Abstract
The aim of this study was to characterise the scintigraphic findings in a large population of hyperthyroid cats in order to determine the location of thyroid pathology in newly diagnosed hyperthyroid cats and those that had previously undergone thyroidectomy. A specific aim was to identify the proportion of cats with ectopic hyperfunctional thyroid tissue and characterise the scintigraphic and clinical features of this subset of cats. Nearly one in five hyperthyroid cats was identified to have multiple areas of hyperfunctional thyroid tissue and/or intrathoracic hyperfunctional thyroid tissue where surgical thyroidectomy would not be curative. In addition, this study demonstrated that scintigraphy cannot reliably distinguish between thyroid carcinoma and adenoma. Owners should always be warned about the possibility of ectopic thyroid tissue before thyroidectomy is performed. In this study, intrathoracic hyperfunctional thyroid tissue and multiple areas of increased radionuclide uptake (IRU) were a common feature of benign thyroid disease and responded well to treatment with low dose radioiodine.
Thyroid scintigraphy is a useful imaging modality that can assist in the diagnosis and further evaluation of feline hyperthyroidism. 1 The technique relies on selective uptake of a radionuclide by thyroid tissue. Pertechnetate (99mTcO4) is the radionuclide routinely used for this purpose. It has similar size and charge to iodine and is taken up by thyroid tissue. Furthermore, it is readily available, inexpensive and has a short half-life making it much safer to use than iodine isotopes. 1–4 Hyperthyroidism is diagnosed by the increased level of uptake of pertechnetate by the thyroid gland in comparison with salivary tissue. 5
Thyroid scintigraphy also demonstrates the location of hyperfunctioning thyroidal tissue and is, therefore, useful in identifying ectopic thyroid tissue, which may be present anywhere from the base of the tongue caudally to within the thoracic cavity. 5,6 Scintigraphy can, therefore, assist with decisions regarding management of hyperthyroidism, in particular identifying patients where thyroidectomy would not be an appropriate treatment. In previous studies, intrathoracic thyroid tissue has been reported to occur in 8–21% of hyperthyroid cats, 2,7–9 often being the result of one thyroid lobe descending into the thoracic cavity, rather than presence of additional areas of isotope uptake discrete from the two lobes of the thyroid gland. More detailed descriptions of scintigraphic features of cats with hyperfunctional ectopic thyroid tissue are lacking.
Scintigraphic imaging has also been described to aid in determination of malignancy and certain scintigraphic features of malignancy have been described. 2,4,10–12 However, these authors have frequently observed cats with ectopic hyperfunctional thyroid tissue, and cases with scintigraphic features of malignancy that appear to have benign thyroid disease.
Therefore, the aim of the present study was to characterise the scintigraphic findings in a large population of hyperthyroid cats in order to determine the location of thyroid pathology in newly diagnosed hyperthyroid cats and those that had previously undergone thyroidectomy. A specific aim was to identify the proportion of cats with ectopic thyroid tissue and characterise the scintigraphic and clinical features of the subset of cats.
Materials and Methods
The database of the University of Bristol (UOB) Feline Centre was searched for cases of feline hyperthyroidism referred between 1994 and 2007. The sole inclusion criterion for the study was that a hyperthyroid cat should have good quality scintigraphic images available for review. Scintigraphic imaging is routinely performed on all hyperthyroid cats referred to UOB, in order to assist with treatment planning. One hundred and twenty cats were identified that met this criterion.
Details of signalment, physical abnormalities (in particular the presence of palpable goitre), whether previous thyroidectomy had been performed, reason for referral, treatment modality and response to treatment were recorded from case records where available.
Scintigraphy was preformed according to the following protocol: 37–111 MBq of sodium 99mTc-pertechnetate (Na99mTcO4) was injected intravenously. Using a Maxi Gamma Camera (General Electric, Horsholm, Denmark) with a low energy, general purpose parallel hole collimator, ventral, right and left lateral images of the cervical region and thorax were obtained 20 min after radionuclide injection. The cats were placed directly on the collimator using propofol sedation (Rapinovet; Schering-Plough Animal Health) to facilitate positioning. A 256×256 matrix was used, and each image was acquired for a total of 150,000 counts. The images were processed using dedicated nuclear medicine software (Micas X-plus Version 5.20, Bartec Medical Systems). Hyperfunctionality of thyroid tissue was measured by using the software to compare the count density obtained from thyroid and zygomatic-molar salivary regions of interest. All areas of hyperfunctional thyroid tissue were termed ‘areas of increased radionuclide uptake (IRU)’.
