Abstract
The subcutaneous (SC) route is often chosen for drug administration in cats because it is easier to perform than intravenous (IV) injection and is perceived as less painful than intramuscular (IM) injection. However, little is known of how the route of administration influences the pharmacodynamics of drugs. This study measured the changes in skin temperature and thermal threshold (TT) and recorded the side-effects after SC injection of 0.1 mg/kg of hydromorphone in six cats. Time to peak TT was 105 min. Skin temperature was elevated at 15 min and between 45 and 360 min. Five cats vomited and two exhibited marked dysphoria. Compared to previously published studies of IV and IM administration of hydromorphone, the SC route results in a slower onset of peak effect, a shorter duration of antinociception and is associated with more undesirable side-effects. As with IV and IM injections, SC administration of hydromorphone at 0.1 mg/kg is associated with a significant elevation in skin temperature. Overall, the SC route appears to have the least utility.
The use of the OP3 (mu)-agonist opioid hydromorphone in cats in both clinical 1–3 and research settings 4–6 has been reported. Hydromorphone is advocated as a perioperative analgesic agent in cats and doses ranging from 0.05 to 0.1 mg/kg given by the intravenous (IV), intramuscular (IM) or subcutaneous (SC) route have been recommended. 7 In a dose response study, Wegner and Robertson 5 reported that 0.1 mg/kg of hydromorphone given intravenously had a more rapid onset of action, longer duration, and more intense antinociceptive effect than 0.05 and 0.025 mg/kg. Based on these findings, the authors concluded that 0.1 mg/kg was likely to be the most appropriate clinical dose. In cats, IM and SC administration is often easier in a clinical setting and the SC route is believed to be less painful. However, little is known about the influence of the route of administration on the pharmacodynamic effects of opioid drugs in this species.
In research cats, an increase in skin temperature has been reported following the use of hydromorphone at 0.1 mg/kg IV. 5,6 In both a retrospective 2 and a prospective 3 clinical study hydromorphone has been implicated as a cause of post-anesthetic hyperthermia (measured by rectal temperature), although a specific dose or route of administration was not identified.
Vomiting associated with opioid administration in cats has been reported. 7,8 In research cats, vomiting was reported after SC and IM but not IV administration of morphine, 9–11 and after IM 4 but not IV administration of hydromorphone. 6
The objective of this study was to measure the changes in skin temperature and thermal threshold (TT) after the administration of 0.1 mg/kg of hydromorphone given by the SC route. The incidence of vomiting, retching and salivation, and behavioral changes such as sedation, euphoria or dysphoria were also recorded. These data were then compared to that previously collected in our laboratory for IV and IM administration.
Materials and Methods
TT studies
All studies were approved by the Institutional Animal Care and Use Committee at the University of Florida.
Six adult (1–2 years of age) cats (four castrated males and two spayed females) were used in this study. Cats weighed an average of 5.8 kg (range 4.9–6.9 kg). A physical examination, complete blood count and serum chemistry analysis were performed on all cats prior to testing. Cats were socialized and familiar with the testing procedures and environment. Ambient temperature was maintained between 22.3 and 22.6°C during the study. Cats were fed a commercial complete dry diet ad libitum and were not fasted prior to testing. Water was available throughout the testing period.
Hydromorphone (Dilaudid, 2 mg/ml; Abbott Laboratories, North Chicago, IL, USA) was administered at 0.1 mg/kg by the SC route, placed under the skin between the shoulder blades using a 23 SWG×1″ needle. TT was tested as previously described. 12 Briefly, a small probe containing a heater element and temperature sensor fixed together in thermally conducting epoxy was held against the shaved skin of the lateral thorax of the cat with an elastic band. A pressure bladder overlying the probe ensured even contact with the skin. Skin temperature was recorded before every test, and then a thermal stimulus was applied at a heating rate of 0.6°C/s. When the cat responded by jumping, flinching, or turning to look at the probe, the stimulus was terminated, and the threshold temperature recorded. To protect the cats from thermal injury, the stimulus was discontinued at 55°C if no response was observed. Baseline (time 0) skin temperature was recorded and three baseline TTs were obtained at 15 min intervals for each cat before administration of hydromorphone, which was given 5 min after the last baseline measurement. Skin temperature was recorded and TT tested at 15, 30, 45, 60, 75, 90, 105, 120, 150, 180, 210, 240, 270, 300, 330, 360, 390, 420, 480 and 720 min after treatment. The tester was not blinded to the treatment.
