Abstract
The objective of the study was to characterize the signalment, clinicopathologic data, and diagnostic imaging of cats with urate urolithiasis, as well as the salts of uric acid present in the uroliths. A retrospective analysis of feline urate uroliths submitted to the GV Ling Urinary Stone Analysis Laboratory between 2000 and 2008 was included. From these data, records were assimilated from referring veterinarians (143); furthermore, all recorded cases from within the William R Pritchard Veterinary Medical Teaching Hospital (16) were included. Median values for the complete blood count and chemistry panels available were within the reference intervals, when provided, with only a few outliers present. Of all cases evaluated, seven had a portosystemic shunt (PSS). Cats with urate uroliths and a PSS were younger than cats without a PSS (2 years vs 7 years). The pathogenesis of urate uroliths in cats is poorly understood. Most cats were not completely evaluated for a PSS, however, clinicopathologic parameters indicating hepatic dysfunction were seldom noted; more sensitive diagnostics such as serum bile acids were rarely performed to confirm or negate the presence of a shunt. Studies are warranted to evaluate pathogenesis of urate uroliths to tailor proper management and breeding strategies.
Urolithiasis is a common and often recurrent problem in cats. Surgery or other techniques 1,2 are usually necessary to remove uroliths so they can be submitted for quantitative crystallographic analysis. The two most common mineral types reported in cats are calcium oxalate (CaOx) and struvite. 3 Urate-containing calculi account for approximately 10% of the uroliths from cats that are submitted to the GV Ling Urinary Stone Analysis Laboratory (UC Davis Stone Lab) each year. 3 Although a slight increase in urate-containing calculi was detected in the late 1980s compared to subsequent years, no significant trends in urolith submissions were detected. In cats, we have reported that urate stones submitted to our laboratory occurred equally in female and male cats; the Siamese breed was overrepresented. 3 In addition to the Siamese breed, Appel et al also noted a strong association between urate urolithiasis and the Egyptian Mau and Birman breeds. 4
In dogs with urate urolithiasis, Dalmatians and other breeds 5 have been reported to be at risk for development of urate uroliths because of a genetic defect that results in hyperuricosuria caused by a defect in uric acid transport in the kidneys and liver.6,7 In addition to the genetic defect, other breeds typically develop urate-containing uroliths as a result of liver disease, namely portosystemic shunts (PSSs). In most dogs with urate-containing calculi the calculi are 100% ammonium hydrogen urate (AHU), however, other salts of uric acid can occur. To date, studies in cats with urate urolithiasis are rare. The aim of this retrospective study was to evaluate the clinicopathologic data from cats whose urate stones were submitted to our laboratory. Because it is reported that cats with urate stones may have a PSS, 8 liver function parameters and imaging studies were also recorded if available. Finally, because the salt(s) of uric acid calculi in cats has not been reported, we analyzed these stones by infrared spectroscopy (IR) whenever possible and analyzed a subset with powder X-ray diffraction (XRD) to accurately report the salt(s) of uric acid present in these calculi.
Materials and methods
Case selection
A computer-assisted search of records from the UC Davis stone lab from January 2000 to December 2008 was used to compile information regarding urate-containing urinary calculi from cats that had stones submitted from practices other than the William R Pritchard Veterinary Medical Teaching Hospital (VMTH) at UC Davis (population A). The signalment, stone location, and any previous urolithiasis episodes were recorded. Furthermore, the veterinarians who submitted these cases were then contacted and asked to submit the entire record pertaining to that cat. From that record, the following information was recorded if available: complete blood count, biochemical profile, urinalysis and urine cultures obtained at the time the stone was removed. Because not all veterinarians used the same commercial laboratory, data were grouped according to which laboratories were utilized. Serum bile acids, liver biopsies and imaging studies including ultrasound and technetium scans were recorded if performed.
In addition to a general search of the stone lab database, a second group of cats with urate calculi submitted from the VMTH from 1982 to 2008 were also analyzed (population B). All cases from the VMTH, a tertiary care facility, were evaluated separately from those cats that presented to their primary care veterinarians.
Calculi analyses
Two methods were implemented to identify the type of urate present in the calculi: IR and XRD. For IR, the KBr disc method was used. The IR spectra were collected immediately after the discs were prepared using a Nicolet Impact 410 (Nicolet Instrument Corporation, Madison, WI) or Nicolet iS10 spectrometer (ThermoFisher Scientific, Madison, WI) as previously described. 9 All sample spectra obtained were compared with that of a reagent grade AHU (ICN Pharmaceuticals, Plainview, NY) which was confirmed to be AHU by XRD. For each sample that was analyzed by XRD as previously described,10,11 the obtained diffraction patterns were compared with the reference pattern for AHU from the International Center for Diffraction Data (ICDD) #52-2414.