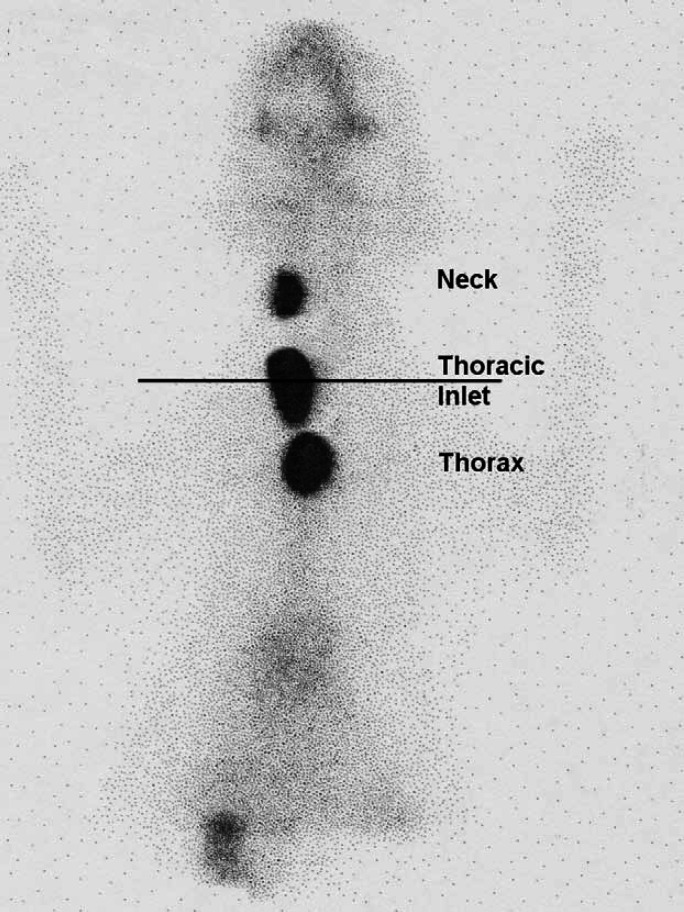
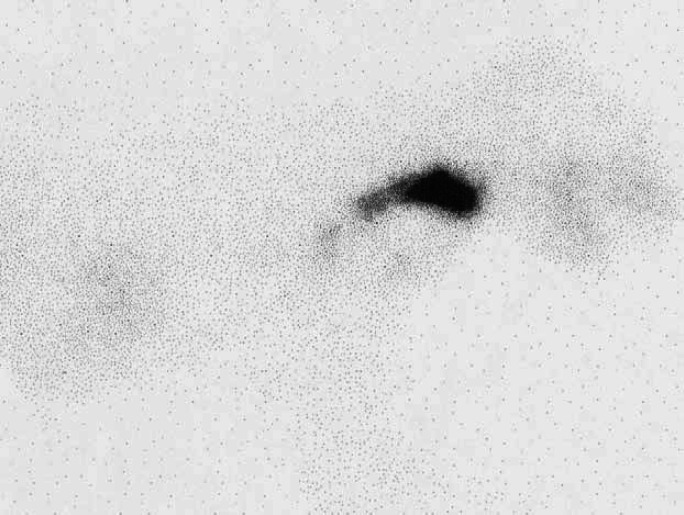
Scintigraphic images from these 120 hyperthyroid cats were evaluated. The ventral images were used to identify the location of areas of IRU. A straight line was drawn between the points of the shoulders. Areas of IRU above this line were classified as being in the neck, areas of IRU on the line were classified as being at the level of the thoracic inlet, and areas of IRU below the line classified as being within the thorax (Fig 1). When a large area of IRU was present extending from the cervical area to the thorax (Fig 2), this was classified as IRU in the neck, thoracic inlet and thorax and a more detailed description describing the pattern of uptake was made.
Fig 1.
Scintigraphy scan, ventral view illustrating how the locations of areas of IRU were classified.
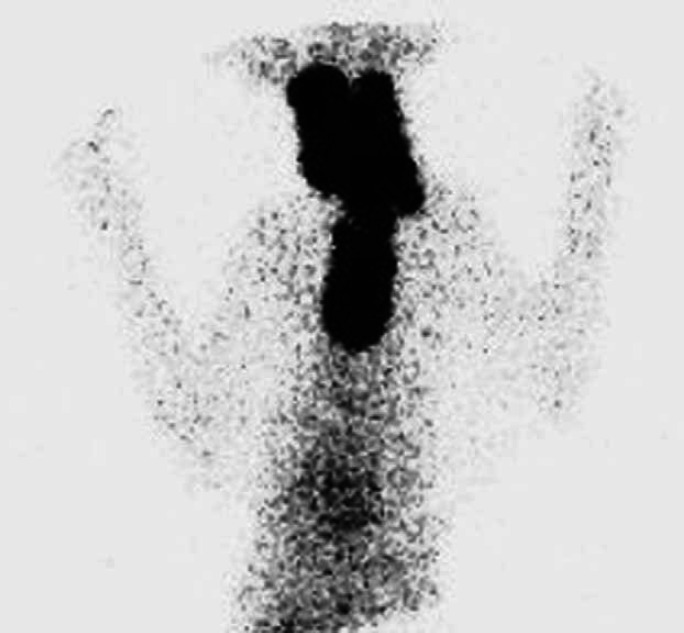
Fig 2.
Scintigraphy scan, ventral view (Table 2; case 24) showing very extensive area of IRU involving both glands in cervical area extending into cranial thorax. In such cases that involved all three areas, the areas of IRU were described as being present in the neck, thoracic inlet and thorax and a more detailed description describing the pattern of uptake was made (Table 2). This thyroid gland had a very cystic appearance on ultrasound.
The number and location of all areas of IRU were recorded, and the presence of ectopic radionuclide uptake was noted. Ectopic hyperfunctional thyroid tissue was defined as uptake of radionuclide in more than two foci, and/or the presence of one or more areas of IRU located within the thorax.
Of particular interest was the subset of cats identified with ectopic hyperfunctional thyroid tissue. In these animals, an analysis was made of the reason for referral, whether the cat was newly diagnosed or had undergone previous surgery, whether the cat had palpable goitre and/or areas of IRU present in the neck and/or thoracic inlet, any histopathological findings, and the treatment and outcome. Scintigraphic images were also evaluated for similarities and differences between cats with carcinoma versus adenoma, and those that responded to radioiodine treatment versus those that did not. The referring veterinarian was contacted by telephone to obtain follow-up information about these cats with ectopic thyroid tissue. Details regarding recurrence of hyperthyroidism, development of any other illness and the time and reason for death or euthanasia were obtained.
In cases where thyroid histopathology had been performed (n=40), the sections were reviewed on one occasion by a single pathologist (MJD) in order to verify the initial histopathological diagnosis and to determine whether any particular histopathological pattern might be associated with the presence of ectopic thyroid tissue.
Results
The age of the cats ranged from 6 to 18 years (mean 12 years). One hundred and fifteen cats were domestic shorthairs, three were domestic longhairs and two were Siamese. Sixty were neutered males, one was an entire male and 59 were neutered females.