Observational studies
Vomiting, retching and salivation
The definitions for vomiting and retching proposed by Scholz et al 13 were used in this study. Vomiting was defined as the forceful expulsion of upper abdominal contents through the mouth. Retching refers to rhythmic activity of the diaphragm and abdominal muscles without expulsion of gastric contents. Salivation was defined as collection of clear, sometimes frothy fluid around the lips, with or without it dripping from the mouth.
Sedation and behavior
A subjective assessment of sedation (yes/no) was made for each cat during the study. Euphoria was defined as a cat that was calm and easy to handle and showed some or all of the following: purring, kneading with its forepaws, rolling and rubbing its head and body on the cage door. A cat was described as dysphoric if it resented being handled, was restless, pacing, agitated, or vocalizing in a plaintive manner.
Statistical analysis
The data for skin temperature and TT were analyzed by means of a two-factor analysis of variance with the fixed factor of time and the random factor of cat (SAS PROC MIXED, SAS Institute, Cary, NC 27513–2414, USA). Post hoc comparisons over time were by means of Bonferroni t test. P<0.05 was considered significant.
Results
Skin temperature (measured before each TT test)
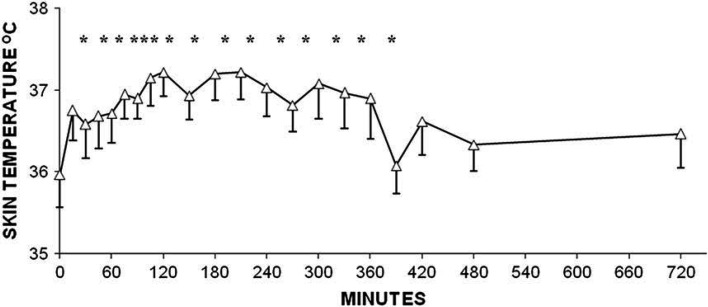
Skin temperature was significantly increased above baseline at 15 min and between 45 and 360 min following administration of hydromorphone. Peak temperature (mean±SD) was 37.2±0.7°C at 120 min (Fig 1).
Fig 1.
Changes in skin temperature (°C) after SC administration of 0.1 mg/kg of hydromorphone to adult cats. Data are shown as mean±SEM. *Denotes significant difference from time 0 (pre-treatment) P<0.05.
TT
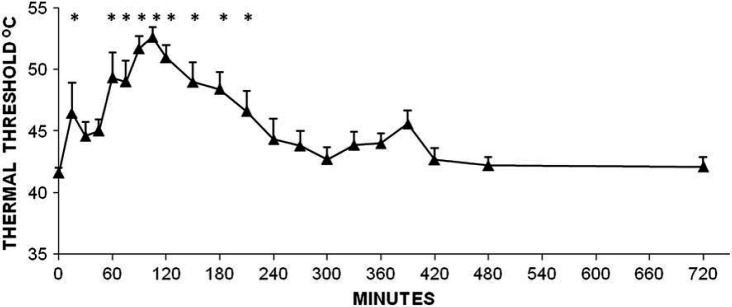
Peak TT (mean±SD) was 52.6±2.0°C and occurred at 105 min. Compared to time 0 (baseline) TT was significantly increased at 15 min and from 60 to 210 min after SC administration (Fig 2).
Fig 2.
Changes in TT (°C) after SC administration of 0.1 mg/kg of hydromorphone to adult cats. Data are shown as mean±SEM. *Denotes significant difference from time 0 (pre-treatment) P<0.05.