Results
Population A cases
Three-hundred-and-fourteen urate-containing calculi from cats were submitted to our laboratory from 2000 to 2008, of these, records were obtained from 143 (45%) and were included for further analysis in this study.
Signalment
Of the 143 cases where records were available for review, the majority of stones were submitted from mixed breeds: domestic shorthair cats (99, 69%), domestic longhair cats (21, 15%) and domestic medium hair cats (nine, 6%). There was one submission from an unidentified breed, the remainder were from purebred cats including Siamese (five, 3%), Ocicat (two, 1%), Persian (two, 1%), Birman (two, 1%), Himalayan (one, 1%), and Manx (one, 1%). The median age was 7 (range 1–14 years) and weight was 5.5 kg (range 1.8–9.1 kg). Sixty-nine cats (48%) were male and 74 cats (52%) were female.
Presentation and physical examination
Seventy-five cats presented for hematuria, the remainder presented for other lower urinary tract signs (n = 45, eg, pollakiuria, stranguria, dysuria, urinary incontinence) or systemic signs (vomiting (four), anorexia (two), lethargy (two), other systemic signs (two)). No presenting complaints or medical histories were reported for 13 cats. Twelve cats had physical exam findings consistent with urethral obstruction (large, turgid, painful bladders). Forty-eight cats had small undetectable bladders, while five cats had palpable cystic calculi. There was no mention of bladder size in the remaining records (78). Within this time period six cats had recurrence of urate-containing stones (all 100% urate); all six cats were domestic shorthair.
Clinicopathologic data
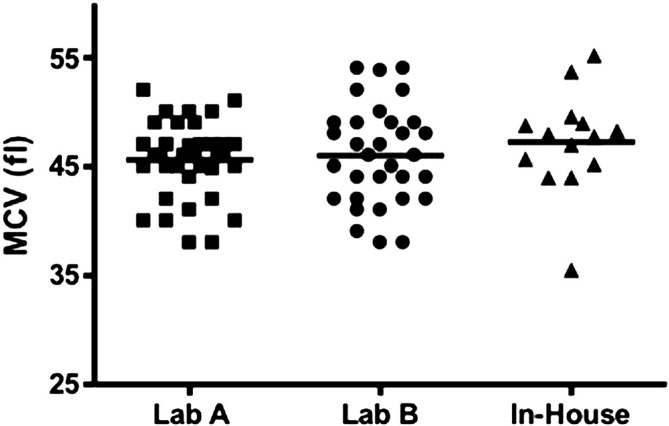
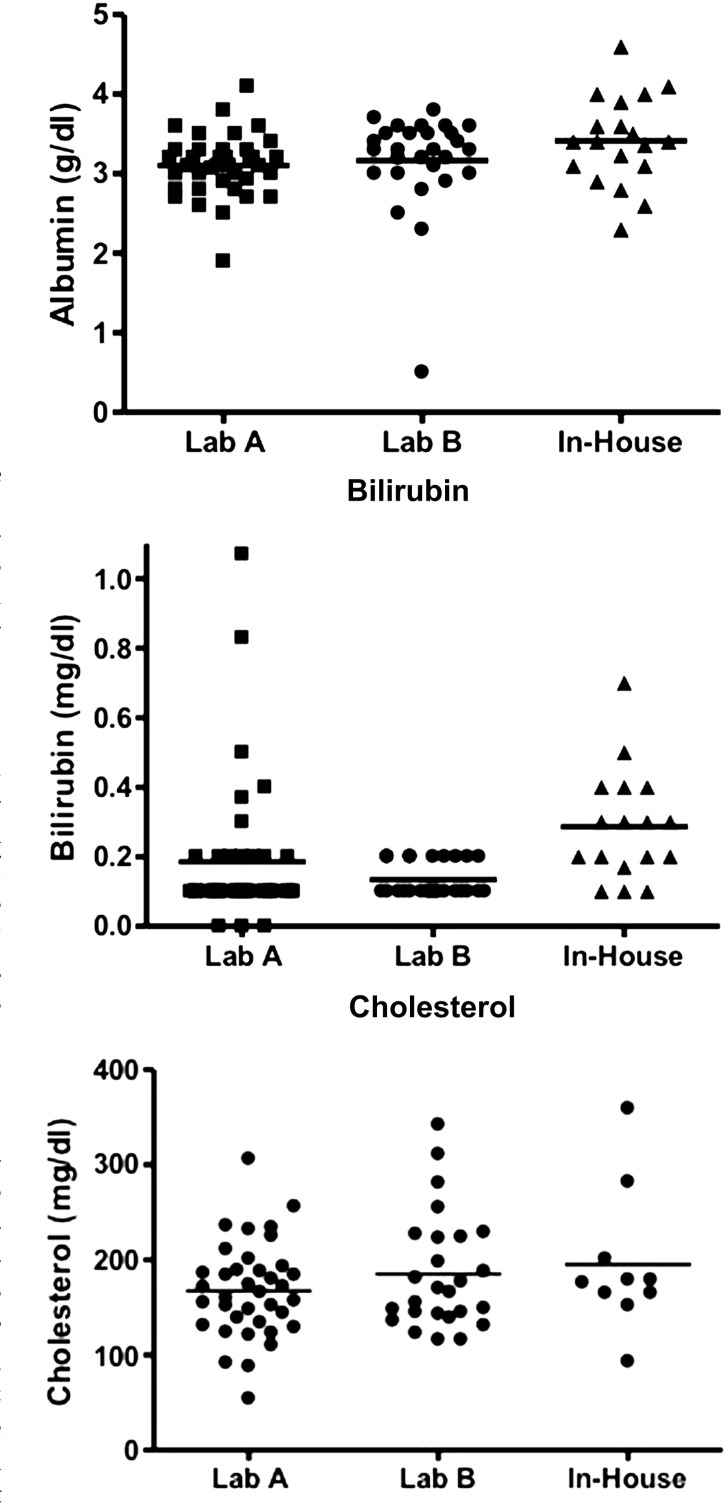
Complete blood counts were reported in 97/143 (68%) records available for review. All median values were within the reference intervals provided by outside commercial laboratories. However, of the 85 cats with recorded mean corpuscular volume (MCV), 10 cats did have microcytosis; the most severe being from an in house laboratory analyzer (Fig 1). Biochemical profiles were available from 112/143 (78%) records. The median values for liver enzymes and liver function tests were in the reference interval; two cats were hypoalbuminemic (89 reported), six were hyperbilirubinemic (85 reported), and one was hypocholesterolemic (76 reported) (Figs 2–4, respectively). Of the hypoalbuminemic cats, one was hypocholesterolemic. None of the hyperbilirubinemic cats had other clinicopathologic evidence of liver dysfunction. In 22 cats, bile acids were analyzed; five were increased. Three of these five cats also had microcytosis. Two of these cats were diagnosed with a PSS (Table 1).
