Abstract
Recurrent constipation is a common problem in cats. Laxatives often are the cornerstone for management of recurrent constipation; however, there is a paucity of published research on laxative use in cats. This study investigated the safety and palatability of polyethylene glycol (PEG3350) in normal cats. All cats consumed the PEG3350 laxative for 4 weeks without changes in weight or food intake. In all cats soft stools were achieved. Effective doses varied widely in experimental cats, so individualized dosing is important. Mild, non-clinical hyperkalemia was noted although the cause is unknown.
Recurrent constipation is a common problem in cats. Affected cats consistently require laxatives as part of daily management to improve their quality of life. A variety of laxatives have been recommended for use in cats with constipation, including soluble and insoluble fiber, lactulose, and polyethylene glycol (PEG3350). 1 Despite the common use of laxatives, there is limited data in veterinary medicine regarding efficacy, dosage and safety of oral laxatives.
PEG3350 is a large molecular weight, water-soluble polymer used widely in human medicine as an osmotic laxative. It is not metabolized by the intestinal bacterial flora and is minimally absorbed by the intestines; therefore, it is retained inside the intestinal lumen. 2 It forms hydrogen bonds with 100 molecules of water per molecule, which creates high osmotic pressures within the bowel lumen. 2 The osmotic pressure opposes absorption of water out of the lumen, and therefore PEG3350 acts as both a bulking and softening agent for the treatment of constipation. Use of PEG3350 as an oral laxative has been studied extensively in humans and is one of the mainstay treatments for chronic constipation both in adults and children.3–9 This is a result of the superior efficacy of PEG3350 products for relief of constipation as well as the relative lack of adverse side-effects. Multiple meta-analyses have shown that PEG3350 is more effective laxative than lactulose and many other oral laxatives.3,10,11 Generally, side-effects are self-limiting and include abdominal distension and pain, nausea and excessive diarrhea. However, a single case of fatal hyponatremia has been reported when PEG3350 was used as a bowel preparation solution for colonoscopy. 12 This has been attributed to the development of a syndrome of inappropriate secretion of antidiuretic hormone and excess free water intake. Clinical hyponatremia has not been documented in patients who use daily low-dose PEG for treatment of chronic constipation.
Lactulose is another commonly used osmotic laxative. It is fermented by colonic bacteria and results in fecal volume expansion to help relieve constipation. 10 However, lactulose can also alter bowel flora, causing bloating and flatulence. There is a moderate amount of evidence in human medicine that lactulose is efficacious and safe for chronic constipation. 5 However, there are no veterinary studies to support its use.
Both soluble and insoluble dietary fibers can theoretically be helpful in chronically constipated patients. 1 The clinical evidence to support their use as laxatives is weak in humans and no clinical studies to date support their use in cats. Their use in chronically constipated adult humans is graded only as fair in systematic reviews, as only a few randomized controlled studies have shown any benefits with treatment.3,13 Also, the few controlled studies of dietary fiber use in chronically constipated pediatric patients showed no statistically significant benefit. 13 Therefore, while dietary fiber is anecdotally recommended for the management of chronic constipation, the evidence to support its use is very limited.
PEG3350 is readily available in a powder form, which can be added to a cat's regular food. It is sometimes recommended anecdotally by veterinarians and discussed on veterinary communities, such as the Veterinary Information Network (VIN, www.vin.com). PEG3350 without electrolytes (Miralax, RestoraLAX) was also recently approved as an over-the-counter medication in both United States and Canada and, therefore, is easily accessible to most owners. The aim of this study was to evaluate the safety and palatability of PEG3350 as a laxative in cats.
Materials and methods
Animals
Six healthy cats, aged 1–4 years, housed in a closed colony, were included in this study. Physical examination and routine bloodwork, including complete blood count and biochemistry profile were performed prior to the study. No history of diarrhea or constipation was noted in the colony. During the study, the animals were housed individually indoors. The animals were fed twice daily with a commercial diet (Iams, ProActive Adult formula, canned and dry). Water was available ad libitum. All animal use in this study complied with Canadian Council for Animal Care guidelines, and was approved by the University of Saskatchewan Animal Research Ethics Board.