Vomiting and salivation
Five cats vomited; time of vomiting was 3, 3, 6, and 7 min after hydromorphone administration, and one cat vomited at 11 and 18 min. One cat retched 7 min after treatment. Salivation occurred in five cats and was marked in two of the cats, which produced large volumes of saliva that resulted in the fur on their neck, chest and forelimbs becoming soaked. Salivation began between 3 and 9 min and lasted for up to 170 min after administration of hydromorphone.
Behavioral changes
Two cats exhibited marked dysphoria which began within 15 min of injection and lasted for 210 min in one cat and 240 min in the other. These were the same two cats that salivated profusely. The other four cats showed signs of sedation beginning within 15 min of treatment. Duration of sedation was variable between cats but ranged from 120 to 300 min.
Discussion
In the current study, SC administration of hydromorphone resulted in significant antinociception at 15 min and from 60 to 210 min. The duration of antinociception after IV administration of hydromorphone at 0.1 mg/kg was from 15 to 450 min in one study 6 and from 5 to 200 min in another. 5 Intramuscular administration resulted in a significant increase in TT from 15 to 345 min. 4 After SC administration, the peak threshold of 52.6°C occurred 105 min after treatment. Peak TT was at or close to instrument cut-out (55°C) and occurred at 20 min 5 and 45 min 6 after IV dosing. Peak TT after IM injection was 49.0°C, occurring at 30 min. 4 SC administration of hydromorphone results in a slow onset of peak effect and a shorter duration of significant antinociception than the other two routes. The results for each route of administration are summarized in Table 1
Table 1.
Comparison of thermal antinociceptive effects of hydromorphone (0.1 mg/kg) given by the IV, IM and SC routes
The time to onset of action and peak effect after IV dosing in cats 5,6 is similar to that reported in humans. 14 Pharmacokinetic data are available for IV, but not IM and SC administration of hydromorphone in cats. 6 Therefore, it is not possible to determine if the differences in thermal antinociception described in this, and previous studies are due to differences in bioavailability, uptake, distribution and/or metabolism. In humans, the bioavailability of SC hydromorphone is 78%. 15 Very few studies have compared the pharmacokinetic profiles of opioids given by different routes in animals. In pigs, pethidine (meperidine) uptake was complete after IM and SC administration, 16 but these authors suggested that after SC administration, uptake was slower than elimination resulting in blunting of peak plasma levels.
In cats, the differences in antinociception between the routes of administration of hydromorphone may be related to the concentration gradient of drug between plasma and the central nervous system (CNS). After IV bolus administration a large initial concentration gradient would exist and hydromorphone would diffuse rapidly across the blood brain barrier and bind to opioid receptors in the CNS. Plasma concentrations of drug are expected to increase more slowly after IM and SC injections than for IV administration, and, therefore, the maximum gradient created between blood and the site of action would be smaller. In addition, drug may be removed from the plasma predominantly via hepatic uptake before crossing the blood brain barrier leading to occupation of fewer opioid receptors in the CNS. In pigs, the median tmax (time to peak plasma concentration) after IM and SC injections of pethidine (meperidine) was 10.4 and 21.4 min, respectively. Cmax (peak plasma concentration) was 2000 ng/ml after IM injection but only 880 ng/ml after SC administration. 16 No pharmacodynamic measurements were made in the 16 study. Therefore, no assumptions can be made about the relationship between plasma concentration of pethidine and its analgesic effect. In cats, the time to peak antinociceptive effects after SC hydromorphone was 105 min as compared to 30 min after IM injection. 4 This discrepancy may reflect a prolonged tmax and lower Cmax following SC administration compared with IM delivery as was observed in pigs.
Individual variation in the antinociceptive response to opioids has been reported in cats. 17,18 Because four of the six cats used in the current study were not included in previous IV and IM studies, such variation could have contributed to the observed differences in TT seen between studies. However, two cats received all three treatments, and both showed responses similar to the group response for each treatment. Therefore, it is likely pharmacokinetic parameters related to the route of administration played a greater role in resultant antinociceptive responses than did individual variation.