Fig 1.
MCV values obtained from cats at the time a urate stone was removed. Lab A (Idexx) and Lab B (Antech) were used most often by veterinarians for lab work submission. Normal MCV for Lab A: (41–58 fl) and for Lab B (37–61 fl). Values from the other laboratories were obtained primarily from use of in house chemistry analyzers; therefore, normal reference intervals could not be obtained.
Figs 2–4.
Albumin, total bilirubin and cholesterol values obtained from cats at the time of urate stone removal. Lab A (Idexx) and Lab B (Antech) were used most often by veterinarians for lab work submission. Normal alkaline phosphatase (0–62, 6–102 IU/l), albumin (2.3–3.9, 2.5–3.9 g/dl), total bilirubin (0.0–0.4, 0.1–0.4 mg/dl) and cholesterol (82–218, 75–220 mg/dl) for Lab A and for Lab B, respectively. Values from the other laboratories were obtained primarily from use of in house chemistry analyzers; therefore, normal reference intervals could not be obtained.
Table 1.
Biochemical parameters from cats with confirmed hepatopathy. No clinicopathologic data were recorded for two cats with hepatopathies in population B as it was performed at the primary veterinarian (cat 4) or the urolith was found at necropsy and no clinical evaluation was performed (cat 5). Values in bold indicate results outside of the reference ranges provided.
| MCV (fl) | Bile acids (pre, post — mg/dl) | Albumin (g/dl) | t. Bili (mg/dl) | Dx | |
|---|---|---|---|---|---|
|
| |||||
| Population A | |||||
| Cat 1 | – | 16, 22 | — | — | PSS |
| Cat 2 | 41 | 10.5, 108 | 2.5 | 0.1 | PSS |
| Cat 3 | 35.5 | — | 2.3 | 0.1 | Hepatopathy |
| Cat 4 | 38 | 18.4, 111 | 2.5 | 0.1 | Hepatopathy |
| Population B | |||||
| Cat 1 | 42.7 | 0, 61.2 | 2.4 | 0 | Hepatopathy |
| Cat 2 | 37 | 10, 17 | 2.7 | 0.3 | PSS |
| Cat 3 | 44.5 | 133.3, 59.8 | 2.7 | 0 | PSS |
| Cat 4 | — | — | — | — | PSS |
| Cat 5 | — | — | — | — | Hepatopathy |
| Cat 6 | 39.2 | — | 2.8 | 0.2 | PSS |
| Cat 7 | 41.9 | 73.8, 91.4 | 2.4 | 0.1 | PSS |
Urinalyses
Urinalyses were reported from 116/143 (81%) cats; of the 71 samples that reported collection method: 60 samples were collected by cystocentesis, four were obtained by catheterization, and seven were voided samples. Crystalluria was identified in 30 cats; seven had urate crystals, 18 had struvite crystals, and five had CaOX crystals. No crystals were reported in the remaining in 87 cats. Urine culture was performed in 75/143 cats (52%); 63 were negative. Escherichia coli (two), unidentified cocci (seven), Clostridium species (one), Corynebacterium species (one), and Pseudomonas species (one) were isolated from the remaining 12 cases. Of those with positive cultures, only one was obtained by a voided sample.