Treatments
A pilot trial was performed in two of the cats to determine an effective initial dose prior to the therapeutic trial. After the pilot trial, all six cats were given an acclimation period of at least 7 days in an individual cage, prior to starting the therapeutic trial. Body weight, food intake and fecal scores were monitored daily during the entire study. After the acclimation period, PEG3350 with electrolytes (Colyte oral solution; Schwarz Pharma)was administered bymixing the powder with the food. A standard weight of canned and dry food was offered each meal. The canned and dry food was weighed separately and combined into a single container. The unconsumed amount was weighed to determine the daily food intake. Water loss from the canned food during the interval between feedings was assumed to be negligible, as all cats consumed most of the food offered at each meal. Fecal scores were assessed daily by one of the authors (FMT) and a trained assistant with a standardized fecal grading scale 14 (see Table 1), and the dosage of PEG3350 was titrated in each cat to achieve soft, but formed stool (grade II—III). The dosage was initiated at 1.9 g of PEG3350 per meal and the animals were fed twice daily. The dose was doubled if no effect was seen within 48 h. All cats were monitored for any adverse effects, including clinical dehydration, vomiting and abdominal discomfort. A complete blood cell count and serum biochemistry panel were obtained prior to, and 2 and 4 weeks after, PEG3350 administration was initiated. The blood samples were submitted to a referral laboratory (Prairies Diagnostic Services, Saskatoon, Saskatchewan) for analysis. After 4 weeks of PEG3350 administration, the medication was discontinued and the cats were monitored until the stool consistency was normal (grade IV—V) for two consecutive defecations. Palatability was indirectly assessed by food intake and body weight during the study.
Table 1.
Fecal grading scale (adopted from Hill's Pet Nutrition 14 ).
| Grade I | Greater than two-thirds of the feces in a defecation are liquid. The feces have lost all form, appearing as a puddle or squirt |
| Grade II | Soft-liquid feces; an intermediate consistency between soft and liquid feces. Approximately equal amounts of feces in a defecation are soft and liquid |
| Grade III | Greater than two-thirds of the feces in a defecation are soft. The feces retain enough form to pile, but have lost their firm cylindrical appearance |
| Grade IV | Firm-soft feces; an intermediate between the grades of firm and soft. Approximately equal amounts of feces in a defecation are firm and soft |
| Grade V | Greater than two-thirds of the feces in a defecation are firm. They have a cylindrical shape with little flattening |
Statistical analysis
The median daily food intake and body weight during the period with and without PEG administration were compared using the paired Wilcoxon signed rank test. Friedman's two-way analysis of variance (ANOVA) was used to compare the bloodwork results from the pretreatment, 2-week, and 4-week time points. All tests were two-tailed, and we used P ≤ 0.05 to indicate significance.
Results
All cats completed the study with no significant side-effects. Stools with consistency of grade I—III were achieved in all animals. Sporadic vomiting was observed in one cat during the treatment and the episodes of vomiting did not appear to be associated with the dosage of PEG3350 or any changes in the dose of PEG3350. No significant findings were noted on the cats' daily physical examination. Diarrhea (fecal grade less than II) was occasionally observed during the 4 weeks and the dosage of PEG3350 was decreased accordingly. Four out of the six cats had their dosage decreased during the study due to diarrhea. Food intake (P = 0.86) and body weight (P = 0.92) were not significantly influenced by PEG3350 administration. The cats had the target fecal grade in a median of 21.5 of the 28 days, with a range of 20–26 days. The median daily dose of PEG3350 powder for all cats to achieve target fecal grade was 3.0 ± 1.1 g. The median daily doses for individual cats to achieve target fecal grade were 0.8, 1.5, 3.0, 3.0, 3.8 and 3.8 g. The median time to return to normal fecal consistency after discontinuation of PEG was 5.5 ± 3.4 days with a range of 4–13 days.