The primary reason for referral was recorded for 51 of the 120 cases and included difficulty medicating (16 cases), owners interest in radioiodine treatment (10 cases), persistence or recurrence of hyperthyroidism following thyroidectomy (10 cases), inability to stabilise with medical management (five cases), difficulty medicating together with lack of palpable goitre (three cases), adverse effects of methimazole (three cases), presence of a very large palpable goitre (two cases) and treatment of thyroid carcinoma (two cases).
On evaluation of the scintigraphic scans, the number of areas of IRU ranged from 1 to 5 (mean 2), with 14 cats (12%) having more than two areas of IRU. Areas of IRU were located in the neck in 73 cats (61%), the thoracic inlet in 64 cats (53%) and the thorax in 26 cats (22%) (Table 1). The cats with areas of IRU in the thorax included 13 of the 14 cats that had more than two areas of IRU. The remaining cat with multiple areas of IRU had two in the neck and one in the thoracic inlet. Forty-four cats (37%) had areas of IRU in the neck only, 36 cats (30%) had areas of IRU in the thoracic inlet only, and nine cats (8%) had areas of IRU in the thorax only. Fifteen cats (13%) had areas of IRU in both the neck and thoracic inlet, five cats (4%) had areas of IRU in both the neck and thorax and three cats (3%) had areas of IRU in both the thoracic inlet and thorax. Nine cats (8%) had areas of IRU in all three locations (neck, thoracic inlet and thorax).
Table 1.
Table showing number and locations of areas of IRU
| Total number of areas of IRU | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Total number of cats | 60 | 46 | 8 | 4 | 2 |
| Number of cats with area(s) IRU in neck | 29 | 32 | 7 | 4 | 2 |
| Number of cats with area(s) IRU in thoracic inlet | 25 | 27 | 5 | 4 | 2 |
| Number of cats with area(s) IRU in thorax | 6 | 7 | 7 | 4 | 2 |
Eighty-six cats (72%) had palpable goitre on examination, of which 50 had areas of IRU in the neck and 51 in the thoracic inlet. There were, therefore, 20 cats with areas of IRU in the neck and 10 cats with areas of IRU in the thoracic inlet which were not palpable. Ten of the cats that did have a palpable nodule (12%) also had one or more areas of IRU in the thorax.
Twenty-eight cats (23%) had undergone previous thyroidectomy. Of these, 13 animals had previously undergone a single unilateral thyroidectomy (UT), three had undergone two unilateral thyroidectomies (one on each side) 8 months to 2 years apart, one had undergone three previous unilateral thyroidectomies (twice on one side and once on the other) approximately 1 year apart and one cat had undergone a previous bilateral thyroidectomy (BT). Two of these 18 cats had been reported to have remained persistently hyperthyroid following initial surgery and 11 of the cats were reported to have initially become euthyroid but had experienced recurrence of hyperthyroidism 6 weeks to 2 years later. The nature of the previous surgery was unrecorded in the remaining 10 cats. Of the cats that had previously undergone thyroidectomy, hyperfunctioning thyroid tissue was found unilaterally in the neck in six cats, unilaterally in the thoracic inlet in three cats, unilaterally in the thoracic inlet and/or neck and in the thorax in four cats, bilaterally in the neck and/or thoracic inlet in two cats, bilaterally in the neck and/or thoracic inlet and in the thorax in eight cats, and in the thorax alone in five cats. In total, ectopic hyperfunctional thyroid tissue was, therefore, present in 17 of the 28 cats (61%) that had previously undergone thyroidectomy.
Twenty-seven cats (23%) were found to have ectopic hyperfunctional thyroid tissue (Table 2). Twenty-six of these cats had intrathoracic areas of IRU, 13 of which also had more than two areas of IRU. One of the cats had three areas of IRU located in the neck and thoracic inlet. Of these 27 cats, 17 (63%) had undergone previous thyroidectomy. Twelve of these 17 animals had been referred for further investigation because of persistence or recurrence of hyperthyroidism following surgery. The remaining five of these 17 cats had been referred for treatment of a thyroid carcinoma (two cases), suspicion of a carcinoma (one case), owner's interest in radioiodine treatment (one case) and lack of response to radioiodine (one case). At least six of the cats that had undergone previous surgery only had a single UT, so presence of ectopic hyperfunctional thyroid tissue was not necessarily suspected, and 10 of the 27 cats (37%) with ectopic thyroid tissue had not had any previous surgery, having been referred as newly diagnosed hyperthyroid cats either because of owners specific interest in radioactive iodine treatment or difficulty in medicating the cats.
Table 2.
