Abstract
Two groups of feline panleukopenia virus (FPV), feline calicivirus (FCV), and feline herpesvirus-1 (FHV-1) seronegative cats (five cats per group) were administered one of two modified live feline viral rhinotracheitis, calicivirus, and panleukopenia virus (FVRCP) vaccines and the serological responses to each agent were followed over 28 days. While all cats developed detectable FPV and FCV antibody titers; only two cats developed detectable FHV-1 antibody titers using the criteria described by the testing laboratory. For FPV and FHV-1, there were no differences in seroconversion rates between the cats that were administered the intranasal (IN) FVRCP vaccine and the cats that were administered the parenteral FVRCP vaccine on any day post-inoculation. For FCV, the cats that were administered the IN FVRCP vaccine were more likely to seroconvert on days 10 and 14 when compared to cats that were administered the parenteral FVRCP vaccine.
Feline panleukopenia virus (FPV), feline calicivirus (FCV), and feline herpesvirus-1 (FHV-1) are prevalent in cat populations around the world and each of the viruses can cause significant morbidity and mortality in cats without specific immunity. Cats housed in humane societies, pet stores, and animal shelters usually have the greatest risk because of high risk of exposure, stress, and potential for co-infections. In a previous study, we showed that kittens inoculated with a single dose of a modified live FPV, FCV, and FHV-1 vaccine feline viral rhinotracheitis, calicivirus, and panleukopenia virus (FVRCP) for intranasal (IN) administration (UltraNasal FVRCP, Heska Corporation, Loveland, Colorado) had significantly less severe clinical signs of FHV-1 infection than unvaccinated control cats when challenged with virulent FHV-1 as soon as 4 days after inoculation. 1 However, to our knowledge, similar data is not available for FCV or FPV.
While it is currently unknown how rapid the onset of protection against FPV is following administration of any formulation of FVRCP vaccine (parenteral or IN; inactivated or modified live), some clinicians believe that parenteral administration of modified live vaccines is optimal, particularly in high risk environments like animal shelters. 2 Resistance against FPV is mediated predominantly via humoral immune responses and so serum antibody responses can be used to predict protection. 3 In a previous study of specific pathogen-free kittens on days 0 and 28 after a single inoculation, we showed UltraNasal FVRCP to induce FPV serum antibody responses that were comparable to two modified live FVRCP vaccines for subcutaneous (SQ) administration and that were superior to those induced by two formulations of an inactivated FVRCP vaccine for SQ administration. 4 However, samples between day 0 and day 28 were not collected and so it is unknown whether the three modified live vaccines induce similar responses over time. Additional information concerning the temporal appearance of FPV, FCV, and FHV-1 antibodies in the serum of vaccinated cats is needed to help determine optimal vaccination protocols. The purpose of this study was to determine when seroconversion occurs in seronegative adult cats after administration of one dose of a commercially available modified live FVRCP vaccine administered IN or a commercially available modified live FVRCP vaccine administered SQ.
Mixed sex, 10-month-old, specific pathogen-free cats (n=10) were studied. The cats had never been vaccinated and were shown to be negative for FPV antibodies by hemagglutination inhibition (HI) and both FCV and FHV-1 antibodies by serum neutralization (SN) at the New York State Diagnostic Laboratory in Ithaca, NY. The serum dilution first assessed by the reference laboratory was 1:10, 1:8, and 1:4 for FPV, FCV, and FHV-1, respectively. In addition, all cats were shown to be negative for FPV, FCV, and FHV-1 antibodies by enzyme-linked immunosorbent assay (ELISA). 3 The cats were randomly divided into two groups and housed in separate rooms. On day 0, one group was administered one dose of UltraNasal FVRCP IN and the other group was administered one dose of a commercially available FVRCP vaccine (Purevax Feline 3, Merial, Athens, Georgia) SQ. Blood was collected by jugular venepuncture on days 0, 3, 7, 10, 14, 17, 21, 24, and 28. Serum collected from clotted blood was stored at −70°C until shipped by overnight express to New York State Veterinary Diagnostic Laboratory for performance of FPV HI, FCV SN, and FHV-1 SN assays. Cats developing FPV HI titers, FCV SN titers, and FHV-1 SN titers of >20, >8, and >8, respectively, were considered positive based on recommendations from the laboratory. Numbers of cats positive for each respective antibody were compared between groups on each day after inoculation by Fisher's exact test with significance defined as P<0.05. There is no evidence that titer magnitudes above these cutoffs denote greater protection and so the range and median values for each assay for each group are presented descriptively. The study was approved by a campus wide Animal Care and Use Committee and the cats were adopted to private homes at the completion of the study.