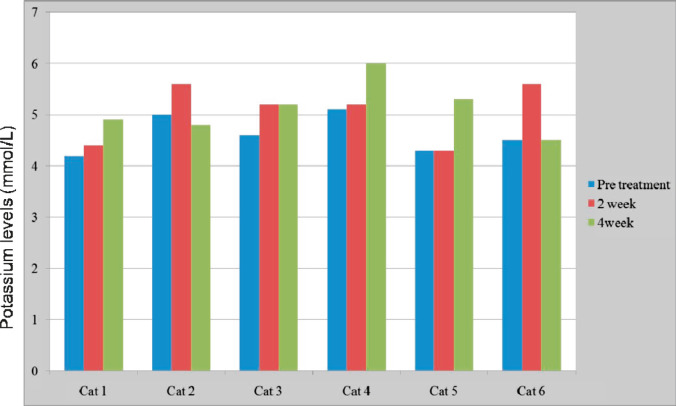
Mild erythrocytosis was found in 1/6 cats before and after the treatment (red blood count 11.3–12 × 1012/l, reference interval (RI) 6.89–10.8 × 1012/l). No other significant findings or changes with treatment were noted on the complete blood counts. No changes in serum sodium were found in any of the cats; however, three cats developed mild hyperkalemia (5.6–6.0 mmol/l, RI 3.9–5.5 mmol/l). The change in serum potassium was not statistically significant between any time points (P = 0.10; see Fig 1). Visual inspection of tabulated potassium concentration and dose vs time did not reveal an obvious association between dose and hyperkalemia. No clinical signs related to hyperkalemia were observed in any of the cats.
Fig 1.
Serum potassium level during treatment.
Discussion
Our study demonstrated that PEG3350 with electrolytes is a safe and palatable oral laxative in healthy cats when used for 4 weeks. Potential side-effects include hyperkalemia; however, the changes we noted were not clinically or statistically significant. The cause of the observed hyperkalemia is unknown. The electrolytes in the preparation were unlikely to be a concern due to the low concentration of potassium (0.14 mEq of potassium per gram). Renal dysfunction was not seen based on the biochemistry profile. Volume depletion due to subclinical dehydration from the diarrhea may have played a role in the hyperkalemia. This is not evidence that metabolic acidosis contributed to the hyperkalemia as both bicarbonate concentrations and the anion gaps were normal in all cats. However, blood gas analysis might be helpful to evaluate the acid—base status of these cats. Epithelial sodium channels, Na+K+2Cl- cotransporters and H+K+ATPase are presented on the colonic epithelial cells and function to regulate the serum potassium concentration in conjunction with the kidneys and the interaction of PEG3350 with these proteins is unknown. Determination of the transtubular potassium gradient might help to further characterize the cause of hyperkalemia. However, based on our findings, it is reasonable to recommend the routine monitoring of serum electrolyte concentrations and hydration status in cats receiving PEG3350. This is especially true if there are concurrent diseases (ie, chronic renal failure), or if medications (ie, ACE inhibitors, diuretics) are being used that may affect electrolyte balance. We also determined that the dosages necessary to achieve soft stools in these cats were highly variable. Individual dosage titration is likely to be needed in clinical patients.
No changes in food intake were observed during the study and the medication appeared well tolerated by all cats. In cats, palatability is an important factor to consider as administering oral medications to cats can be a major challenge. The ease of delivery can have a substantial impact on owner compliance and, therefore, the success of long-term management for constipation. Anecdotally, it has been noted that lactulose is not accepted by some cats. PEG3350 appears well suited as a chronic laxative in cats given the ease of administration and efficacy of the product. This study used PEG3350 with electrolytes, which in humans, was reported to be less palatable than PEG3350 without electrolytes when administered in water and other beverages, due to the salty taste. 15 It may not be relevant in our species as PEG3350 is administered with food. However, if an individual patient is not tolerating the addition of PEG3350 with electrolytes preparation in its diet, PEG3350 without electrolytes may be an attractive alternative choice. The efficacy and safety of PEG3350 with or without electrolytes is similar in human studies. 15 Although no similar studies were performed in cats, the amount of sodium and potassium in a standard PEG3350 with electrolytes preparation is 1.8mEq/g and 0.14mEq/g, respectively, which is insignificant compared to the daily requirement for our patients.
PEG3350 has the potential to play an essential role in the medical management of chronically constipated cats. Prospective clinical trials in patients with chronic constipation are indicated to establish the safety, as well as efficacy in clinical patients. Furthermore, comparison of the efficacy, of PEG3350 to other commonly used oral laxatives, such as lactulose will provide more insights for the management of chronic constipation in cats.