Details of cats identified with ectopic hyperfunctional thyroid tissue
| Case number | Number of hotspots | Additional features on imaging | Palpable nodule | Previous surgery | Reason for referral | Histopathology | Treatment | Outcome | Long-term follow-up | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neck | TI | Thorax | |||||||||
| 1 | 1 | 1 | Mediastinal 1 cm diameter ST opacity on XR | Yes | R then L UT 2 years apart | Recurrence of hyperthyroidism | ND | LD radioiodine | Euthyroid within 4 weeks post treatment | Still alive and euthyroid 4.5 years post treatment | |
| 2 | 1 | 2 | Yes | No | Owners interest in iodine treatment | ND | LD radioiodine | Euthyroid within 4 weeks post treatment | Lost to long-term follow-up | ||
| 3 | 2 | 1 | 2 | No | L then R UT | Recurrence of hyperthyroidism | ND | Carbimazole | Well controlled with medical management | Euthanased 5 months later with pancreatic neoplasia | |
| 4 | 2 | 1 | Yes | UT | Recurrence of hyperthyroidism, large palpable goitre | ND | Carbimazole | Well controlled with medical management | Euthanased 10 months later for unknown reason; euthyroid at time of euthanasia | ||
| 5 | 1 | 1 | 1 | Very large thoracic area of IRU | Yes | No | Difficulty medicating | ND | LD radioiodine | T4 reducing but not yet euthyroid after 4 weeks | Developed CHF, died 4 weeks post treatment |
| 6 | 1 | 1 | Mediastinal ST opacity on XR | No | UT | Carcinoma and concurrent disease | Carcinoma | No treatment | Severe concurrent cystitis and liver disease, euthanased at time of diagnosis | NA | |
| 7 | 1 | 1 | 2 | Yes | BT | Recurrence of hyperthyroidism | Adenoma | LD radioiodine | Remained hyperthyroid, successfully controlled with carbimazole treatment | Euthanased 2.5 years later with renal failure | |
| 8 | 2 | No | Yes | Recurrence of hyperthyroidism | ND | Carbimazole | Stabilised with medical management | Lost to follow-up | |||
| 9 | 1 | 1 | Yes | Yes | Recurrence of hyperthyroidism | Adenoma | Carbimazole | Stabilised with medical management | Lost to follow-up | ||
| 10 | 1 | No | Yes | Recurrence of hyperthyroidism | ND | Carbimazole | Stabilised with medical management | Lost to follow-up | |||
| 11 | 3 | 1 | 1 | On initial scintigraphy multiple areas of IRU in neck/TI/thorax, raising suspicions of carcinoma. Scintigraphy repeated after removal of further nodules for histopathology | No | UT 3 months previously, further thyroid nodules removed when hyper-thyroid-ism recurred | Recurrence of hyperthyroidism and suspicion of carcinoma | Adenoma | LD radioiodine | T4 significantly reduced 5 weeks post treatment but not yet euthyroid | Lost to follow-up |
| 12 | 2 | 1 | Clinician suspicious of carcinoma because of multiple areas of IRU | Yes | 2 L UT | Recurrence of hyperthyroidism | 2 Nodules removed – both adenoma | Methimazole | Well controlled with medical management | Developed laryngeal squamous cell carcinoma a few months later, time of euthanasia unknown | |
| 13 | 2 | Large area of IRU extending from inside thoracic inlet to heart base, clinician suspicious of carcinoma | Yes | UT 4 years previously | Persistence of hyperthyroidism following thyroidectomy | Adenoma | Surgical biopsy for histopathology, followed by LD radioiodine | Became hypothyroid; stabilised with thyroxine | Euthyroid (still on thyroxine) 2 years later then lost to further follow-up | ||
| 14 | 1 | No | No | Not recorded | ND | LD radioiodine | Unknown | Lost to follow-up | |||
| 15 | 2 | 1 | Extremely large area of IRU within mediastinum, connected to bilateral areas of IRU in neck | Yes | No | Owners interest in iodine treatment | ND | No apparent response to LD radioiodine, then treated with HD radioiodine | Euthyroid within 10 weeks post treatment | Lost to follow-up | |
| 16 | 1 | No | No | Poor response to methimazole | ND | LD radioiodine | Euthyroid within 4 weeks post treatment | Still alive and euthyroid 3 years post-treatment | |||
| 17 | 2 | 1 | 1 | Yes | No | Lack of response to LD radioiodine | ND, FNA suggestive of carcinoma | HD radioiodine | Euthyroid within 10 weeks post treatment, became hypothyroid | Still alive and hypothyroid (on thyroxine) 2.5 years later | |
| 18 | 2 | No | No | Poor response to methimazole and no palpable thyroid | ND | LD radioiodine | Euthyroid within 4 weeks post treatment | Still alive and euthyroid 21 months post treatment | |||
| 19 | 1 | No | 3 UT 1 year apart | Persistence of hyperthyroidism following 3rd thyroidectomy | ND | LD radioiodine | Euthyroid within 4 weeks post treatment | Still alive and euthyroid 27 months post treatment | |||
| 20 | 1 | Very large thoracic area of IRU | No | Yes | Recurrence of hyperthyroidism | ND, FNA suggestive of carcinoma | HD radioiodine | Died 2 weeks following treatment, cause unknown | NA | ||
| 21 | 1 | 1 | 1 | Widespread uptake of pertechnetate throughout pulmonary fields | Yes | UT | Treatment for carcinoma | Carcinoma | HD radioiodine | Euthyroid within 10 weeks post treatment | Remained euthyroid, euthanased 25 weeks post treatment due to pancytopenia (FIV positive) |
| 22 | 1 | 1 | 1 | Yes | UT | Difficulty medicating and owners interest in iodine treatment | ND | LD radioiodine | Euthyroid within 4 weeks post treatment | Lost to follow-up | |
| 23 | 1 | 1 | Yes | UT 8 months previously | Recurrence of hyperthyroidism and very large goitre led to suspicion of carcinoma | ND | LD radioiodine | T4 had reduced within 4 weeks post treatment but not euthyroid | 5 Months post treatment still not euthyroid but T4 continuing to decrease | ||
| 24 | 2 | 1 | 1 | Very extensive area IRU involving both glands in cervical area extending into cranial thorax cystic appearance on ultrasound | Yes | Yes | Poor response to medical treatment and large goitre suspicious of carcinoma | FNA suggestive of carcinoma, biopsy planned but died before performed | Methimazole | Euthanased due to development of CHF during investigations | NA |
| 25 | 2 | 1 | 1 | 1 Large area IRU involving both glands, down cervical region into thorax | Yes | No | Poor response to methimazole, large goitre | Adenomatous hyperplasia | LD radioiodine | No reduction in T4 | Stabilised more easily on lower dose methimazole compared with pre-iodine, despite no reduction in T4 with radioiodine alone. Alive and euthyroid 15 months post treatment |
| 26 | 1 | No | No | Difficulty medicating | ND | LD radioiodine | Euthyroid within 4 weeks post treatment | Still alive and euthyroid 7 months post treatment | |||
| 27 | 2 | 1 | Yes | No | Difficulty medicating | Adenomatous hyperplasia | Surgery | Euthyroid post surgery | Lost to follow-up | ||
R=right sided, L=left sided, ND=not done, FNA=fine needle aspirate, NA=not applicable.
