Abstract
This paper describes a cat with severe localised infections with Cryptococcus neoformans/gattii species complex and Mycobacterium avium affecting the subcutis and underlying fascia and bone of the right pelvic limb. The simultaneous isolation of both pathogens in this patient was unexpected and posed unique issues concerning both diagnosis and clinical management. The aetiopathogenesis of this infection is discussed in relation to aspects of diagnosis and therapy.
Cryptococcal rhinosinusitis and mycobacterial panniculitis are two well-recognised entities in feline medicine. Deep soft tissue infection with either Cryptococcus species or mycobacteria is, however, exceedingly unusual. The diagnosis and management of a challenging case in which both these saprobes were present concurrently is described and an attempt is made to decipher the causal pathomechanisms of the infection.
A 15-year-old, neutered male domestic shorthair cat (6 kg) was referred to the Veterinary Specialist Centre with a 6-month history of a ‘non-healing wound’ on the right medial thigh. The lesion allegedly started as a small draining sinus that had progressed, despite veterinary interventions, to a large deficit that extended deep into the limb. There was no history of prior trauma. The cat lived on a farm in Richmond, New South Wales and had been previously diagnosed with chronic kidney disease (IRIS stage 3, creatinine 263 μmol/l [reference interval 71–212], urine specific gravity (USG) 1.025). Empirical oral antimicrobials were trialled initially, including; orbifloxacin (Orbax; Schering Plough) 25 mg q 24 h for 7 days, clindamycin (Antirobe; Pfizer) 75 mg q 24 h for 7 days (two courses) and doxycycline (Vibravet; Pfizer) 25 mg q 12 h for 4 weeks.
Cytological and histological examination of representative material from the wound by the referring veterinarian demonstrated capsulated yeast-like organisms suggestive of the Cryptococcus neoformans/gattii species complex. Once cryptococcosis was suspected, itraconazole (Sporanox; Janssen Cilag) 30 mg q 12 h for 8 weeks, amoxicillin—clavulanate (Clavulox palatable drops; Pfizer) 31.25 mg q 12 h for 7 days, enrofloxacin (Baytril; Bayer) 50 mg q 24 h for 7 days and intralesional itraconazole (emptied from capsules) and various ointments (Panolog ointment; Fort Dodge and Otomax ear drops; Schering Plough) were administered with limited success and the patient was referred.
At referral, 6 months after the lesion was first apparent, the patient was bright and alert and able to bear weight on the affected limb. Vital signs were within normal limits, rectal temperature was 38.5°C and the kidneys were considered a normal size on palpation. The lesion on the right pelvic limb took the form of a ‘non-healing wound’ and extended along the medial aspect from the distal femur and inguinal region to the proximal tibia. The subcutis, fascia, inguinal panniculus and the ligamentous structures of the medial stifle were extensively exposed (Fig 1).
Fig 1.
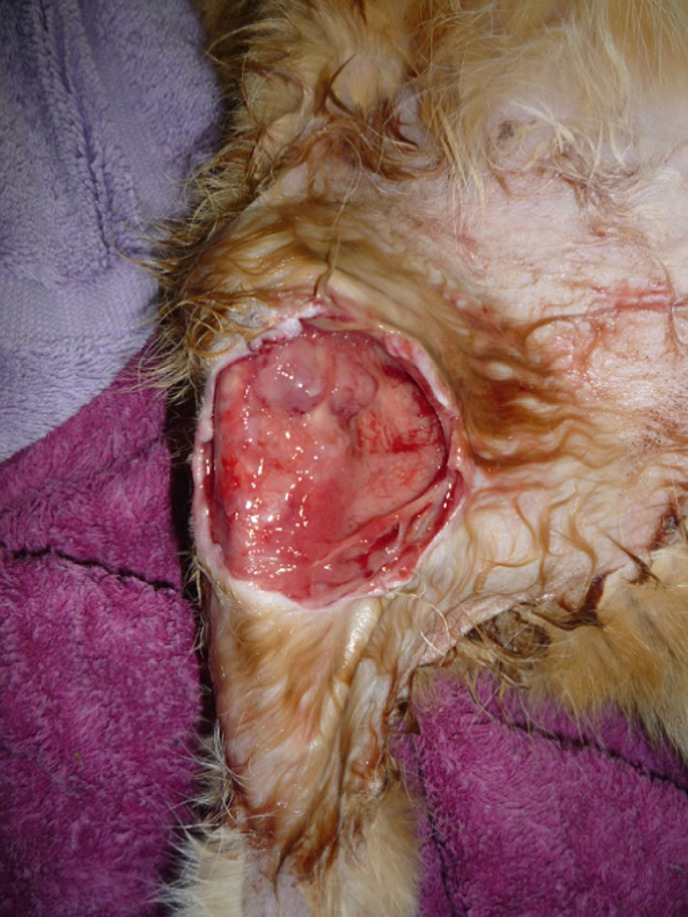
‘Non-healing wound’ of the craniomedial aspect of the right pelvic limb. The appearance of the lesion is reminiscent of the ‘non-healing wounds' associated with rapidly growing mycobacteria such as M smegmatis.