Conclusion
This study showed that polyethylene glycol 3350 is a safe and palatable oral laxative in cats for long-term use. It is also well suited as a chronic laxative in cats due to the ease of administration. Serum electrolyte levels and hydration status should be monitored closely when PEG3350 is used in clinical patients. Also, the dosage can be highly variable among cats and, therefore, titration is important. Further studies are needed to establish the safety and efficacy in cats with chronic constipation to improve our knowledge and ability to manage chronic constipation in these patients.
Acknowledgements
This study was fully funded by Companion Animal Health Fund (CAHF), Western College of Veterinary Medicine, University of Saskatchewan.
References
- 1.Zoran DL. Diet and drugs: the keys to managing feline colonic disease. Compend Contin Educ Pract Vet 1999; 21: 731–48. [Google Scholar]
- 2.Schiller LR, Emmett M, Santa Ana CA, Fordtran JS. Osmotic effects of polyethylene glycol. Gastroenterology 1988; 94: 933–41. [DOI] [PubMed] [Google Scholar]
- 3.Candy D, Belsey J. Macrogol (polyethylene glycol) laxatives in children with functional constipation and faecal impaction: a systematic review. Arch Dis Child 2009; 94: 156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao SS, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging 2010; 5: 163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramkumar D, Rao SSC. Efficacy and safety of traditional medical therapies for chronic constipation: systemic review. Am J Gastroenterol 2005; 100: 936–71. [DOI] [PubMed] [Google Scholar]
- 6.Di Palma JA, Cleveland MVB, McGowan J, Herrera JL. An open label study of chronic polyethylene glycol laxative use in chronic constipation. Aliment Pharmacol Ther 2006; 25: 703–8. [DOI] [PubMed] [Google Scholar]
- 7.Di Palma JA, Cleveland MVB, McGowan BS, Herrera JL. A randomized, multicenter comparison of polyethylene glycol laxative and tegaserod in treatment of patients with chronic constipation. Am J Gastroenterol 2007; 102: 1964–71. [DOI] [PubMed] [Google Scholar]
- 8.Dipalma JA, Cleveland MV, McGowan J, Herrera JL. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am J Gastroenterol 2007; 102: 1436–41. [DOI] [PubMed] [Google Scholar]
- 9.Candy DCA, Edwards D, Geraint M. Treatment of faecal impaction with polyethelene glycol plus electrolytes (PGE + E) followed by a double-blind comparison of PEG + E versus lactulose as maintenance therapy. J Pediatr Gastroetnerol Nutr 2006; 43: 65–70. [DOI] [PubMed] [Google Scholar]
- 10.Lee-Robichaud H, Thomas K, Morgan J, Nelson RL. Lactulose versus polyethylene glycol for chronic constipation (review). Cochrane Database Syst Rev 2010; 7: 1–38. [DOI] [PubMed] [Google Scholar]
- 11.Belsey JD, Geraint M, Dixon TA. Systematic review and meta analysis: polyethylene glycol in adults with non-organic constipation. Int J Clin Pract 2010; 64: 944–55. [DOI] [PubMed] [Google Scholar]
- 12.Nagler J, Popper D, Turetz M. Severe hyponatremia and seizure following a polyethylene glycol-based bowel preparation for colonscopy. J Clin Gastroenterol 2006; 40: 558. [DOI] [PubMed] [Google Scholar]
- 13.Johnson DA. Treating chronic constipation: how should we interpret the recommendations? Clin Drug Invest 2006; 26: 547–57. [DOI] [PubMed] [Google Scholar]
- 14.Strickling JA, Harmon DL, Dawson KA, Gross KL. Evaluation of oligosaccharide addition to dog diets: influences on nutrient digestion and microbial populations. Anim Feed Sci Technol 1999; 86: 205–19. [Google Scholar]
- 15.Sienela L, Sairanen U, Laine T, Kurl S, Pettersson T, Happonen P. Comparison of polyethylene glycol with and without electrolytes in the treatment of constipation in elderly institutionalized patients: a randomized, double-blind, parallel-group study. Drugs Aging 2009; 26: 703–13. [DOI] [PubMed] [Google Scholar]