching limited to the face’ 3 or ‘glazed look with facial twitching’ 4 but the aetiology has never been investigated separately. In recent years we have observed a high number of cats with different kinds of seizures with different aetiology. 5 A group of cats, however, showed unique and very similar clinical presentation. The main aim of the study was to summarise the results of work-up, therapy and outcome in this group of cats.
Materials and methods
The medical database of our clinic was analysed for the following inclusion criteria in cats: (1) acute onset of complex partial seizures (CPSs) with orofacial signs (salivation, facial twitching, lip smacking, chewing, licking and swallowing), (2) repeated over 24 h, (3) complete work-up including brain magnetic resonance imaging (MRI) or pathohistology. Seventeen cats met the inclusion criteria. The diagnostic work-up in all cases included signalment and medical history, physical examination, neurological examination, haematology and serum biochemistry. Ancillary diagnostic tests included dynamic bile acid tests (12), activity of acethylcholinesterase (11), feline leukaemia virus (FeLV) antigen and feline T4 concentration (eight), cerebrospinal fluid (CSF) analysis (13), electroencephalography (EEG) (one), MRI (0.23 Tesla, Outlook, Gold Performance, Philips Medizinische Systeme, Vienna, Austria) of the brain (six) and pathological examination of the animal, including pathohistology of the brain (11). For recording of the EEG (Micromed, Italy), subcutaneous needle electrodes and an eight-channel montage were used according to Redding. 6
Results
All patients were domestic shorthair cats. There was no gender predisposition including eight male (seven neutered) and nine female spayed cats. The mean age of onset of clinical signs was 6.5 years (range 1–11 years). The mean body weight was 3.97 kg (range 3.5–4.8 kg). Four cats were outdoor cats and 13 were indoor cats.
All cats showed acute onset of CPSs with confusion and orofacial signs. The orofacial signs were salivation, facial twitching, lip smacking, chewing, licking and swallowing with some variation. Typically the first reported sign was unusual behaviour. Patients started staring motionless often in a sitting position, often considered as ‘looking in the air’ by the owner (video available as Supplementary data). These short episodes were followed by twitching of the facial muscle uni- or bilaterally or by jawing and swallowing for few seconds to 1 min. The majority of cats showed excessive salivation as well (video available as Supplementary data). Usually cats stayed in a sitting position during these episodes and seemed not to be aware of the environment. Other signs, such as mydriasis, secondary generalised seizures, vocalisation, defecation and urination were also commonly observed (Table 1). Interictal and postictal behavioural changes were commonly reported by the owner and observed during hospitalisation. The signs are summarized in Table 1.
Table 1.
Ictal, interictal and postictal signs and abnormalities at the first clinical presentation of cats with ‘FEPSO-HN’.
| Ictal signs | Number of cats |
|---|---|
|
| |
| Orofacial motor signs | 17 |
| Mydriasis | 14 |
| Arrest (motionless starring) | 14 |
| Salivation | 13 |
| Secondary generalised seizure | 12 |
| Vocalisation | 10 |
| Stress-induced episodes | 10 |
| Urination | 8 |
| Defecation | 5 |
| Interictal and postictal signs | |
| Behavioural changes | 14 |
| Aggression | 9 |
| Ataxia | 6 |
| Rapid running | 4 |
| Blindness/deafness | 2 |
| Weakness | 2 |
| Polyphagia | 2 |
| Polydipsia | 2 |
| Circling | 2 |
| Abnormalities at the first clinical presentation | |
| Aggression | 12 |
| Decreased menace response | 11 |
| Confusion | 8 |
| Weakness | 6 |
| Apathy | 5 |
| Elevation of body temperature | 5 |
| Salivation | 3 |
| Ataxia | 3 |
| Head turn | 3 |
| Circling | 2 |
At the first presentation in 12 cats physical and neurological examinations were abnormal and the most common clinical and neurological changes were: aggression, decreased menace response, confusion, weakness, apathy, elevation of body temperature (above 39.2°C) (Table 1).
Two cats showed moderately elevated liver enzymes and two others showed a mild elevation of creatinine (2 mg/dl and 2.1 mg/dl; reference level <1.6). Elevation of creatine kinase activity was detected in 11 cases and was considered as a consequence of muscle twitching. Otherwise, the blood work was normal in all cats.