Capsulate yeast-like organisms consistent with a diagnosis of cryptococcosis had been described by the referring clinician. The gross appearance of the wound, however, appeared atypical for a cryptococcal infection and further diagnostic tests were suggested to establish whether there was an additional disease process occurring. Our plan was to determine if mycobacteria were present deeper within the lesion and to conduct haematology, serum biochemistry, urinalysis and retroviral screening to search for potential underlying predispositions, such as diabetes mellitus or long-standing feline immunodeficiency virus (FIV) infection. Haematology was unremarkable. Biochemistry revealed moderate azotaemia (urea 17.5 mmol/l [5.0–15.0]; creatinine 271 μmol/l [80–200]) and mild hypoalbuminaemia (23 g/l [25–38]). Urine was minimally concentrated (USG 1.022) with an inactive sediment and negative routine aerobic culture. Further evaluation of the chronic kidney disease, including a urine protein—creatinine ratio and abdominal ultrasound, was not performed. The patient was FIV antibody and feline leukaemia virus (FeLV) antigen negative (Witness; Agen Biomedical). Impression smear cytology of the wound demonstrated large numbers of round to ovoid, narrow-necked budding capsulate yeasts within ‘strands’ of macrophages. The latex cryptococcal antigen agglutination test (LCAT) was positive with a titre of 512.
Deep biopsies were obtained under anaesthesia a few days later and a heavy growth of a Cryptococcus species was isolated on bird-seed agar at 28°C after 48–72 h incubation. Additional smears were made from the deeper samples and were stained with Diff-Quik, Gram and a modified acid-fast stain. Acid-fast bacilli (AFB) were not visualised initially but because the presentation suggested that a mycobacterial aetiology was possible, the specimen was also cultured aerobically at 37°C on soybean-based medium (BBL Mycosel agar; Becton, Dickinson) incorporating actidione and chloramphenicol to prevent cryptococcal and staphylococcal growth, respectively. The Cryptococcus species grew at 28° and 37°C and the colonies were typically brown due to production of melanin by phenol oxidase activity on bird-seed agar. The isolate was susceptible to itraconazole, fluconazole amphotericin B and flucytosine. Nasal swab cytology, performed at the same time as the biopsies, was negative for cryptococcal organisms, suggesting the cryptococcal wound infection was localised. Nasal culture for Cryptococcus species was not performed.
The cat was thus considered to have localised subcutaneous cryptococcosis and concurrent chronic kidney disease. Amputation was suggested as the treatment most likely to resolve the issue, due to the severity and extent of the disease process, but was declined by the owners. Immediately after biopsies were obtained the wound was cleaned and debrided and terbinafine soaked gauze swabs were sutured loosely into the region. Fluconazole (50 mg orally q 12 h; Bova Compounding) was started. Additionally, terbinafine cream (1%; Lamisil; Novartis) was used topically in an attempt to deliver targeted local therapy. The patient was sent home the following day.