The association between hydromorphone use and post-anesthetic hyperthermia has been reported in cats in clinical settings. 2,3 Under research conditions, skin temperature was elevated between 105 and 270 min 6 and between 35 and 140 min 5 after IV administration. Intramuscular hydromorphone resulted in elevated skin temperatures between 150 and 240 min and in the current study a significant increase was seen at 15 min and between 45 and 360 min. Change in the mean skin temperature was 1.5°C at 135 min 6 and 0.9°C at 50 min 5 after IV dosing and 0.9°C at 150 min following IM injection. In the current study the peak rise in skin temperature of 1.2°C occurred at 120 min (Fig 1, Table 2). The findings of the current and previous studies suggest that the degree of hyperthermia associated with hydromorphone is not influenced by the route of administration, but is related to the dose. 5 However, these studies do suggest that the duration of hyperthermia may be related to route of administration, with the SC route resulting in the longest duration of elevated skin temperature.
Table 2.
Comparison of changes in skin temperature after administration of hydromorphone (0.1 mg/kg) by the IV, IM and SC routes
Because nausea is a subjectively unpleasant sensation associated with the urge to vomit 13 , we avoided using this term even though two cats in the SC group looked miserable and salivated profusely and may well have felt nauseous. Vomiting was not reported after IV administration. 5,6 Three of the six cats in the IM study vomited. 4 This occurred at 1 min after injection in two cats and after 5 min in the third cat (unpublished data). Mild, short lived (<5 min) salivation accompanied by lip licking was noted in four out of six cats after IV treatment, and mild salivation occurred between 45 and 120 min in one cat after IM treatment (unpublished data).
Systemically administered opioids can have both pro- and anti-emetic effects. Opioids stimulate the chemoreceptor trigger zone (CTZ) which is located outside the blood brain barrier in the area postrema of the medulla, thus promoting nausea and vomiting. 13,19 However, opioids also have anti-emetic effects related to their action on both emetic and anti-emetic brain centers located in the lateral reticular formation within the blood brain barrier. 13 The rate of diffusion across membranes is related to the lipid solubility of a drug as well as the trans-membrane concentration gradient. Rapid transfer following high doses of more lipophilic opioids is thought to directly inhibit the vomiting center. 20,21 Opioid induced nausea and vomiting is a well recognized clinical problem in humans with an incidence ranging from 10 to 61%. 22 In humans, no single opioid is consistently more emetogenic than another although there is individual variation. Switching from one opioid to another can influence the incidence of vomiting. 19,21 A review of the veterinary literature suggests that the incidence of opioid associated vomiting may be related to the species, drug used, dose, route of administration, and the use of co-administered drugs. In dogs, there was no significant difference in the incidence of vomiting after IM administration of morphine, oxymorphone or hydromorphone. 23 No vomiting was seen after IM administration of 0.22 mg/kg hydromorphone to dogs, 24 but occurred in 44% of dogs when a lower IM dose (0.1 mg/kg) was used. 23 In cats, both the opioid used and route of administration appear to be factors that influence vomiting. Intramuscular and SC but not IV injection of morphine caused most cats to vomit. 9–11 Pethidine (meperidine) is not recommended for IV use in cats because of adverse effects that include hypotension, excitement and convulsions, 25 but IM dosing is not associated with vomiting. 9,12 Buprenorphine did not cause vomiting in cats regardless of dose or route of administration. 9,10,26,27 Butorphanol and fentanyl are not reported to cause emesis in cats. 4,10,17,28 These reports suggest a correlation between the incidence of vomiting and the lipid solubility of the compound such that the more lipophilic opioids cause less vomiting. The lipid solubility of the specific opioids are characterized by the n-octanol/water partition coefficients which are as follows: morphine 0.7, hydromorphone 1.28, pethidine (meperidine) 38.9, 29 butorphanol 180 (Stadol (butorphanol tartrate) injection, USP; available at www.fda.gov), fentanyl 717.0, 29 buprenorphine 1943. 30 In vitro studies show that opioid transfer across isolated dura mater is a simple diffusion process independent of lipid solubility, related only to the initial drug concentration and thus to the concentration gradient across the membrane. 31 This could explain the antinociceptive onset differences between the routes of hydromorphone administration seen in cats as well as differences in the incidence of vomiting. A high plasma concentration of hydromorphone after IV bolus injection could lead to a rapid transfer across the blood brain barrier esulting in direct inhibition of the vomiting center. The lower concentration gradients expected after IM and SC dosing would result in slower blood brain barrier penetration, thereby allowing the emetic effects at the CTZ to predominate. In cats there are no published studies of hydromorphone given alone by the IM or SC route at doses greater than 0.1 mg/kg, therefore, correlations between dose and incidence of vomiting cannot be made.