Radiographic findings
Findings of preoperative imaging studies were available for 98/143 (68%). Abdominal radiographs were obtained in 77/143 cases (54%); uroliths were identified in 69/77 (90%), suspected in three and not identified in five cases. Thirty-five radiodense stones were 100% urate, the remainder were mixed with either struvite or a small percentage of CaOx. Abdominal ultrasounds were performed in 30/143 cases (21%); uroliths were identified in 26/30 (87%). In six of these cases, uroliths were also identified on radiographs. In five cases, a urolith was identified sonographically but not radiographically. In four cases, uroliths were suspected, but not confirmed.
Liver evaluation
Two cats were diagnosed with a PSS via laparotomy and liver biopsy. Three other cats had liver biopsies submitted and mild, diffuse hepatocellular changes with vacuolar degeneration were revealed histologically, but no suggestion of portal venous anomaly was present. The stone in one cat with a PSS was composed of 100% urate; while urate was present in the other case, the core of the stone was comprised of 90% struvite, neither this cat's urine nor the stone was cultured. However, of the 62 stones with greater than 50% struvite within the core layer, 14 had urine cultures performed, only one of which was positive for a b-hemolytic Staphylococcus species. Furthermore, 18 of these stones were cultured and again only one was positive for a Staphylococcus species. Liver disease was suspected based on laboratory values and clinical signs in four additional cases, however, no liver biopsies or advanced testing for a PSS were performed. Table 1 displays relevant liver function parameters for cats with hepatopathies or a confirmed PSS.
Population B cases
Sixteenurate-containing stones were submitted from patients seen at the UC Davis VMTH from 1982 to 2008. In this population there were seven domestic shorthair cats (44%), three domestic longhair (19%), three Persian (19%), two Siamese (13%) and one Himalayan. The median age was 5 years (range <1–10 years) and median weight was 4 kg (range 2–7.2 kg). Eight of these cats were female and eight were male.
Presentation and physical examination
Ten of the 16 (63%) cats presented for lower urinary tract signs (hematuria, pollakiuria or urethral obstruction), four were referred for evaluation of a PSS, one was referred for neurologic signs (acute blindness, disorientation) and one was seen for anorexia. Four cats had large bladders on physical examination, three were obtunded and nine cats had no physical exam findings consistent with lower urinary tract disease. Three of the stones were collected at necropsy.
Clinicopathologic data
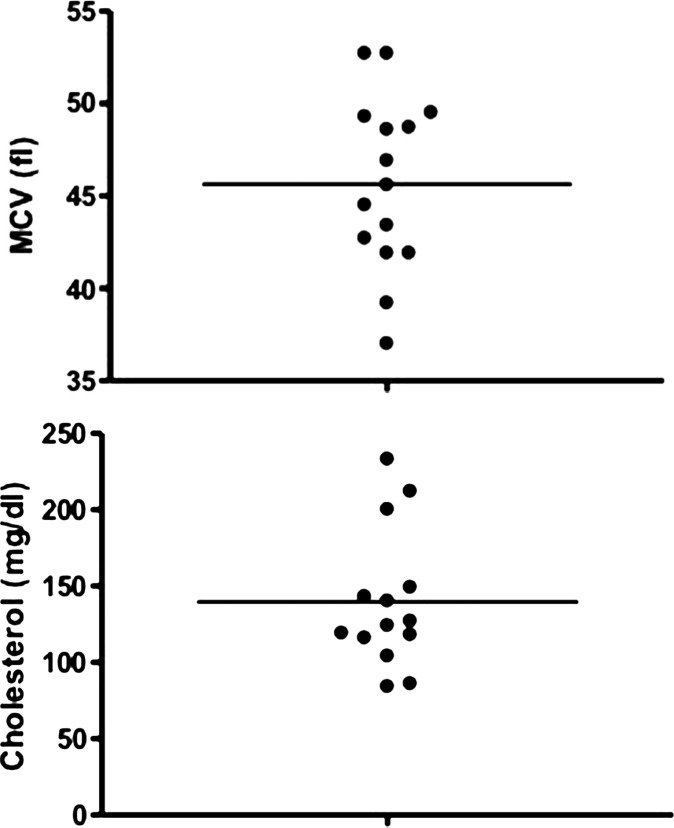
The median values for all hematologic and routine biochemical parameters fell within the reference intervals of the UC Davis VMTH laboratories. However, four cats had microcytosis (Fig 5), and three of these cats were diagnosed with a PSS. The median value of all liver function tests were within the reference interval. Two cats were hypocholesterolemic (Fig 6); microcytosis was also present in these two cats and both had a PSS. Two cats were moderately to severely azotemic (creatinine 2.8 and 6.9, blood urea nitrogen 25 and 87, respectively). Furthermore, bile acids were analyzed in six cats and the median of both the pre- and post-prandial samples were mildly elevated at 16 and 30 μmol/l (pre-prandial range 0–133, post-prandial range 15–91). Bile acids were markedly elevated (pre- or post-prandial greater than 50 μmol/l) in three cats, two of which were diagnosed with a PSS. The third had hepatocellular degeneration and necrosis noted on liver biopsy but no evidence of a shunt.