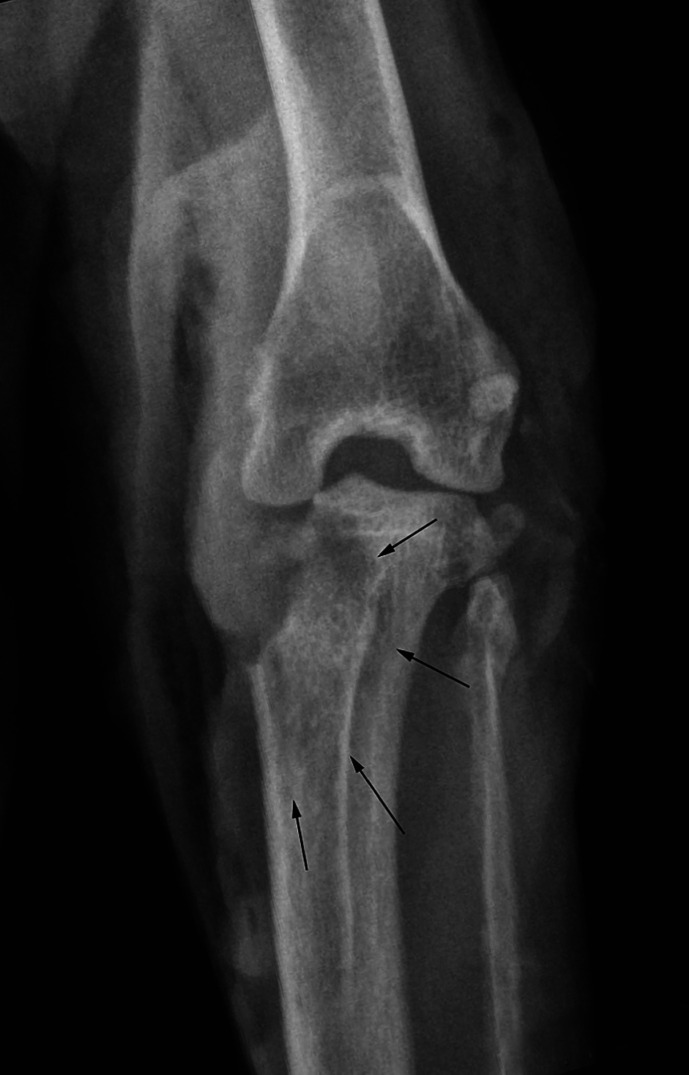
The cat represented 5 days later, earlier than anticipated, with a painful and non-weight bearing right pelvic limb. Although the wound dressing had remained in place, the lesion was malodorous and multiple fly larvae had invaded the wound. Radiographs were taken as the underlying stifle joint cavity and proximal tibia were now exposed. There was extensive bony lysis evident with a complete absence of the medial third of the tibial plateau (Fig 2). The surrounding soft tissues were thickened and contained gas. Reactive sclerosis was present within the cancellous compartment of the proximal tibia, although the periosteum appeared uninvolved. Additional survey radiographs of the entire skeleton were performed but no additional skeletal lesions were identified. The rapid clinical deterioration was difficult to explain. Given the extent of the bony lysis, it was presumed to have been present at initial presentation even though minimal pain was detected at that time. Whether the terbinafine topical therapy contributed, or partial closure of the wound combined with fly larval infestation was responsible, was not known.
Fig 2.
Note the generalised soft tissue swelling on both the lateral and medial aspects of the stifle and gas in the soft tissue planes. The medial third of the tibial condyle is no longer present. There is a ragged medial margin of bone adjacent to some more medially located opacities which may represent residual bone. Within the proximal tibia itself, there is a heterogenous mixture of increased and decreased bone opacity which has an unstructured appearance and it suggests a reactive bone change within the cancellous compartment of the proximal tibia (arrows). The periosteum appears normal.
Considering the extensive bony involvement, amputation was considered the only option to resolve such a chronic, deep seated infection. Amputation of the affected limb was, therefore, performed via coxofemoral disarticulation. This offered a wide surgical margin around the medial inguinal region to minimise the possibility of residual disease persisting along the surgical wound. The procedure proceeded uneventfully and the entire limb was submitted for pathological investigation. Culture was not requested on the limb given previous cultures had been performed; however, in hindsight it may have been useful given the recent rapid clinical decline. The cat recovered rapidly. Interestingly, he had a markedly improved demeanour compared to before the surgery. Fluconazole was continued and a 10-day course of amoxicillin—clavulanate (125 mg PO q 12 h) was administered given the extensive nature of the surgery. The cat was sent home 3 days later.
Additional microbiology results became available 6 days after the amputation from the original deep tissue biopsies. After 11 days incubation, a relatively slow-growing Mycobacterium species was isolated. In light of the positive culture, the initial modified acid-fast stained smears were reviewed; a small number of AFB could be identified (Fig 3). The culture was subsequently forwarded to a mycobacteria reference laboratory (Pathology Queensland, Queensland Health Clinical and Statewide Services) and was identified using 16S rRNA sequencing as Mycobacterium avium. Anti-mycobacterial therapy was not considered necessary at this stage, on the presumption that the infection had been resolved surgically and the patient was doing well postoperatively.
Fig 3.