The cats were observed daily by the trained staff of the laboratory facility and by at least one author on each collection date. Sneezing, coughing, or ocular discharges were not noted in any of the cats. One cat administered the FVRCP vaccine IN had minimal crusting around both nares that was interpreted by the evaluators as dried nasal secretions on days 10 and 14 after inoculation.
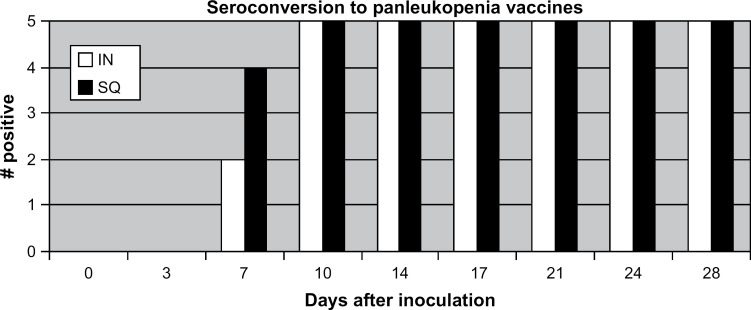
After inoculation, the first detectable positive FPV HI titer was detected on day 3 for two cats in each vaccine group (titer=20 for all four cats). FPV HI titers>20 were first detected on day 7 for two cats administered the FVRCP vaccine IN (titers=40 and 80) and on day 7 for four cats administered the FVRCP vaccine SQ (titers=40, 160, 320, and 320); all cats in both groups ultimately had titers>20 by day 10 (Fig 1). There were no statistical differences between seroconversion rates by group on any day after inoculation. After inoculation, FPV HI titers ranged from 10 to 5120 (median titer=1280) for cats administered the FVRCP vaccine IN and from 10 to 2560 (median titer=320) for cats administered the FVRCP vaccine SQ.
Fig 1.
A positive result was defined by the laboratory performing the test as any HI titer>20. There were no significant differences in seroconversion rates on any day after inoculation. IN=UltraNasal FVRCP, Heska Corporation, Loveland, Colorado; SQ=Purevax Feline 3, Merial, Athens, Georgia.
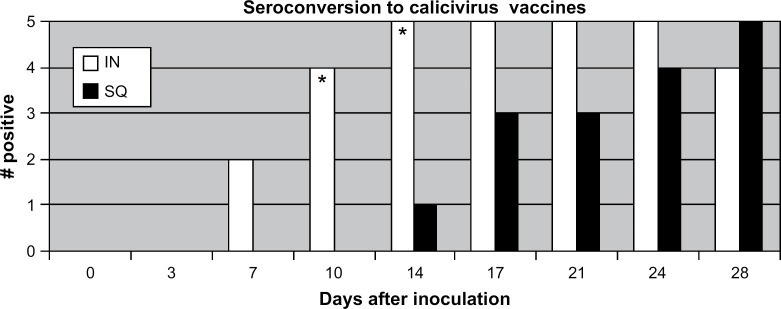
After inoculation, the first detectable positive FCV SN titer was detected on day 7 for two cats administered the FVRCP vaccine IN (titers=16 and 16) and day 10 for three cats administered the FVRCP vaccine SQ (titer=8 for all three cats). FCV SN titers>8 were first detected on day 7 for two cats administered the FVRCP vaccine IN (titers=16 and 16) and on day 14 for one cat administered the FVRCP vaccine SQ (titer=96); all cats ultimately had titers>8 by day 28 (Fig 2). On days 10 and 14 after inoculation, a statistically greater number of cats administered the FVRCP vaccine IN had FCV SN titers>8 than cats administered the FVRCP vaccine SQ. After inoculation, FCV SN titers ranged from 8 to 384 (median titer=32) for cats administered the FVRCP vaccine IN and from 8 to 128 (median titer=8) for cats administered the FVRCP vaccine SQ.