CSF analyses were performed in 13 cats and only one showed mild pleocytosis (9 cells/ μl) with mononuclear predominance. The amount of protein was normal in both cases (13 mg/dl and 23 mg/dl). EEG was performed in one case and visual analysis could not detect single spikes, spike complexes, spikewave complexes, sharp waves or slow waves.
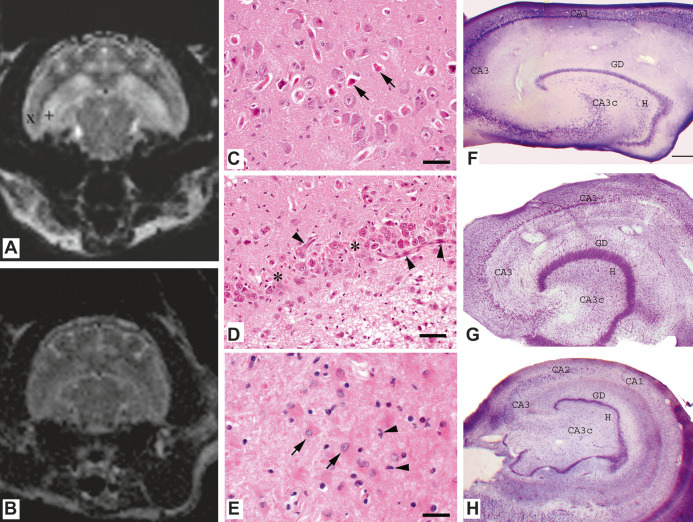
Six cats underwent brain MRI. One patient had a follow-up MR study, 14 months after an initial MR study. MRI of all cats showed bilateral hippocampal T1 hypo/isointensity and T2 hyperintensity (Fig 1A). Mild heterogenic hippocampal contrast enhancement was seen in one cat. The follow-up study performed in one animal did not show progression of disease.
Fig 1.
A/B. (A) Transverse T2 weighted MR images of patient with hyperintense lesions, restricted to the hippocampus (+) and other extrahippocampal regions, such as the piriform lobe (x) can be seen. (B) The same image of a control cat. (C—E). Histology of a cat hippocampus, HE. (C) Neuronal loss and neuronal necrosis (arrows), bar = 50 μm. (D) Neuronal loss (asterisks), extensive capillary proliferation (arrowheads) and gliosis, bar = 50 mm. (E) Gliosis with proliferation of gemistocytes (arrows) and microglia (arrowheads), bar = 25 mm. (F—G). Demonstration of HS in cresyl violet-stained (Nissl staining) hippocampi of a control and epileptic cat. Control cat is introduced in (F), subfields are labelled according to the scheme proposed by Lorente de No. 28 Hippocampus of an epileptic cat displaying characteristic signs of HS (G), significant cell loss is visible in the hilus and CA1. The CA1 subfield is shrunken, gyrus dentatus shows granule cell dispersion. (H) demonstrates a human hippocampus from a patient with TLE. The cell loss pattern is typical for HS. Large amount of cells disappeared from the hilus, CA3c and CA1. The CA1 subfield is a shrunken, granule cell layer of the gyrus dentatus shows local dispersion. Scales: F, G: 400 μm, H: 1, 2 mm CA1, CA2, CA3, CA3c = subfields of the cornu Ammonis according to Lorente de No. GD = gyrus dentatus, H = hilus.
In the acute phase, several medications were used (Table 2). Supportive fluid therapy was administered to all cats. Nine cats were euthanased without treatment or with only treatment for some days (Table 2). Eight cats could be released from the hospital after 4–11 days as 48 h seizure freedom was achieved. Four cats were euthanased 60–120 days after the initial signs due to recurrent seizures and four cats were alive at the time of finishing the manuscript. Normal behaviour gradually returned only after 7–90 days. Two cats showed recurrent seizures after the acute phase of the disease, which was considered to be rare (less than one seizure/month) and another two cats become seizure free for longer period. The overall mean monitoring time of all cats after disease onset was 357 days (2–1640). The mean monitoring time of cats which were not euthanased in the acute phase of the disease (in the first 10 days) was 517 days (60–1640) (Table 2). Problems other than seizures reported or detected at the control examinations were: mild paraparesis in two cats, sedation in two and mild proprioceptive deficits in two cats. One cat showed two focal cluster seizure episodes similar to the initial event and another cats showed episodes of single seizure but did not develop cluster seizure. In all surviving cases, the owners reported a good quality of life. All four surviving cats are on phenobarbital therapy and in two cases therapy was reduced but not withdrawn.