Following scintigraphic evaluation, 56 cats were treated with subcutaneously administered radioactive iodine. Anti-thyroid medication was withdrawn 2 weeks prior to radioactive iodine treatment. Fifty-one cats were treated with a single dose of 110 MBq (3 mCi, ‘low dose (LD)’), and five cats were treated with a single dose of 1100 MBq (30 mCi, ‘high dose (HD)’), either due to confirmed thyroid carcinoma (three cases) or because of a lack of response to a previous single treatment of LD iodine and suspected thyroid carcinoma (two cases). Figure 3 illustrates the scintigraphic scan of one of these cases. The cats treated with LD iodine included 14 of the cats with ectopic hyperfunctional thyroid tissue.
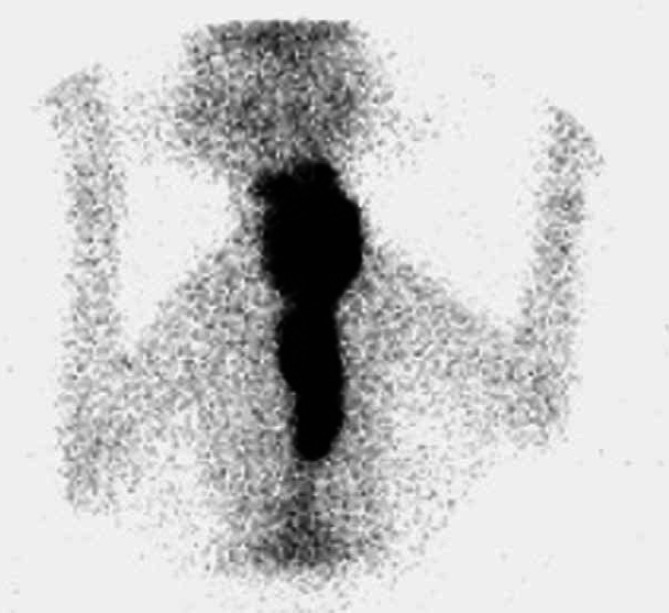
Fig 3.
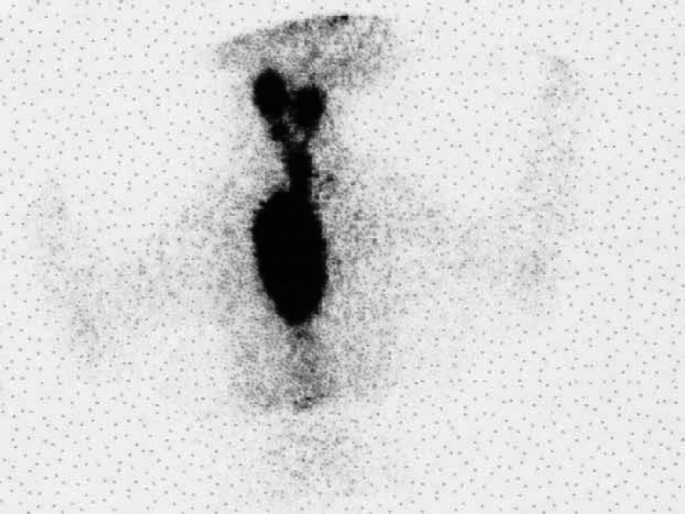
Scintigraphy scan, ventral view (Table 2; case 15) showing an extensive area of IRU involving both glands in the neck extending to a very large intrathoracic area of IRU. Histopathology was not performed in this case. The cat failed to respond to LD radioiodine but became euthyroid following HD radioiodine.
The majority of cats treated with LD radioiodine was euthyroid within 4 weeks post-treatment. In addition to the two cases that went on to receive HD radioiodine, two other cases were recorded as not responding to LD radioiodine. One cat had medium sized bilateral hotspots in the neck and then went on to have a BT; histopathology confirmed thyroid adenoma. The other case (Table 2; case 25) had a very extensive area of isotope uptake involving both glands in the neck and extending down the cervical region into the thorax (Fig 4). This cat also had thyroid biopsies confirming adenoma. The cat went on to be well stabilised with medical management. Three additional cases were also recorded that had not become euthyroid within the follow-up period, but thyroxine concentrations had significantly reduced. All of these cases had ectopic thyroid tissue (Table 2; cases 5, 7 and 11) and two (cases 7 and 11) had microscopically confirmed thyroid adenoma.
Fig 4.
Scintigraphy scan, ventral view (Table 2; case 25) showing an extensive area of IRU involving both glands in the neck and extending down the cervical region into the thorax. Biopsies of the mass in the neck confirmed adenomatous hyperplasia. The cat only partially responded to LD radioiodine treatment and went on to be well stabilised with medical management.
Only one of the cats treated with HD radioiodine did not become euthyroid; this was one of the confirmed carcinoma cases. A further cat (case 20) died shortly after treatment. Nineteen cats were treated with thyroidectomy, six cats were stabilised successfully with medical management and three cats received no further treatment for thyroid disease. Treatment modality in the remaining cases was not recorded. Further, follow-up information was only obtained for the cats with ectopic hyperfunctional thyroid tissue.