Fig 2.
A positive result was defined by the laboratory performing the test as any SN titer>8. On the days marked *, a significantly greater number of cats administered the IN vaccine seroconverted. IN=UltraNasal FVRCP, Heska Corporation, Loveland, Colorado; SQ=Purevax Feline 3, Merial, Athens, Georgia.
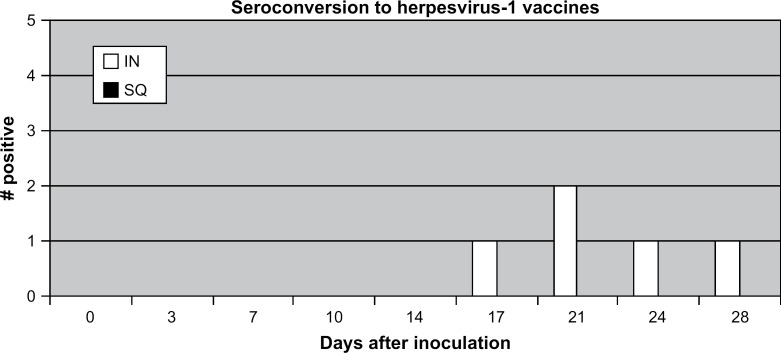
After inoculation, the first detectable positive FHV-1 SN titer was detected on day 14 for two cats administered the FVRCP vaccine IN (titers=4) and day 21 for one cat administered the FVRCP vaccine SQ (titer=4). FHV-1 SN titers>8 were first detected on day 17 for one cat (titer=12) and day 21 for another cat (titer=12) in the group administered the FVRCP vaccine IN. While these were the only two cats in the either group that developed titers>8 (Fig 3), four of the cats administered the FVRCP vaccine IN (titers=6, 8, 12, and 16) and three of the cats administered the FVRCP vaccine SQ (titers=4, 4, and 8) ultimately had detectable FHV-1 antibodies.
Fig 3.
A positive result was defined by the laboratory performing the test as any SN titer>8. There were no significant differences in seroconversion rates on any day after inoculation. IN=UltraNasal FVRCP, Heska Corporation, Loveland, Colorado; SQ=Purevax Feline 3, Merial, Athens, Georgia.
Some cats in this study had the first detectable FPV titers on day 3 after inoculation, all cats had titers that predict resistance to challenge by day 10, and there were no statistically significant differences in numbers of cats with titers>20 between groups administered FVRCP vaccines IN or SQ on the days assessed. However, numerical differences were detected between groups on day 7, with more cats administered the FVRCP vaccine SQ having a titer>20. If additional cats had been available for study and this trend continued, a statistical difference may have been recognized. While this study did not have a challenge, FPV titers of >20 have been predictive of resistance to virulent FPV challenge as long as 7.5 years after final inoculation. 3,5 These results suggest that either vaccine could be used successfully to prevent clinical signs of FPV infection in previously naive, adult cats if exposed to virulent FPV. A limitation to this study is that adult cats were used instead of kittens. However, the seroconversion rates at day 14 in the cats described herein are similar to those reported for the same two vaccines when administered to kittens at 8–10 weeks of age. 6 Both studies purposely used FPV antibody negative cats and so the data cannot be used to determine which route of administration is most likely to break through existing maternal immunity. There are differences between modified live FVRCP vaccines for parenteral administration and so the results of this study cannot be directly extrapolated to other commercially available vaccines. However, the seroconversion rates for other FVRCP vaccines for parenteral administration have been similar at 2 weeks 6 and 4 weeks. 4
The FVRCP vaccine administered IN induced FCV titers>8 more quickly than the FVRCP administered SQ. These results suggest that the FVRCP vaccine administered IN should be considered in populations of FCV naive cats with high risk of exposure. However, because the cats also develop cell-mediated immune responses to FCV and were not challenged in this study, it is unknown whether these findings are clinically significant.