Table 2.
Treatment, monitoring time and outcome of cats with feline CPS with orofacial automatism associated with hippocampal necrosis.
| Patient | Acute treatment | Chronic treatment | Monitoring time | Outcome |
|---|---|---|---|---|
|
| ||||
| 1 | Pheno/Gaba | Pheno reduced | 908 | Seizure free |
| 2 | No treatment | No treatment | 2 | Euth |
| 3 | Diaz/Pheno/Gaba/Clava | Pheno reduced | 730 | Seizure free |
| 4 | Pheno/Pred/Gaba | Pheno | 732 | Rare seizures |
| 5 | Pheno/Pred/Gaba | Pheno | 549 | Rare seizures |
| 6 | No treatment | No treatment | 10 | Euth |
| 7 | Midaz/Pheno/Gaba/Pento | Pheno | 60 | Euth |
| 8 | Pheno | Pheno | 108 | Euth |
| 9 | Diaz/Pheno/Gaba/Pred | No chronic treatment | 8 | Euth |
| 10 | Gaba/KBr/Lactulose/Neomy | No chronic treatment | 5 | Euth |
| 11 | Gaba/KBr/Lactulose/Neomy | No chronic treatment | 5 | Euth |
| 12 | Pheno/Gaba/Pento | No chronic treatment | 3 | Euth |
| 13 | Pheno/Gaba | No chronic treatment | 9 | Euth |
| 14 | Pento | Pheno | 120 | Euth |
| 15 | Pheno/Gaba/Pred | No chronic treatment | 4 | Euth |
| 16 | Pheno/Gaba/Pred/Midaz/Clava | No chronic treatment | 4 | Euth |
| 17 | Pheno/Diaz | Pheno | 60 | Euth |
Pheno = phenobarbital, Diaz = diazepam, Gaba = gabapentin, Pred = prednisolone, Midaz = midazolam, Clava = amoxicillin—clavulanic acid, Neom = neomycin, Pento = pentobarbital, Euth = euthanasia. Doses of medications: phenobarbital (3–5 mg/kg IV, q 12 h), gabapentin (10–19 mg/kg PO, q 8 h), diazepam (0.3–2 mg/kg rectal/IV/PO), midazolam (0.1–0.2 mg/kg IV), pentobarbital (4–8 mg/kg IV), prednisolone (0.2–2 mg/kg IV/PO/SC, q 12 h), amoxicillineclavulanic acid (15–20 mg/kg IV/PO, q 12 h), clindamycin (10 mg/kg PO, q 12 h) and neomycin (15 mg/kg PO, q 12 h).
Pathological lesions (Fig 1C—E) were usually distributed bilaterally, consisting of single to generalised degeneration, necrosis and loss of neurons within the pyramidal cell layer. In most cases (n = 7), lesions were more or less localised in all segments of the hippocampus. In 10 cases they could be found within the CA1 segment, whereas they were restricted to the CA1, CA2 and CA3 segments in two cases and to the CA1 and CA4 segments in one cat. Additionally, neuronal degeneration was evident in the dentate gyrus in three cases. Mild inflammatory infiltration, mainly localised in the perivascular region and within adjacent leptomeninges, primarily composed of lymphocytes and histiocytes, could be found in 10 cats. A conspicuous microgliosis was evident in all cases, whereas numerous swollen astrocytes, so-called gemistocytes, could be found in five cases as well. In eight cases microglial alterations were additionally accompanied by an intense capillary proliferation. Finally, in some cases neuronal necrosis led to dilution. In some sections a complete loss of neurons within the hippocampus was observed. Extrahippocampal pathological changes, usually localised adjacent to the hippocampus, concerning regions such as the entorhinal cortex, subiculum and parahippocampal gyrus, were found in seven cats. In single cases the cerebral cortex region, including the temporal neocortex, basal ganglia and diencephalon was involved additionally.
The immunohistochemical examination revealed that inflammatory infiltrates showed an intense expression of human leukocyte antigen (HLA)-protein in all cats, indicating a high profile of histiocytes. In 11 cases immunohistochemistry against the CD3-antigen revealed the presence of T-lymphocytes, mainly located within perivascular infiltrates and leptomeninges, as well as singly interspersed within the hippocampus.