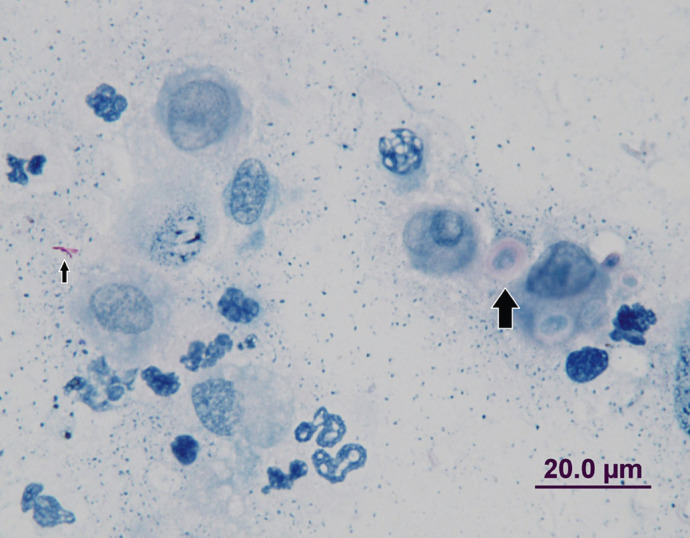
Cytology (Ziehl—Neelsen stain) of the lesion demonstrating acid-fast bacilli (small arrow) and capsulated cryptococcal blastoconidia (large arrow).
Haematoxylin and eosin (H&E)-stained sections demonstrated the synovium surrounding affected articular cartilage and adjacent bone was thickened due to villous proliferation, granulation tissue and an influx of inflammatory cells, predominantly plasma cells and lymphocytes. Periosteal tissues showed areas of granulomatous inflammation, with clusters of neutrophils and, occasionally, plasma cells. Giant cells were evident but sparse, however, sheets of epithelioid macrophages were prominent. The bone marrow was cellular and contained an admixture of megakaryocytes, erythroid and proliferating myeloid cells; some areas also contained clusters of epithelioid cells. Other areas of bone marrow had been completely effaced by granulomatous inflammation and, in many of these areas, budding capsulate yeasts could be detected extracellularly and within macrophages, sometimes in ‘packets’. Ziehl—Neelsen (ZN) stained sections demonstrated that macrophages in the bone marrow contained AFB singly and in packets. The morphological diagnosis was (pyo)granulomatous inflammation of articular cartilage, bone and bone marrow associated with concurrent infection with Cryptococcus species and Mycobacterium species.
Fifteen days after amputation, clinical progress was excellent, renal analytes were stable and the LCAT had decreased to 1:128. Fluconazole therapy was continued. At day 38, significant weight loss was evident due to inappetence. Azotaemia had worsened significantly (urea 27 mmol/l, creatinine 370 μmol/l) and hyperphosphataemia (2.47 mmol/l [1.0–2.3]) had developed. The LCAT was 1:32. The patient was re-admitted to hospital and given intravenous fluid therapy. Despite these measures, the cats' clinical status continued to decline and the owners opted for euthanasia 1 week later with no opportunity for necropsy examination.
This case report describes a non-healing wound in a cat attributable to chronic concurrent deep-seated infection with C neoformans/gattii species complex and M avium. This was considered highly unusual for two reasons. First, neither organism is typically found in non-healing open wounds and secondly, both organisms were present simultaneously, a finding that has not been documented previously. Cryptococcosis is typically a primary mycotic rhinosinusitis that develops subsequent to deposition of infectious basidiospores into the upper respiratory tract, while M avium either causes localised disease in immune competent hosts or widely disseminated disease in immune deficient hosts.
M avium is a slow growing saprophytic organism. It rarely causes disease in cats in Australia, whereas the ‘rapidly growing’ mycobacteria, eg, Mycobacterium fortuitum and Mycobacterium smegmatis not uncommonly give rise to infection of the skin and subcutis of Australian outdoor cats following penetrating traumatic injuries of fatty tissues such as the subcutaneous panniculus of the inguinal region.1–6 M avium is best known as a cause of disseminated disease in acquired immunodeficiency syndrome (AIDS) patients.2,7,8 In cats, it has been reported most often as a cause of disseminated disease affecting the subcutis, lymph nodes and internal organs and is thought to be associated with an immunodeficient state.1,9,10 A familial immunodeficiency has been proposed in families of Abyssinian 2 and Siamese breeds with a predisposition to disseminated disease. 11
Feline cryptococcosis has been extensively reported in the literature, most commonly affecting the rostral nasal cavity. Disseminated, central nervous system, cutaneous and atypical disease occur, although in most cases the nasal cavity is implicated as the primary site of infection.12–15 The patient in this report had no clinical evidence of cryptococcosis outside the pelvic limb. Nasal cytology was negative for capsulated yeasts although more sensitive diagnostic techniques, eg, computed tomography or mycotic culture of nasal washings, were not performed, therefore, subclinical primary disease at this site could not be discounted.