Histopathological examination of thyroid tissue was performed in 40 cases. Thirty cases were diagnosed with adenoma/adenomatous hyperplasia and 10 with carcinoma. Sections from all cats with carcinoma and 13 cats with adenoma were available for review. In all cases, there was agreement with the previous histological diagnosis. The cats identified with thyroid carcinomas have been previously described. 13
Thyroid tissue from eight of the 26 cats with intrathoracic hyperfunctional thyroid tissue was examined microscopically. Two of these eight cats had thyroid carcinoma and six had adenoma/adenomatous hyperplasia (five of these also had more than two areas of IRU). The cat with three areas of IRU in the neck/thoracic inlet had adenomatous hyperplasia (case 27). Of the 18 cats that did not have histopathological assessment of thyroid tissue, at least a further seven cats became euthyroid following treatment with LD radioiodine (including two cats with more than two hotspots) and remained euthyroid with follow-up periods of 21 months to 4.5 years. A further four of these 18 cats, including two cats with more than two hotspots, were successfully controlled with medical management. The remaining seven cats either died (one from congestive heart failure (CHF), two from unknown cause) or were euthanased due to thyroid carcinoma and concurrent disease (one case), lost to follow-up (one case), became euthyroid following HD radioiodine administered due to suspected thyroid carcinoma (one case) or had not yet become euthyroid at the point of follow-up although T4 was continuing to decrease (one case).
Many of the cats in this study identified as having intrathoracic hyperfunctional thyroid tissue were initially suspected to have thyroid carcinoma, particularly if there were very large and/or multiple and/or irregular areas of IRU (Fig 5). In addition, six further cases were initially suspected to have carcinoma. Two of these six cats had pulmonary masses evident on radiographs; both were subsequently diagnosed with thyroid adenoma on histology (one had a single area of IRU in the neck; the other had one area of IRU in the neck and one in the thoracic inlet). One cat had a mediastinal mass evident on radiographs (Fig 6); on ultrasonographic and cytological examinations this was found to be filled with acellular fluid. There was no uptake of technetium in the mediastinum (two areas of IRU in the neck only) and the cat became euthyroid following LD iodine treatment. A further cat had a single very large cervical thyroid mass that was confirmed to be thyroid adenoma on histopathology. The remaining two cats also had a large cervical mass which on scintigraphy had an irregular outline and very patchy appearance (eg, Fig 7); on histopathology one was found to be a cystic adenoma and the other adenomatous hyperplasia.
Fig 5.
Scintigraphy scan, ventral view (Table 2; case 13) showing the presence of large irregular areas of IRU within the thorax. Biopsy of the mass revealed microscopical features consistent with an adenoma and the cat responded well to treatment with LD radioiodine.
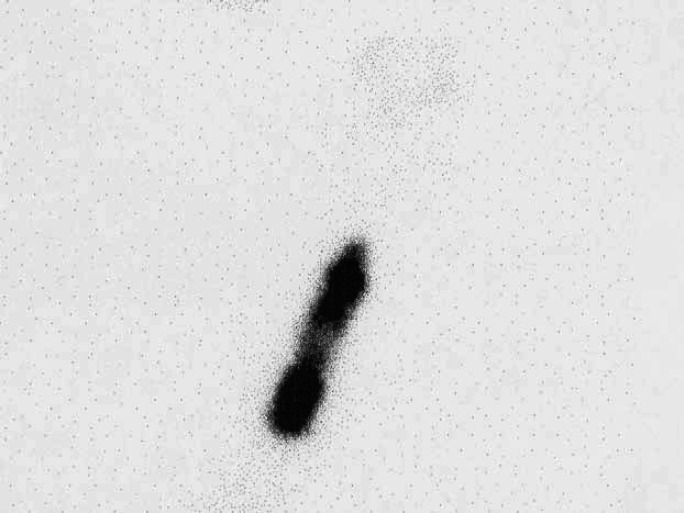
Fig 6.
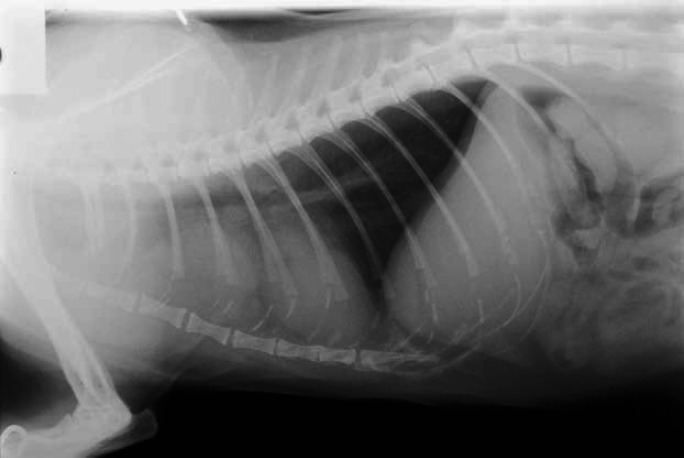
Thoracic radiograph demonstrating the appearance of a mediastinal cyst, which led to the suspicion of a thyroid carcinoma. However, there was no uptake of isotope in the thorax, and the cat responded fully to LD radioiodine.
Fig 7.
Scintigraphy scan, lateral view from a cat with a suspected carcinoma. An irregular outline and patchy appearance can be seen. This was diagnosed as a cystic adenoma on histopathological examination.