Discussion
We observed a very characteristic CPS type with orofacial involvement in 17 cats which were acute, occurred in cluster and it was often associated with hippocampal pathology. The main ictal signs were: salivation, facial twitching, lip smacking, chewing, licking, swallowing, mydriasis, motor arrest (motionless starring), vocalisation accompanied mostly by postictal behavioural changes and aggression. The work-up revealed hippocampal pathology but no other brain lesions. In 11 cats hippocampal necrosis (HN) was confirmed by histopathology, resembling those reported by Fatzer et al. 7 In six cats, T1 weighted iso- to hypointense and T2 weighted hyperintense hippocampal changes were found by MRI.
Our cases showed similar clinical, ictal characteristics to those described by Fatzer et al. 7 However, in this previous study, partial and generalised seizures were observed. Secondary generalised seizures can be very fast and may mimic primary generalised seizures. Furthermore, as the importance of the differentiation was not emphasised before, the initial partial onset could have been missed easily. Another explanation is that it is not only CPSs that are associated with HN. This issue should be examined further.
Schmied et al 8 observed seizures, salivation, hyperactivity, vocalisation, urination, disorientation, aggression, cluster of seizures and mydriasis in four cats with suspected HN. However, the scope of his work was not the clinical findings but the MRI changes. Brini et al 9 reported two cats with HN and described clinical characteristics very similar to the episodes of our cats: ‘The cat had clusters of CPSs which lasted for few seconds and were invariably characterised by the following sequence: sitting down with extended neck, twitching of lips and eyelids, ear movement and profuse salivation.’
Earlier authors already mentioned this specific partial seizure type in cats, describing ‘twitching limited to the face’ 3 or a ‘glazed look with facial twitching’ 4 but none of the authors suggested the seizure might stem from a similar localisation in the brain. In veterinary medicine, unlike human medicine, this kind of seizure does not have real diagnostic value. Only some authors suggest that partial seizures are more often associated with focal brain pathology but no further differentiation was attempted.
The most important finding of our study is that the observed complex partial ictal clinical signs were always associated with the same pathological localisation. This could lead to the conclusion that this kind of CPS in cat may originate from the limbic region and be associated with HN. In recent years we observed a high number of cats with different kinds of seizures of different aetiology. 5 We included into the study all cats with orofacial seizures and in none of the cases could be identified pathologic changes elsewhere than in hippocampus or in associated structures.
Koestner 10 suggested that ‘temporal lobe epilepsy (TLE) is a special form of epilepsy in dogs and cats and devotes special attention to its neurological and neuropathological characteristics’. To the best of the authors' knowledge there is no published veterinary clinical study about the correlation between temporal lobe lesions and seizure types in client owned cats. Experimentally, however, facial seizures similar to those in our cases, were reported in cats due to a hippocampal lesion. 11 Tanaka et al 12 reported very similar characteristic limbic seizure in cats after kainic acid injection into the amygdala. Behavioural changes, facial twitching, mastication and salivation occurred. 12 Recently, a possible spontaneous model of human genetic epilepsy was detected in laboratory cats with a similar ictal pattern. 13
The majority of our cases were euthanased in the first days after onset due to lack of good response to the treatment. Four cases, however, survived and two of them are seizure free for long-term follow-up. The previously published 44 cases of feline HN suggested an unfavourable prognosis as all cases died or were euthanased.7–9 We could also observe an initial resistance to the antiepileptic therapy which may result in the conclusion that this disease cannot be treated. Surviving cases became well-controlled (seizure free) only after 4–11 days of treatment and that normal behaviour gradually returned only after 7–90 days. It can be assumed that surviving cases would have shown similar presumably less severe pathohistological lesions in the brain as cases confirmed. We cannot prove this conclusion because of a lack of histopathological confirmation in the surviving cases. Diagnostic imaging suggested hippocampal pathology in all surviving cases. The prognosis of this condition seems to be much better than reported in the literature previously with supportive and antiepileptic treatment (Table 2).