The presumed pathogenesis of infection in this case was interesting. Intuitively, simultaneous inoculation of both organisms in large numbers via a penetrating wound would seem the most plausible explanation for such a deep seated dual infection. This theory would have been strengthened had we conclusively proven no systemic involvement for both organisms. The cryptococcal infection was likely to have been localised to the limb based on the decreasing LCAT titre post amputation and negative nasal cytology. It should be noted, however, that the patient was receiving anti-fungal therapy at that time which will also cause LCAT titres to decrease. Polysaccharide antigen persists in circulating macrophages after successful therapy and thus declines slowly over time.
In humans, cryptococcosis of the subcutis or bone is rare except in HIV-positive individuals with AIDS.16–19 The patient in this case report had chronic renal insufficiency and as a consequence may have had reduced immune function, although direct evidence supporting an immunodeficient state in cats with chronic kidney disease is lacking. Circumstantiated evidence has implicated renal disease as a potential cause of ‘feline leprosy’ due to the novel east coast species described by Hughes et al. 20 Humans with end-stage renal disease develop an immunodeficient state caused by altered cytokine bioavailability, the presence of uraemic toxins, 21 increased free radical production and antioxidant depletion. 22
Treatment with amphotericin B was avoided due to the increased potential for nephrotoxicity in a patient such as ours with known kidney disease. Fluconazole was selected based on previous reports of efficacy and safety. 23 The decision to use terbinafine cream topically on an open wound was without evidence based support and is not known whether it had a positive or negative impact, although the depth of tissue involvement would have greatly limited its therapeutic efficacy.
A potential deficiency in managing this case may have been the lack of antimicrobials directed at the M avium once a definitive diagnosis was made. However, by the time the isolate was identified, the limb had been amputated, the patient was lively and appeared well and systemic involvement with M avium was not suspected. Without abdominal ultrasonography and/or necropsy examination, it is impossible to determine whether systemic mycobacteriosis was present, especially given the bone marrow involvement seen on histopathology. The eventual cause of decline in this patient could not be established definitively, however, we propose that the progressive renal dysfunction was the most significant factor, possibly promoted by increased tissue catabolism subsequent to longstanding infection and surgery. Chronic fluconazole therapy may have contributed to inappetence. There was no significant biochemical change to incriminate fluconazoleinduced hepatotoxicity, which is indeed rare in cats.
Cryptococcosis and mycobacteriosis occur occasionally in cats and usually have a reasonably predictable syndromic presentation. Cutaneous cryptococcosis is uncommon in the absence of nasal cavity disease and mycobacterial panniculitis is usually caused by ‘rapidly growing’ mycobacteria. This report describes a cat with co-infections of C neoformans/gattii species complex and M avium causing a deep soft tissue and bone infection that progressed to involve the bone marrow. We propose a transcutaneous mode of infection via a penetrating injury.
Acknowledgements
The authors would like to thank Dr Greg Thompson for the initial diagnostic and therapeutic clinical work with this patient and subsequent referral.