Discussion
The most appropriate treatment option for any individual hyperthyroid cat is determined by various factors, and the presence of multiple foci of abnormal thyroid tissue or ectopic thyroid tissue is an important consideration. The commonly used treatment options for feline hyperthyroidism are medical management with anti-thyroid drugs, surgical thyroidectomy and radioactive iodine treatment (131I). Radioiodine treatment is often considered to be the best treatment option for many cats because it has the greatest potential to cure the disease with a single treatment, it is not dependent on the location of the hyperfunctional thyroid tissue, no general anaesthesia is required and there are minimal side effects. 2,14–16 However, in the United Kingdom there is relatively limited availability of this treatment modality and the major disadvantage is the necessity, for health and safety reasons, for a long period of hospitalisation in isolation facilities following administration of 131I. Until recently the required isolation time at UOB was 5 weeks (now reduced to 3 weeks), which made radioactive iodine treatment less than ideal. For these reasons, thyroidectomy remains a common first choice of treatment for feline hyperthyroidism and is a useful treatment modality when there is no ectopic hyperfunctional thyroid tissue present. However, based on this study between 1/4 and 1/5 hyperthyroid cats have multiple areas of hyperfunctional thyroid tissue and/or intrathoracic hyperfunctional thyroid tissue where surgical thyroidectomy would not be curative. Veterinary surgeons should, therefore, routinely warn owners about the possibility of ectopic thyroid tissue before thyroidectomy is performed. Particular consideration should be given to cats that have recurrence of hyperthyroidism following previous thyroid surgery, and those without palpable goitre. Sixty-eight percent of those cats in the present study which had recurrence of hyperthyroidism following thyroid surgery had ectopic hyperfunctional thyroid tissue.
The proportion of cats with palpable goitre was relatively low in this study (72%) compared with previous reports. 17 However, palpable goitre may have been underestimated as this was a retrospective study in which identification of this change was reliant upon annotation of the patient case records. Whilst lack of palpable goitre may increase suspicion of intrathoracic thyroid tissue, the presence of palpable goitre in a hyperthyroid cat does not exclude the possibility of additional ectopic thyroid tissue. In this study 12% of cats with a palpable goitre also had ectopic intrathoracic thyroid tissue.
In 97–99% of hyperthyroid cats thyroid pathology is benign, characterised histologically as adenomatous hyperplasia, adenoma or mutinodular adenoma. Thyroid carcinomas are recognised less frequently, with reported incidence of less than 3% of hyperthyroid cats. 2,8,17–19 It is important that thyroid carcinomas are distinguished as higher doses of radioisotope are required for successful radiotherapy. 2,20 Because malignant cells concentrate and retain 131I less efficiently. 21
It has been proposed that scintigraphic imaging may aid in determination of malignancy. Typical scintigraphic features of thyroid malignancy include distortion of the lobes, activity extending beyond the confines of the lobes, multiple foci of radionuclide uptake and uptake extending caudally or towards the thoracic inlet, multiple and extensive hotspots, a heterogeneous pattern of uptake with irregular, spiculated margins, linear multifocal patterns (suggestive of tumour extension along fascial planes) and uptake within the cranial mediastinum. 2,4,10,12,16 However, a recent retrospective review of 10 cases of feline thyroid carcinoma suggested that many cases did not display these typical scintigraphic findings. 13 Furthermore, multifocal and irregular uptake of radionuclide can occur in multinodular adenoma, cystic adenoma and cats that have had prior intracapsular thyroidectomy. 12 This finding was corroborated in the current study where many cats with benign disease had features such as multiple and extensive areas of IRU, areas of IRU in the mediastinum, heterogeneous uptake, irregular margins and distortion of the lobes. This suggests that although some scintigraphic features may increase the suspicion of malignancy, these are not definitive scintigraphic features that can reliably distinguish between malignant and benign thyroid disease.
It is not known why some of the cats in this series failed to respond clinically to administration of a single dose of 110 MBq (3 mCi) radioiodine. Radioactive iodine is considered to be a highly effective treatment for feline hyperthyroidism associated with benign thyroid pathology. It is however, recognised that up to 5–6% of cats require re-treatment; 21 the reported success rate after a single treatment is up to 94%. 14
At the UOB a fixed dosing regime of 110 MBq (3 mCi) is used routinely for the treatment of benign thyroid disease, which is the median effective dose reported with individualised dosing methods. 4,8,14,15 However, it has been suggested that cats with extremely elevated thyroxine and cats with significantly larger volumes of hyperfunctional thyroid tissue as measured on scintigraphy, require higher doses of radioiodine. 8 Individualised dosing has been evaluated, attempting to take into consideration these factors, in addition to the biological half-life and thyroid uptake of iodine. 8,22 Other studies conclude that a fixed dose protocol of 148–185 MBq (4–5 mCi) is most appropriate for routine use. 15,23 Presence of large volumes of hyperfunctional thyroid tissue and/or marked thyrotoxicoxis may be the reason for a lack of response in some of the cats in this study. This was not a consistent finding as other cats with large volumes of hyperfunctional thyroid tissue did have a complete response, whilst another cat that had no response had only two small areas of pertechnetate uptake in the neck. The presence of thyroid carcinoma is another possibility in some cases where histopathology was not performed. As with this case, cats with thyroid carcinoma may respond very well to HD radioiodine. 2,13,20
Another potential reason for the variation in response to a single LD of 131I is that pertechnetate may not accurately reflect uptake or organification of 131I and, therefore, may not reliably predict the response to 131I. 2 It seems more likely that cats with ectopic tissue or very large volumes of abnormal tissue have a higher risk of not responding to a single LD of 131I, but owners should be warned prior to treatment that a lack of response is possible in a small number of cats, regardless of the scintigraphic findings.
In conclusion, the results of the present investigation indicate that the potential for the presence of ectopic thyroid tissue should be discussed with all owners of hyperthyroid cats prior to performing thyroidectomy. Furthermore, if hyperthyroidism recurs following thyroidectomy, scintigraphic evaluation should be seriously considered prior to performing a second surgical procedure. The identification of ectopic hyperfunctional thyroid tissue and/or multiple areas of IRU generally reflects the presence of benign thyroid disease and animals thus affected can respond well to treatment with LD radioiodine.
Acknowledgements
Andrea Harvey and Angie Hibbert hold positions funded by the Feline Advisory Bureau (FAB). The authors would like to thank all the clinicians at The University of Bristol involved in the management of these cases and the referring veterinary surgeons for referring the cases and providing follow-up information.