Figs 5 and 6.
MCV and cholesterol values obtained from cats presenting to the UC Davis VMTH (population B) at the time of urate stone removal. Reference interval for MCV (42–53 fl), cholesterol (89–258 mg/dl).
Urinalyses
Urinalyses were performed in 11 cats. Crystalluria was identified in four samples (one ammonium biurate, one CaOx and two amorphous crystals). Urine cultures were performed on 13 samples, all of which were negative. Antibiotic history of these cats was not recorded.
Radiographic findings
In five cases radiographs were obtained and uroliths were identified; uroliths were seen sonographically in eight cases. No clear urolith was identified in two cases, but evidence of ureteral obstruction was noted in both. Shunts were confirmed via either technetium (two) or ultrasound (one) in three cases.
Liver evaluation
Five of 16 cats (31%) from population B were diagnosed with a PSS (three via imaging, two at necropsy).
Histologic evidence of portovascular anomalous vessels (portal arteriolar hyperplasia) was present in three cases and evidence of hepatic fibrosis and inflammation was identified in four cases. Three of the cats with a PSS had stones composed of 100% urate while the other two contained 10–40% struvite in either the core or outer layer. The median age of cats diagnosed with a PSS in population B was 3 years old. Two other cats in population B were diagnosed with hepatopathy. Two cats with liver abnormalities (PSS and hepatocellular necrosis) were Siamese.
Crystallographic analysis
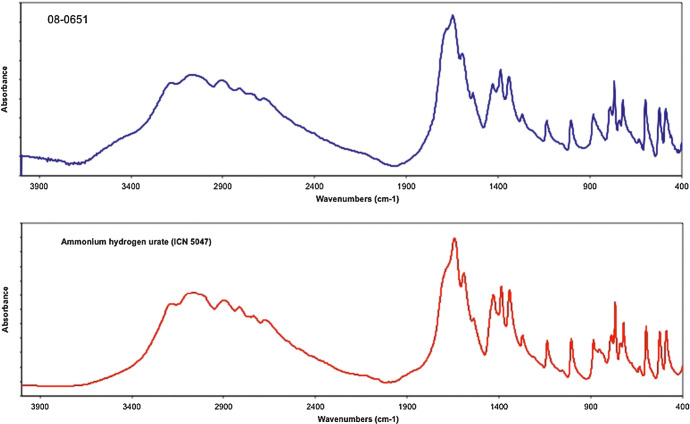
Of the stones from referring veterinarians, 125/143 (87%) were analyzed by IR and identified as 100% AHU. In five of these cases, AHU could not be confirmed by IR due to the coexistence of other minerals in a higher content than urate; in 13 cases IR was not performed because there was not enough urate present within the sample or the stones were too small to obtain IR results. Fifteen out of 16 (94%) of the stones from population B were identified by IR as 100% AHU. AHU could not be confirmed in one of the 15 stones due to the coexistence of other minerals. Figure 7 compares the IR spectrum of a representative sample composed of only AHU to that of the reference standard for AHU confirming that the calculus specimen is composed of AHU.
Fig 7.
Infrared spectra of a representative sample (laboratory number 08-0651) (top) and reagent grade AHU (bottom) confirming that the sample is composed of AHU. Abs = absorbance.