Of all the vaccinated cats in this study, only two cats developed FHV-1 antibody titers>8. However, seven of the 10 cats developed detectable FHV-1 of ≥4. These results suggest that more than one immunization should be given to naive cats. However, specific cell-mediated immune responses also play a role in inducing protection against FHV-1. In a previous study, we showed statistically greater lymphocyte blastogenesis after exposure to concanavalin A (non-specific immune stimulation) or FHV-1 antigens in cats vaccinated with this FVRCP vaccine administered IN when compared to four FVRCP vaccines administered SQ (including the vaccine used here) at several time points after vaccination. 7 The FVRCP vaccine for IN administration was used previously in kittens challenged 2, 4, or 6 days after a single vaccination; significant differences in clinical scores were detected in vaccinated kittens compared to unvaccinated control kittens at 4 and 6 days after vaccination. 1 In that study, serological responses were not measured but based on the results of the study described here, it is likely the kittens were seronegative for FHV-1 antibodies on days 4 and 6. Results of that study emphasize the importance that non-specific and cell-mediated immune responses play in protecting cats against FHV-1.
Abnormal clinical signs were present in one cat administered the FVRCP vaccine IN, but signs were mild and transient. However, when this vaccine is to be used, the owners should be informed about the potential development of clinical signs of upper respiratory tract disease like sneezing, coughing, and nasal discharge.
References
- 1.Lappin M.R., Sebring R.W., Porter M., Radecki S.J., Veir J. Effects of a single dose of an intranasal feline herpesvirus 1, calicivirus, and panleukopenia vaccine on clinical signs and virus shedding after challenge with virulent feline herpesvirus 1, J Feline Med Surg 8, 2006, 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richards J.R., Elston T.H., Ford R.B., Baskell R.M., Harmann K., Hurley K.F., Lappin M.R., Levy J.K., Rodan I., Scherk M., Schultz R.D., Sparkes A.H. The 2006 American Association of Feline Practitioners Feline Vaccine Advisory Panel report, J Am Vet Med Assoc 229, 2006, 1405–1441. [DOI] [PubMed] [Google Scholar]
- 3.Lappin M.R., Andrews J., Simpson D., Jensen W.A. Use of serologic tests to predict resistance to feline herpesvirus 1, feline calicivirus, and feline parvovirus infection in cats, J Am Vet Med Assoc 220, 2002, 38–42. [DOI] [PubMed] [Google Scholar]
- 4.Lappin MR, Jensen WA. Panleukopenia antibody responses following a single inoculation with one of five FVRCP vaccines. In: Proceedings of the American College of Veterinary Internal Medicine 25th Annual Forum 2007.
- 5.Scott F.W., Geissinger C.M. Long-term immunity in cats vaccinated with an inactivated trivalent vaccine, Am J Vet Res 60, 1999, 652–658. [PubMed] [Google Scholar]
- 6.Patterson E.V., Reese M.J., Tucker S.J., Dubovi E.J., Crawford P.C., Levy J.K. Effect of vaccination on parvovirus antigen testing in kittens, J Am Vet Med Assoc 230, 2007, 359–363. [DOI] [PubMed] [Google Scholar]
- 7.Lappin MR, Veir J, Sebring R, Radecki SV. Feline lymphocyte blastogenesis in response to feline herpesvirus 1 antigens and concanavalin A after vaccination with five FVRCP vaccines. In: Proceedings of the American College of Veterinary Internal Medicine 23rd Annual Forum 2005.