Behavioural changes and aggression were frequently described previously in cats with HN and were observed in our cats too, which is not surprising as the limbic system is involved.7,8
CPSs are epileptic seizure types that originate from a distinct area of the brain and cause impaired consciousness. Jackson and Colman, 14 who were pioneers of epileptology, described the first case with characteristic symptomatology of the TLE with impaired consciousness (dreamy state) and automatism. The oroalimentary automatism was found an important ictal signs in TLE in a later study. 15 CPS in people often starts with motor arrest (motionless starring) typically followed by oroalimentary automatisms which are: lip smacking, lip pursing, chewing, licking, tooth grinding, or swallowing.1,16 In man, oroalimentary automatisms are suggestive of TLE, originating in the limbic portion of the lobe; however, no single sign can be considered as specific. Aura, motor arrest, amnesia and automatism are key features of mesial temporal lobe epilepsy (MTLE), but depending on seizure spread and duration, ictal motor signs and postictal confusion can be found as well. 17 We observed very similar orofacial signs in cats. Facial twitching, lip smacking, chewing, licking and swallowing were observed in all cases and summarised as orofacial motor signs (Table 1). Complex seizure suggested (contrary to simple seizure) impaired consciousness which is frequently difficult to characterise in humans and much more difficult, or even impossible, in cats. Our cases, however, frequently arrested (Table 1) and seemed to be impaired even in the absence of motor signs.
The association between ictal clinical signs and hippocampal localisation could not be confirmed by EEG in our cats. However, EEG was performed only in one cat, therefore, no conclusion can be achieved. Kuwabara et al could localise the epileptic activity to the temporal-parietal region by interictal EEG, examining similar complex partial orofacial seizures in cats. 13 EEG examination should be undertaken in the future. However, human ictal scalp EEG is not always diagnostic and can be normal in 60% of cases of TLE at seizure onset. 17 Seizure activity may be restricted to temporal lobe structures and not penetrating the scalp, therefore, special recording and restraint techniques may be necessary to detect temporal lobe epileptic discharges in cats.
The aetiology of seizures in cats was reported to be secondary in most cases. 18 In contrast other studies suggested a high proportion of seizures to be primary.3,5,19 However, histopathology in later studies frequently found HN. It seems that different authors had various explanations for HN. Some categorise HN to symptomatic epilepsy whilst others to primary epilepsy. A recent review paper categorised HN as a possible differential diagnosis for secondary epilepsy. 20 This uncertainty is how to ascribe HN may be the reason for controversies in the veterinary literature regarding primary versus secondary epilepsy in cats as we considered recently. 5
In the original description in Switzerland 7 the aetiology for HN was suspected an environmental factor, probably a toxin, however, most cases were sporadic, which does not support environmental aetiological factors. HN was reported in two Italian cats and intoxication appeared rather unlikely. 9 Toxic aetiology seems unlikely based on our findings too; as the majority of the cats (13/17) were kept indoors.
Our observation in cats resembled a special human syndrome among TLE: mesial temporal lobe epilepsy with hippocampal sclerosis (MTLE-HS). The most important features of human MTLE-HS are: arrest, oral automatism, hippocampal MRI changes and neuronal cell loss and gliosis of the hippocampus, most severely in the CA1 region (Fig 1. Plate H). Similar findings were detected in the cats of the present study, however, there are several differences (Table 3). Our cases were clinically acute and inflammatory changes could be found in the hippocampal region (Table 3). The cause of inflammation is unclear but it was observed in earlier reports as well.7,8 One explanation may be that acute neuronal loss leads to mild local reactive encephalitis in cats. Another explanation may be that a primary immune-mediated process causes limbic encephalitis (LE) and hippocampal neuronal loss is secondary. Such human LE is associated with the potassium channel antibody.21,22 At the disease onset our cats were adult in contrast to human MTLE-HS but similar to human LE. 21
Table 3.
Comparison between feline CPS with orofacial automatism associated with hippocampal necrosis, and human mesial temporal lobe epilepsy with hippocampal necrosis.