When given prior to an opioid, acepromazine reduces the incidence of vomiting in dogs. 23 This may also be true in cats. In two clinical studies, acepromazine was used in combination with hydromorphone and vomiting was not reported as a common side-effect. 2,3
In humans and other species, hydromorphone is metabolized predominantly to hydromorphone-3-glucuronide (H-3-G) which has been shown to have potent neuro-excitatory effects in rats. 32 The pharmacokinetics of hydromorphone but not its major metabolite H-3-G have been studied in cats. 6 However, H-3-G is a close structural analog of morphine-3-glucuronide 32 which could not be detected in cats after IV or IM administration of morphine. 9 It is unlikely that cats produce large quantities of H-3-G because of the deficiency in their glucuronosyl transferase pathway. 33,34 Therefore, it is unlikely that H-3-G was responsible for the dysphoria and agitation seen in two cats after SC administration but more data are required to substantiate this statement. The dysphoric behavior may have been related to the distress of salivation and perhaps nausea. There were no adverse behavioral side-effects after IV administration, and all cats remained easy to handle, calm, and quiet. Sedation was not marked in any cat. 6 All cats in the IM study exhibited calm euphoria 4 and mild sedation ranging in duration from 15 to 240 min.
In summary, when hydromorphone is used in cats, the IV route is preferred over IM and SC administration because it provides a faster onset of antinociception without emesis or salivation. The duration of antinociception following SC administration of hydromorphone reported here is shorter than for IM 4 or the range of effect reported after IV administration. 5,6 All cats should be monitored for a rise in body temperature when 0.1 mg/kg hydromorphone is used regardless of the route of administration. Of the three routes studied, SC administration appears to have the least utility.
Acknowledgments
The authors would like to thank Dr J Hauptman, Michigan State University for statistical analysis.
References
- 1.Pettifer G., Dyson D. Hydromorphone: A cost-effective alternative to the use of oxymorphone, Can Vet J 41, 2000, 135–137. [PMC free article] [PubMed] [Google Scholar]
- 2.Niedfeldt R.L., Robertson S.A. Postanesthetic hyperthermia in cats: A retrospective comparison between hydromorphone and buprenorphine, Vet Anaesth Analg 33, 2006, 381–389. [DOI] [PubMed] [Google Scholar]
- 3.Posner L.P., Gleed R.D., Erb H.N., Ludders J.W. Post-anesthetic hyperthermia in cats, Vet Anaesth Analg 34, 2007, 40–47. [DOI] [PubMed] [Google Scholar]
- 4.Lascelles B.D., Robertson S.A. Antinociceptive effects of hydromorphone, butorphanol, or the combination in cats, J Vet Int Med 18, 2004, 190–195. [DOI] [PubMed] [Google Scholar]
- 5.Wegner K., Robertson S.A. Dose-related thermal antinociceptive effects of intravenous hydromorphone in cats, Vet Anaesth Analg 34, 2007, 132–138. [DOI] [PubMed] [Google Scholar]
- 6.Wegner K., Robertson S.A., Kollias-Baker C., Sams R.A., Muir W.W., 3rd Pharmacokinetic and pharmacodynamic evaluation of intravenous hydromorphone in cats, J Vet Pharmacol Ther 27, 2004, 329–336. [DOI] [PubMed] [Google Scholar]