References
- 1.Feeney D.A., Anderson K.L. Nuclear imaging and radiation therapy in canine and feline thyroid disease, Vet Clin North America: Small Anim Pract 37, 2007, 799–821. [DOI] [PubMed] [Google Scholar]
- 2.Peterson M.E., Becker D.V. Radionuclide thyroid imaging in 135 cats with hyperthyroidism, Vet Radiol Ultrasound 25 (1), 1984, 23–27. [Google Scholar]
- 3.Kintzer P.P., Peterson M.E. Thyroid scintigraphy in small animals, Sem Vet Med Surg Small Anim 6, 1991, 131–139. [PubMed] [Google Scholar]
- 4.Broome M.R. Thyroid scintigraphy in hyperthyroidism, Clin Tech Small Anim Pract 21, 2006, 10–16. [DOI] [PubMed] [Google Scholar]
- 5.Beck K.A., Hornof W.J., Feldman E.C. The normal feline thyroid: Technetium of pertechnetate imaging and determination of thyroid to salivary gland ratios in 10 normal cats, Vet Radiol and Ultrasound 26, 1985, 10–16. [Google Scholar]
- 6.Jones T.C., Hunt R.D. Veterinary Pathology, 5th edn, 1983, Lea and Febiger: Philadelphia, 1597–613. [Google Scholar]
- 7.Swalec K.M., Birchard S.J. Recurrence of hyperthyroidism after thyroidectomy in cats, J Am Anim Hosp Assoc 26, 1990, 433–437. [Google Scholar]
- 8.Forrest L.J., Baty C.J., Metcalf M.R., Thrall D.E. Feline hyperthyroidism: Efficacy of treatment using volumetric analysis for radioiodine dose calculation, Vet Radiol Ultrasound 37 (2), 1996, 141–145. [Google Scholar]
- 9.Naan E.C., Kirpensteijn J., Kooistra H.S., Peters M.E. Results of thyroidectomy in 101 cats with hyperthyroidism, Vet Surg 35 (3), 2006, 287–293. [DOI] [PubMed] [Google Scholar]
- 10.Cook S.M., Daniel G.B., Walker M.A., Maddux J.M., Jenkins C.C., Klebanow E.R., Bouley D.M., Dean D.F., Peterson M.G. Radiographic and scintigraphic evidence of focal pulmonary neoplasia in three cats with hyperthyroidism: Diagnostic and therapeutic considerations, J Vet Int Med 7 (5), 1993, 303–308. [DOI] [PubMed] [Google Scholar]
- 11.Turrel J.M., Feldman E.C., Hays M., Hornof W.J. Radioactive iodine therapy in cats with hyperthyroidism, J Am Vet Med Assoc 184, 1984, 554–559. [PubMed] [Google Scholar]
- 12.Daniel G.B., Brawner W.R. Thyroid scintigraphy. Daniel G.B., Berry C.R. Textbook of Veterinary Nuclear Medicine, 2nd edn, 2006, American College of Veterinary Radiology: Harrisburg, PA, 181–199. [Google Scholar]
- 13.Hibbert A., Barrett E.L., Harvey A.M. Thyroid carcinoma in cats: Clinical and scintigraphic findings, treatment and long-term follow-up of 10 cases, Proceedings of BSAVA Congress, 2007, 483.
- 14.Peterson M.E., Becker D.V. Radioiodine treatment of 524 cats with hyperthyroidism, J Am Vet Med Assoc 207 (11), 1995, 1422–1428. [PubMed] [Google Scholar]
- 15.Meric S.M., Rubin S.I. Serum thyroxine concentrations following fixed dose radioactive iodine treatment in hyperthyroid cats: 62 cases (1986–1989), J Am Vet Med Assoc 197, 1990, 621–623. [PubMed] [Google Scholar]
- 16.Turrel J.M., Feldman E.C., Nelson R.W., Cain G.R. Thyroid carcinoma causing hyperthyroidism in cats: 14 cases (1981–1986), J Am Vet Med Assoc 193, 1988, 359–364. [PubMed] [Google Scholar]
- 17.Feldman E.C., Nelson R.W. Feline hyperthyroidism (thyrotoxicosis), Canine and Feline Endocrinology and Reproduction, 3rd edn, 2004, Elsevier Science: USA, 152–218. [Google Scholar]
- 18.Lucke V.M. A histological study of thyroid abnormalities in the domestic cat, J Small Anim Pract 5, 1964, 351–358. [Google Scholar]
- 19.Milner R.J., Channell C.D., Levy J.K., Schaer M. Survival times for cats with hyperthyroidism treated with iodine 131, methimazole or both: 167 cases (1996–2003), J Am Vet Med Assoc 228 (4), 2006, 559–563. [DOI] [PubMed] [Google Scholar]
- 20.Guptill L., Scott-Moncrieff C.R., Janovitz E.B., Blevins W.E., Yohn S.E., DeNicola D.B. Response to high-dose radioactive iodine administration in cats with thyroid carcinoma that had previously undergone surgery, J Am Vet Med Assoc 207 (8), 1995, 1055–1058. [PubMed] [Google Scholar]
- 21.Peterson M.E. Radioiodine treatment of hyperthyroidism, Clin Tech Small Anim Pract 21, 2006, 34–39. [DOI] [PubMed] [Google Scholar]
- 22.Slater M.R., Komkov A., Robinson L.E., Hightower D. Long-term follow-up off hyperthyroid cats treated with iodine131, Vet Radiol Ultrasound 35 (3), 1994, 204–209. [Google Scholar]
- 23.Chun R., Garrett L.D., Sargeant J., Sherman A., Hoskinson J.J. Predictors of response to radioiodine therapy in hyperthyroid cats, J Vet Radiol Ultrasound 43 (6), 2002, 587–591. [DOI] [PubMed] [Google Scholar]