| FEPSO-HN | Human MTLE-HS | |
|---|---|---|
|
| ||
| Possible causative factors | Unknown | Genetic predisposition for febrile seizures, sodium-channel defects, genetic defect |
| Age at the first seizure | 1–11 years (adult) | 6–14 years (childhood) |
| History of IPI | Unknown, may be LE | Frequent (30–60%) |
| Interictal EEG pattern | Unknown | Normal or temporal paroxysms or intermittent slow waves |
| Clinical course | Acute | Chronic |
| Neurological signs | No focal neurological abnormalities | No focal neurological abnormalities |
| Seizure semiology | Orofacial automatism, autonomic signs, arrest, behavioural changes | Orofacial automatism autonomic signs, arrest, amnesia, manual automatism |
| Postictal signs | Bizzare behaviour, staring, aggression | Cognitive impairment, memory deficits, mood changes, language deficits |
| MRI changes | Hippocampal T1 hypo-intensity and T2 and FLAIR hyperintensity (Schmied 8 ) | Hippocampal atrophy on T1 and/or increased signal on T2 and FLAIR |
| Histological hippocampal changes | Bilateral neuronal loss, gliosis, proliferation of capillaries and mild non-suppurative perivascular inflammatory infiltrates | Uni- or bilateral neuronal loss, gliosis, astrocytosis, mossy fibre sprouting |
Hippocampal sclerosis (HS) is the most frequent abnormality in human TLE.23,24 Neuronal loss is particularly found in the CA1 segment, also known as the ‘vulnerable’ or Sommer's sector, 25 which was the most severely affected region in our study (Fig 1).
It has to be considered that feline HN may not have a unique aetiology, but that it may be the consequence of different events. Retrospective studies in human MTLE demonstrated a high incidence of febrile seizures, trauma, hypoxia and intracranial infections, usually before the age of 5 years. 17 It is easy to miss such previous events in cats as the accuracy of history in veterinary medicine is much lower.
In general, HN could be the consequence of seizures and cause as well. Experimental data suggest that the type and severity of seizures are of importance in the formation of the neuronal cell loss pattern, rather than the duration of epilepsy itself. Therefore, severe HN can even occur in susceptible individuals after a single acute seizure.26,27 That's why the authors hold possible that idiopathic epilepsy can cause HN in some susceptible cats, leading to the development of the typical partial seizures. Perhaps if the cat is not individually susceptible or is treated early and does not develop severe lesions with refractory seizures, the outcome can be improved. However, aetiology versus consequence dilemma cannot be easily solved. Even the International League Against Epilepsy (ILAE) could not determine whether HS is a nonspecific result of primary epileptogenic lesion or a coexistence. 17
The presented condition (feline CPS with orofacial automatism associated with hippocampal necrosis, FEPSO-HN), should be not confused with feline orofacial pain syndrome (FOPS). FOPS is a condition with acute oral discomfort and automutilation to the face or tongue. Affected cats are commonly presented with exaggerated licking and chewing movement but no seizure-like mowement. 29 Furthermore none of our cats showed signs of discomfort or automutilation of tongue and face during the study.
We conclude that complex partial cluster seizures with orofacial involvement in cats are often associated with HN without other structural brain disease. The aetiology remains unknown, and may be heterogenous. It seems that HN is not necessarily fatal and long-term outcome can be good to excellent.
Acknowledgement
The authors are thankful to Dr Magloczky (Institute of Experimental Medicine, Hungarian Academy of Science, Budapest, Hungary) for her help.
Supplementary material
Supplementary data associated with this article can be found in the online version, at doi:10.1016/j.jfms.2011.05.014.
References
- 1.Commission on Classification and Terminology of International League Against Epilepsy Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989; 30: 389–99. [DOI] [PubMed] [Google Scholar]
- 2.Jan MM, Girvin JP. Seizure semiology: value in identifying the seizure origin. Can J Neurol Sci 2008; 35: 22–30. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz-Porsche D, Kaiser E. Feline epilepsy. In: Inderi RJ, ed. Problems in veterinary medicine. Vol 1: Philadelphia: Lippincott, 1989: 628–49. [PubMed] [Google Scholar]