References
- 1.Malik R, Gabor L, Martin P, Mitchell DH, Dawson DJ. Subcutaneous granuloma caused by Mycobacterium avium complex infection in a cat. Aust Vet J 1998; 76: 604–7. [DOI] [PubMed] [Google Scholar]
- 2.Baral RM, Metcalfe SS, Krockenberger MB, et al. Disseminated Mycobacterium avium infection in young cats: overrepresentation of Abyssinian cats. J Feline Med Surg 2006; 8: 23–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horne KS, Kunkle GA. Clinical outcome of cutaneous rapidly growing mycobacterial infections in cats in the south-eastern United States: a review of 10 cases (1996–2006). J Feline Med Surg 2009; 11: 627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kipar A, Schiller I, Baumgartner W. Immunopathological studies on feline cutaneous and (muco)cutaneous mycobacteriosis. Vet Immunol Immunopathol 2003; 91: 169–82. [DOI] [PubMed] [Google Scholar]
- 5.Jang SS, Hirsh DC. Rapidly growing members of the genus Mycobacterium affecting dogs and cats. J Am Anim Hosp Assoc 2002; 38: 217–20. [DOI] [PubMed] [Google Scholar]
- 6.Malik R, Wigney DI, Dawson D, Martin P, Hunt GB, Love DN. Infection of the subcutis and skin of cats with rapidly growing mycobacteria: a review of microbiological and clinical findings. J Feline Med Surg 2000; 2: 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson DJ. Infection with Mycobacterium avium complex in Australian patients with AIDS. Med J Aust 1990; 153: 466–8. [DOI] [PubMed] [Google Scholar]
- 8.Guthertz LS, Damsker B, Bottone EJ, Ford EG, Midura TF, Janda JM. Mycobacterium avium and Mycobacterium intracellulare infections in patients with and without AIDS. J Infect Dis 1989; 160: 1037–41. [DOI] [PubMed] [Google Scholar]
- 9.Sieber-Ruckstuhl NS, Sessions JK, Sanchez S, Latimer KS, Greene CE. Long-term cure of disseminated Mycobacterium avium infection in a cat. Vet Rec 2007; 160: 131–2. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson K, Howie FE, Low JC, Cameron ME, Porter J, Sharp JM. Feline skin granuloma associated with Mycobacterium avium. Vet Rec 1998; 143: 109–10. [DOI] [PubMed] [Google Scholar]
- 11.Jordan HL, Cohn LA, Armstrong PJ. Disseminated Mycobacterium avium complex infection in three Siamese cats. J Am Vet Med Assoc 1994; 204: 90–3. [PubMed] [Google Scholar]
- 12.Malik R, Krockenberger MB, O'Brien CR, et al. Veterinary insights into cryptococcosis caused by Cryptococcus neoformans and Cryptococcus gattii. Cryptococcus: from human pathogen to model yeast. Washington DC: ASM Press, 2011; 489–505. [Google Scholar]
- 13.McGill S, Malik R, Saul N, et al. Cryptococcosis in domestic animals in Western Australia: a retrospective study from 1995–2006. Med Mycol 2009; 47: 625–39. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien CR, Krockenberger MB, Wigney DI, Martin P, Malik R. Retrospective study of feline and canine cryptococcosis in Australia from 1981 to 2001: 195 cases. Med Mycol 2004; 42: 449–60. [DOI] [PubMed] [Google Scholar]
- 15.Malik R, Wigney DI, Muir DB, Gregory DJ, Love DN. Cryptococcosis in cats: clinical and mycological assessment of 29 cases and evaluation of treatment using orally administered fluconazole. J Med Vet Mycol 1992; 30: 133–44. [DOI] [PubMed] [Google Scholar]
- 16.Moosbrugger EA. Cutaneous cryptococcosis in a patient on corticosteroid therapy for rheumatoid arthritis. Int J Dermatol 2008; 47: 630–2. [DOI] [PubMed] [Google Scholar]
- 17.Goldshteyn N, Zanchi A, Cooke K, Agha R. Cryptococcal osteomyelitis of the humeral head initially diagnosed as avascular necrosis. South Med J 2006; 99: 1140–41. [DOI] [PubMed] [Google Scholar]
- 18.Murphy SNT, Parnell N. Fluconazole treatment of cryptococcal rib osteomyelitis in an HIV-negative man. A case report and review of the literature. J Infect 2005; 51: e309–11. [DOI] [PubMed] [Google Scholar]
- 19.Xiujiao X, Ai'e X. Two cases of cutaneous cryptococcosis. Mycoses 2005; 48: 238–41. [DOI] [PubMed] [Google Scholar]
- 20.Hughes MS, James G, Taylor MJ, et al. PCR studies of feline leprosy cases. J Feline Med Surg 2004; 6: 235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Descamps-Latscha B. The immune system in end-stage renal disease. Curr Opin Nephrol Hypertens 1993; 2: 857–1012. [DOI] [PubMed] [Google Scholar]
- 22.Roudebush P, Polzin DJ, Ross SJ, Towell TL, Adams LG, Dru Forrester S. Therapies for feline chronic kidney disease. What is the evidence? J Feline Med Surg 2009; 11: 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Brien CR, Krockenberger MB, Martin P, Wigney DI, Malik R. Long-term outcome of therapy for 59 cats and 11 dogs with cryptococcosis. Aust Vet J 2006; 84: 384–92. [DOI] [PubMed] [Google Scholar]