- 7.Lamont L.A. Feline perioperative pain management, Vet Clin North Am Small Anim Pract 32, 2002, 747–763. [DOI] [PubMed] [Google Scholar]
- 8.Lukasik V. Premedication and sedation. Seymour C., Gleed R.D. Manual of Small Anim Anaesth and Analgesia, 1999, British Small Animal Veterinary Association: Cheltenham, 71–85. [Google Scholar]
- 9.Taylor P.M., Robertson S.A., Dixon M.J., Ruprah M., Sear J.W., Lascelles B.D., Waters C., Bloomfield M. Morphine, pethidine and buprenorphine disposition in the cat, J Vet Pharmacol Ther 24, 2001, 391–398. [DOI] [PubMed] [Google Scholar]
- 10.Robertson S.A., Taylor P.M., Lascelles B.D., Dixon M.J. Changes in thermal threshold response in eight cats after administration of buprenorphine, butorphanol and morphine, Vet Rec 153, 2003, 462–465. [DOI] [PubMed] [Google Scholar]
- 11.Steagall P.V., Carnicelli P., Taylor P.M., Luna S.P., Dixon M., Ferreira T.H. Effects of subcutaneous methadone, morphine, buprenorphine or saline on thermal and pressure thresholds in cats, J Vet Pharmacol Ther 29, 2006, 531–537. [DOI] [PubMed] [Google Scholar]
- 12.Dixon M.J., Robertson S.A., Taylor P.M. A thermal threshold testing device for evaluation of analgesics in cats, Res Vet Sci 72, 2002, 205–210. [DOI] [PubMed] [Google Scholar]
- 13.Scholz J., Steinfath M., Tonner P.H. Antiemetics. Evers A.S., Maze M. Anesthetic Pharmacology: Physiologic Principles and Clinical Practice: a Companion to Miller's Anesthesia, 2004, Churchill Livingston: Philadelphia, 777–791. [Google Scholar]
- 14.Coda B., Tanaka A., Jacobson R.C., Donaldson G., Chapman C.R. Hydromorphone analgesia after intravenous bolus administration, Pain 71, 1997, 41–48. [DOI] [PubMed] [Google Scholar]
- 15.Moulin D.E., Kreeft J.H., Murray-Parsons N., Bouquillon A.I. Comparison of continuous subcutaneous and intravenous hydromorphone infusions for management of cancer pain, Lancet 337, 1991, 465–468. [DOI] [PubMed] [Google Scholar]
- 16.Ranheim B., Hoiset J., Framstad T., Horsberg T.E., Skaare J.U., Soli N.E. Pharmacokinetics of pethidine in pigs following intravenous, intramuscular and subcutaneous administration, J Vet Pharmacol Ther 21, 1998, 491–493. [PubMed] [Google Scholar]
- 17.Lascelles B.D., Robertson S.A. Use of thermal threshold response to evaluate the antinociceptive effects of butorphanol in cats, Am J Vet Res 65, 2004, 1085–1089. [DOI] [PubMed] [Google Scholar]
- 18.Johnson J.A., Robertson S.A., Pypendop B.H. Antinociceptive effects of intramuscular butorphanol, buprenorphine or their combination in cats, Am J Vet Res 68, 2007, 699–703. [DOI] [PubMed] [Google Scholar]
- 19.Bailey P.L., Egan T.D., Stanley T.H. Intravenous opioid anesthetics. Miller R.D. Anesthesia, 5th edn, 2000, Churchill Livingstone: New York, 273–376. [Google Scholar]
- 20.Blancquaert J.P., Lefebvre R.A., Willems J.L. Emetic and antiemetic effects of opioids in the dog, Eur J Pharmacol 128, 1986, 143–150. [DOI] [PubMed] [Google Scholar]