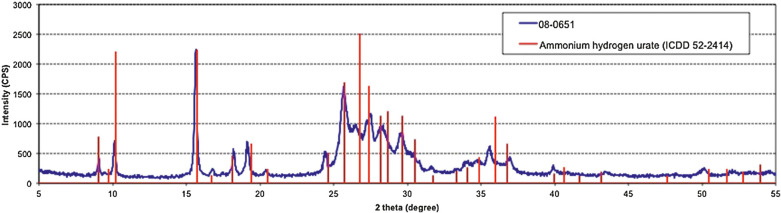
XRD was implemented as an additional methodology in 20 stones to confirm the IR identification of AHU in the calculus samples. The urate in these samples was identified as AHU by XRD. Figure 8 compares a representative diffraction pattern (from the same calculus depicted in Fig 7) showing the matching peak positions and relative intensities of AHU (reference material from the ICDD database) confirming by XRD that the calculus is composed of AHU.
Fig 8.
XRD pattern of the same representative sample in Fig 7 (laboratory number 08-0651). Vertical straight lines depict peak positions and relative intensity of AHU obtained from a reference diffraction database standard (ICDD 52-2414), thus confirming the stone to be composed of AHU. CPS = counts per second.
Discussion
Urate-containing uroliths in cats account for approximately 10% of calculi submitted to our laboratory each year; 3 others report a slightly lower incidence. 4 It has been stated and anecdotally reported that many cases of urate calculi in cats are found in young cats that have a PSS, 8 even though PSS in cats has been reported to be rare. 12 Unfortunately, most cats in our study had very little data regarding liver evaluation. Although only two cases from population A were diagnosed with a PSS, it was impossible to identify which cats within this retrospective series had an underlying liver disorder because very few cases had full liver function evaluations such as serum bile acids and imaging studies. Primary and secondary care veterinarians do not appear to evaluate cats for this issue, perhaps because most of these cats were reported to have no history or clinical signs (eg, ptyalism, neurological signs, or lethargy) suggestive of a PSS as well as normal physical examinations, biochemical panels. However, a normal biochemical panel cannot exclude a diagnosis of a PSS. If one suspects a PSS in a cat, serum bile acids are much more sensitive for the diagnosis. 13 Post-prandial serum bile acids have been reported to have the highest sensitivity for evaluating hepatobiliary disease, including a PSS, in the cat. 14 When reviewing the records from earlier cases, no cats were reported to develop clinical signs related to liver disease in subsequent years, suggesting that if an underlying hepatopathy such as a PSS was present in these cases, it was not severe enough to warrant evaluation for it by the owner or primary care veterinarian.
As mentioned, very few cats had serum bile acids performed and only 5/21 cats (24%) evaluated had elevated pre- or post-prandial bile acids, making hepatopathies in the remaining 16 cats unlikely. 13 In our study, only two cats (1.2%) from population A had confirmed shunts; 7/144 (4.9%) may have had an underlying hepatopathy. Although the median values for all hematologic and biochemical parameters fell within chosen reference intervals, there was a small subset of cats whose values were outside of these ranges. Although not all of these cats had imaging or liver biopsy to verify the presence of hepatopathy, it was suspected by the primary clinician in three cases. Based on this study, if clinicopathologic findings (eg, microcystosis, or rarely hypoalbuminemia) are suggestive for liver dysfunction in cats with urate stones, additional diagnostics such as serum bile acids and imaging studies should be performed. In a case series evaluating 23 cats with a PSS, all cats had at least one variable, excluding electrolytes, on the serum biochemical panel out of the reference range. 12 Therefore, if abnormalities are detected, further evaluation of the liver is warranted. We cannot state that cats with normal complete blood counts and biochemical profiles excluded the diagnosis of a shunt. A combination of clinical signs, history, as well as other ancillary tests (eg, serum bile acids, and imaging studies) would be needed to confirm or negate the presence of a PSS or other hepatopathy in cats with urate stones and normal laboratory values.
Clinicopathologic data obtained in this study were from various laboratories and submitted by many different veterinarians. The number of abnormalities detected needs to be interpreted with caution; the quality and quantity of the samples submitted were not reported.
In population B, we found that 31% had a PSS confirmed. We examined this population of cats separately to evaluate the differences between primary care veterinary cases and those seen at a tertiary care hospital. Many of the cats referred to the VMTH for evaluation had abnormal physical examinations or histories suggestive of a PSS. Overall, the cats diagnosed with a PSS were generally younger (median 2 years vs 7 years), which is similar to what has previously been reported; 12 nine had laboratory values suggestive of a PSS (eg, microcytosis and elevated bile acids). Lastly, cats with a PSS did not necessarily have stones composed of 100% AHU. While urate was present, three of these stones had AHU with other minerals present. Therefore, cats with mixed urate stones do not exclude the possibility of a PSS present in that cat.