- 4.Parent JM, Quesnel AD. Seizures in cats. Vet Clin North Am Small Anim Pract 1996; 26: 811–25. [PubMed] [Google Scholar]
- 5.Pakozdy A, Sarchachi Ali A, Leschnik M, Tichy A, Thalhammer JG. Clinical comparison of primary versus secondary epilepsy in 125 cats. J Feline Med Surg 2010; 12: 910–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redding RW. A simple technique for obtaining the electroencephalogram of the dog. Am J Vet Res 1964; 25: 854–7. [PubMed] [Google Scholar]
- 7.Fatzer R, Gandini G, Jaggy A, Doherr M, Vandevelde M. Necrosis of hippocampus and piriform lobe in 38 domestic cats with seizures. A retrospective study on clinical and pathologic findings. J Vet Intern Med 2000; 14: 100–4. [DOI] [PubMed] [Google Scholar]
- 8.Schmied O, Scharf G, Hilbe M, Michal U, Tomsa K, Steffen F. Magnetic resonance imaging of feline hippocampal necrosis. Vet Radiol Ultrasound 2008; 49: 343–9. [DOI] [PubMed] [Google Scholar]
- 9.Brini E, Gandini G, Crescio I, Fatzer R, Casalone C. Necrosis of hippocampus and piriform lobe: clinical and neuropathological findings in two Italian cats. J Feline Med Surg 2004; 6: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koestner A. Neuropathology of canine epilepsy. Probl Vet Med 1989; 4: 516–34. [PubMed] [Google Scholar]
- 11.Blum B, Liban E. Experience with experimental temporal lobe epilepsies produced by the tungstic acid method. Isr J of Exp Med 1963; 11: 7–17. [Google Scholar]
- 12.Tanaka S, Tanaka T, Kondop S, et al. Magnetic resonance imaging in kainic acid-induced limbic seizure status in cats. Neurol Med Chir 1993; 33: 285–9. [DOI] [PubMed] [Google Scholar]
- 13.Kuwabara T, Hasegawa D, Ogawa F, et al. A familial spontaneous epileptic feline strain: a novel model of idiopathic/genetic epilepsy. Epilepsy Res 2010; 92: 85–8. [DOI] [PubMed] [Google Scholar]
- 14.Jackson JH, Colman WS. Case of epilepsy with tasting movements and ‘dreamy state': very small patch of softening in the left uncinate gyrus. Brain 1898; 21: 580–90. [Google Scholar]
- 15.Feindel W, Penfield W. Localization of discharge in temporal lobe automatism. Arch Neur Psych 1954; 72: 605–30. [PubMed] [Google Scholar]
- 16.Blume WT, Lüders HO, Mizhari E, Tassinari C, van Emde Boas W, Engel J. Glossary of descriptive terminology for ictal semiology: report of the ILAE task force classification and terminology. Epilepsia 2001; 42: 1212–8. [DOI] [PubMed] [Google Scholar]
- 17.Wieser HG. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 2004; 45: 695–714. [DOI] [PubMed] [Google Scholar]
- 18.Quesnel AD, Parent JM, McDonell W, Percy D, Lumsden JH. Diagnostic evaluation of cats with seizure disorders: 30 cases (1991–1993). J Am Vet Med Assoc 1997; 210: 65–71. [PubMed] [Google Scholar]
- 19.Rusbridge C. Diagnosis and control of epilepsy in the cat. In Pract 2005; 27: 208–14. [Google Scholar]
- 20.Bailey KS, Dewey CW. The seizuring cat. J Feline Med Surg 2009; 11: 385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bien CG, Urbach H, Schramm J, et al. Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy. Neurology 2007; 69: 1236–44. [DOI] [PubMed] [Google Scholar]
- 22.Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 2004; 127: 701–2. [DOI] [PubMed] [Google Scholar]
- 23.Margerison JH, Corsellis JAN. Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain 1966; 89: 499–530. [DOI] [PubMed] [Google Scholar]
- 24.Babb TL, Brown WJ. Pathological findings in epilepsy. In: Engel J, ed. Surgical treatment of epilepsies. New York, USA: Raven Press, 1987: 511–40. [Google Scholar]
- 25.Sommer W. Erkrankung des Ammonhorns als aetiologisches Moment der Epilepsie. Arch Psych Nerv 1880; 10: 631–75. [Google Scholar]
- 26.Pitkanen A, Nissinen J, Nairismagi J, et al. Progression of neuronal damage after status epilepticus and during spontaneous seizures in a rat model of temporal lobe epilepsy. Progr Brain Res 2002; 135: 67–83. [DOI] [PubMed] [Google Scholar]
- 27.Maglocky Zs. Sprouting in human temporal lobe epilepsy: excitatory pathways and axons of interneurons. Epilepsy Res 2010; 89: 52–9. [DOI] [PubMed] [Google Scholar]
- 28.de Lorente No R. Studies on the structure of the cerebral cortex. II: continuation of the study of the ammonic system. J Psychol Neurol 1934; 46: 113–77. [Google Scholar]
- 29.Rusbridge C, Heath S, Gunn-Moore DA, et al. Feline orofacial pain syndrome (FOPS): a retrospective study of 113 cases. J Feline Med Surg 2010; 12: 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]