- 21.Stein C., Rosow C.E. Analgesics: Receptor ligands and opiate narcotics. Evers A.S., Maze M. Anesthetic Pharmacology: Physiologic Principles and Clinical Practice: a Companion to Miller's Anesthesia, 2004, Churchill Livingstone: Philadelphia, 457–471. [Google Scholar]
- 22.Kovac A.L. Prophylaxis of postoperative nausea and vomiting: Controversies in the use of serotonin 5-hydroxytryptamine subtype 3 receptor antagonists, J Clin Anest 18, 2006, 304–318. [DOI] [PubMed] [Google Scholar]
- 23.Valverde A., Cantwell S., Hernandez J., Brotherson C. Effects of acepromazine on the incidence of vomiting associated with opioid administration in dogs, Vet Anaesth Analg 31, 2004, 40–45. [DOI] [PubMed] [Google Scholar]
- 24.Smith L.J., Yu J.K., Bjorling D.E., Waller K. Effects of hydromorphone or oxymorphone, with or without acepromazine, on preanesthetic sedation, physiologic values, and histamine release in dogs, J Am Vet Med Assoc 218, 2001, 1101–1105. [DOI] [PubMed] [Google Scholar]
- 25.Thurmon J.C., Tranquilli W.J., Benson G.J. Preanesthetics and anesthetic adjuncts. Thurmon J.C., Tranquilli W.J., Benson G.J. Lumb and Jones' Veterinary Anesthesia, 3rd edn, 1996, Williams and Wilkins: Baltimore, 183–209. [Google Scholar]
- 26.Robertson S.A., Taylor P.M., Sear J.W. Systemic uptake of buprenorphine by cats after oral mucosal administration, Vet Rec 152, 2003, 675–678. [DOI] [PubMed] [Google Scholar]
- 27.Robertson S.A., Lascelles B.D., Taylor P.M., Sear J.W. PK–PD modeling of buprenorphine in cats: Intravenous and oral transmucosal administration, J Vet Pharmacol Ther 28, 2005, 453–460. [DOI] [PubMed] [Google Scholar]
- 28.Robertson S.A., Taylor P.M., Sear J.W., Keuhnel G. Relationship between plasma concentrations and analgesia after intravenous fentanyl and disposition after other routes of administration in cats, J Vet Pharmacol Ther 28, 2005, 1–7. [DOI] [PubMed] [Google Scholar]
- 29.Roy S.D., Flynn G.L. Solubility and related physicochemical properties of narcotic analgesics, Pharm Res 5, 1988, 580–586. [DOI] [PubMed] [Google Scholar]
- 30.Ohtani M., Kotaki H., Sawada Y., Iga T. Comparative analysis of buprenorphine- and norbuprenorphine-induced analgesic effects based on pharmacokinetic–pharmacodynamic modeling, J Pharmacol Exp Therap 272, 1995, 505–510. [PubMed] [Google Scholar]
- 31.McEllistrem R.F., Bennington R.G., Roth S.H. In vitro determination of human dura mater permeability to opioids and local anaesthetics, Can J Anaesth 40, 1993, 165–169. [DOI] [PubMed] [Google Scholar]
- 32.Wright A.W., Mather L.E., Smith M.T. Hydromorphone-3-glucuronide: A more potent neuro-excitant than its structural analogue, morphine-3-glucuronide, Life Sci 69, 2001, 409–420. [DOI] [PubMed] [Google Scholar]
- 33.Court M.H., Greenblatt D.J. Biochemical basis for deficient paracetamol glucuronidation in cats: An interspecies comparison of enzyme constraint in liver microsomes, J Pharm Pharmacol 49, 1997, 446–449. [DOI] [PubMed] [Google Scholar]
- 34.Court M.H., Greenblatt D.J. Molecular basis for deficient acetaminophen glucuronidation in cats. An interspecies comparison of enzyme kinetics in liver microsomes, Biochem Pharmacol 53, 1997, 1041–1047. [DOI] [PubMed] [Google Scholar]