We have previously reported that Siamese cats have significantly more urate-containing calculi, whereas Persian cats had significantly less. 3 Other laboratories have reported a higher prevalence of urate urolithiasis not only in Siamese but also the Egyptain Mau and Birman breeds. 4 Two of the cats from population B with a confirmed hepatopathy (one PSS, one hepatocellular necrosis) were Siamese cats. No Siamese cats from population A were diagnosed with a PSS, but not all were fully evaluated for this disorder. It is suggested in some reports that Siamese cats are overrepresented when evaluating cats with a PSS15,16 but the numbers appear too low to draw any conclusions regarding risk. Therefore, while the Siamese may be predisposed to urate stones as we previously reported, the reasons for this risk are still unclear. We recommend that Siamese be evaluated for liver pathology if clinically indicated.
The pathogenesis of urate stone formation in the Dalmatian has been well described7,17 and a genetic basis for Dalmatian hyperuricosuria has also been documented. 18 This mutation has been found in other non-Dalmatian dogs, including the English Bulldog and Black Russian Terriers. 19 To our knowledge, the etiopathogenesis and any genetic studies in cats with urate stones have not been reported and future studies should be conducted to evaluate at risk breeds such as the Siamese, Egyptian Mau, and Birman breeds for genetic mutations related to purine metabolism.
In this study, only six cats (4%) had recurrent episodes of urate urolithiasis, which is lower than previously reported rates of 13.1%. 20 The differences between these two studies are likely due to the fact that we did not have complete records available to us for all cats, and we were limited to what was submitted to us by the referring veterinarian. The recurrence rate of urate urolithiasis in Dalmatians has been reported to be high, with over 22% presenting with a second episode, 21 which is higher than recurrence rates reported for cats that had stones submitted to the Minnesota Urolith Center. 20 Further studies are necessary to document the number of cats with recurrent urate urolith formation.
Unlike humans, who primarily form pure uric acid stones which can precipitate in acidic urine, in this study the type of urate identified was AHU. Urine pH can affect crystal formation; depending on which salt of uric acid is present, the solubility of the urate stone may be pH dependent. 22 Because ammonium and hydrogen ions may precipitate with uric acid in urine with a pH approximately <7.0, alkalinization of the urine (via diet or alkalinizing agents) may be warranted to help prevent acid metabolites from increasing tubular production of ammonia. 23 However, increasing urine production by increasing water intake is still a cornerstone of therapy to dilute solutes and help prevent recurrence of urate uroliths.
Due to the retrospective nature of this paper, limited data were available for each cat. The diagnostics were determined and performed by the various attending veterinarians and consistent evaluations of liver function were not performed. Therefore, the exact number of cats with hepatopathies could not be determined. Furthermore, different laboratories were used to evaluate the clinicopathologic data. We grouped the data by laboratory, and reference intervals were similar between the two laboratories that were primarily utilized. When we reported abnormalities from these data, reference intervals were provided from each in-house analyzer. Quality and quantity of all blood and urine samples were not provided.
To our knowledge, this is the first paper in which the clinicopathologic data as well as the salt of uric acid present in stones from cats with urate-containing uroliths has been reported. Very rarely, abnormalities related to liver dysfunction were present on routine screening blood work in both populations of cats we evaluated, however, the exact number with underlying liver pathology could not be determined from our study because ancillary tests such as serum bile acids and advanced imaging were not performed on most cats with urate stones. Specific breeds such as the Egyptian Mau, Birman, and Siamese have been reported to be at increased risk for urate stones. Prospective studies are needed to see if full liver evaluations are warranted in breeds that are predisposed to urate stones, as well as in cats without a history, clinical signs and screening blood work suggestive of a PSS.
References
- 1.Lulich JP, Osborne CA, Carlson M, et al. Nonsurgical removal of urocystoliths in dogs and cats by voiding urohydropropulsion. J Am Vet Med Assoc 1993; 203: 660–3. [PubMed] [Google Scholar]
- 2.Adams LG, Berent AC, Moore GE, Bagley DH. Use of laser lithotripsy for fragmentation of uroliths in dogs: 73 cases (2005–2006). J Am Vet Med Assoc 2008; 232: 1680–7. [DOI] [PubMed] [Google Scholar]
- 3.Cannon AB, Westropp JL, Ruby AL, Kass PH. Evaluation of trends in urolith composition in cats: 5,230 cases (1985–2004). J Am Vet Med Assoc 2007; 231: 570–6. [DOI] [PubMed] [Google Scholar]
- 4.Appel SL, Houston DM, Moore AE, Weese JS. Feline urate urolithiasis. Can Vet J 2010; 51: 493–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Bannasch D, Henthorn PS. Changing paradigms in diagnosis of inherited defects associated with urolithiasis. Vet Clin North Am Small Anim Pract 2009; 39: 111–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trimble HC, Ce K. The inheritance of ‘high uric acid excretion’ in dogs. J Hered 1938; 29: 280–9. [Google Scholar]
- 7.Giesecke D, Tiemeyer W. Defect of uric acid uptake in Dalmatian dog liver. Experientia 1984; 40: 1415–6. [DOI] [PubMed] [Google Scholar]
- 8.Ling GV. Urinary stone disease. In: Ling GV, ed. Lower urinary tract diseases of dogs and cats. St Louis, MO: Mosby, 1995. 170. [Google Scholar]
- 9.Westropp JL, Ruby AL, Bailiff N, Kyles AE, Ling GV. Dried solidified blood calculi in cats. J Vet Intern Med 2006; 20: 828–34. [DOI] [PubMed] [Google Scholar]
- 10.Neumann RD, Ruby AL, Ling GV, Schiffman P, Johnson DL. Ultrastructure and mineral composition of urinary calculi from horses. Am J Vet Res 1994; 55: 1357–67. [PubMed] [Google Scholar]
- 11.Grant DC, Werre SR, Gevedon ML. Holmium: YAG laser lithotripsy for urolithiasis in dogs. J Vet Intern Med 2008; 22: 534–9. [DOI] [PubMed] [Google Scholar]
- 12.Kyles AE, Hardie EM, Mehl M, Gregory CR. Evaluation of ameroid ring constrictors for the management of single extrahepatic portosystemic shunts in cats: 23 cases (1996–2001). J Am Vet Med Assoc 2002; 220: 1341–7. [DOI] [PubMed] [Google Scholar]
- 13.Ruland K, Fischer A, Hartmann K. Sensitivity and specificity of fasting ammonia and serum bile acids in the diagnosis of portosystemic shunts in dogs and cats. Vet Clin Path/Am Soc Vet Clin Pathol 2010; 39: 57–64. [DOI] [PubMed] [Google Scholar]
- 14.Center SA, Erb HN, Joseph SA. Measurement of serum bile acids concentrations for diagnosis of hepatobiliary disease in cats. J Am Vet Med Assoc 1995; 207: 1048–54. [PubMed] [Google Scholar]
- 15.Lipscomb VJ, Jones HJ, Brockman DJ. Complications and long-term outcomes of the ligation of congenital portosystemic shunts in 49 cats. Vet Rec 2007; 160: 465–70. [DOI] [PubMed] [Google Scholar]
- 16.Blaxter AC, Holt PE, Pearson GR, Gibbs C, Gruffydd-Jones TJ. Congenital portosystemic shunts in the cat: a report of nine cases. J Small Anim Pract 1988; 29: 631–45. [Google Scholar]
- 17.Kuster G, Shorter RG, Dawson B, Hallenbeck GA. Uric acid metabolism in Dalmatians and other dogs. Role of the liver. Arch Intern Med 1972; 129: 492–6. [PubMed] [Google Scholar]
- 18.Bannasch D, Safra N, Young A, Karmi N, Schaible RS, Ling GV. Mutations in the SLC2A9 gene cause hyperuricosuria and hyperuricemia in the dog. PLoS Genet 2008; 4: e1000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karmi N, Safra N, Young A, Bannasch DL. Validation of a urine test and characterization of the putative genetic mutation for hyperuricosuria in Bulldogs and Black Russian Terriers. Am J Vet Res 2010; 71: 909–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albasan H, Osborne CA, Lulich JP, et al. Rate and frequency of recurrence of uroliths after an initial ammonium urate, calcium oxalate, or struvite urolith in cats. J Am Vet Med Assoc 2009; 235: 1450–5. [DOI] [PubMed] [Google Scholar]
- 21.Case LC, Ling GV, Ruby AL, Johnson DL, Franti CE, Stevens F. Urolithiasis in Dalmatians: 275 cases (1981–1990). J Am Vet Med Assoc 1993; 203: 96–100. [PubMed] [Google Scholar]
- 22.Bowyer RC, Brockis JG, McCulloch RK. The role of common urinary constituents in the precipitation of ammonium acid urate. Clin Chim Acta 1979; 99: 221–7. [DOI] [PubMed] [Google Scholar]
- 23.Bartges JW, Osborne CA, Lulich JP, et al. Canine urate urolithiasis. Etiopathogenesis, diagnosis, and management. Vet Clin North Am Small Anim Pract 1999; 29: 161–91. xii–xiii. [DOI] [PubMed] [Google Scholar]