Abstract
The purpose of this study was to evaluate the effects of dietary modification in addition to twice daily insulin glargine. Cats were treated with insulin glargine twice daily and randomized to receive either a low carbohydrate, high protein (LCHP) diet (n=6) or a control diet (n=6) for 10 weeks. Re-evaluations of clinical signs, blood glucose curves, and serum fructosamine concentrations were performed at weeks 1, 2, 4, 6, and 10. Two of 12 cats achieved complete remission by the end of the study but remission rate was not different between diet groups. Using twice daily insulin glargine and frequent monitoring, all cats in both diet groups achieved successful glycemic control. Frequent monitoring is key to achieving glycemic control in diabetic cats; potential benefits of dietary modification require further evaluation.
Diabetes mellitus (DM) is one of the most common endocrine diseases in cats, and is typically managed with a combination of exogenous insulin administration and dietary modification. Long-acting insulins, often given twice daily, are usually required to provide adequate glycemic control. The choice of insulin is often dependent on veterinarian preference, but the ideal insulin is one that restores euglycemia, potentially reversing β cell toxicity, thus allowing endogenous insulin secretion to recover.
Insulin glargine (Lantus; Sanofi-Aventis, Bridgewater, NJ) is a long-acting recombinant human insulin that has a relatively constant systemic absorption profile in people. 1 However, a pharmacodynamic study in healthy cats presented in abstract form has shown that there are definite peaks in insulin concentration and glucose lowering effects. 2 A recent study comparing efficacy of glargine and lente insulins in cats with DM fed a low carbohydrate, high protein (LCHP) diet found no difference between once daily glargine and twice daily lente. 3
Dietary modification is an important adjunct to insulin therapy in cats with DM. Traditionally the approach has been to feed a high fiber, low fat diet based upon data extrapolated from past human and canine studies. 4–9 Results from a study in cats with naturally occurring DM also supported the benefits of a diet high in insoluble fiber compared to a low fiber diet for maintaining glycemic control. 10 However, just as concepts regarding dietary management of people with DM are evolving, 11 investigators are reassessing what constitutes the ideal diet for the feline diabetic. 12 Data from recently published studies have suggested that an LCHP diet may have advantages in the management of some cats with DM. One of these studies evaluated nine cats with DM that were initially fed a high fiber, moderate fat canned diet for 1–2 months then transitioned to an LCHP canned diet for 3 months. 13 In this study, the insulin dose was decreased in all cats and could be discontinued in 3/9 cats. Another study evaluated 18 cats with DM that were treated with insulin or glipizide and fed a low carbohydrate diet or a low carbohydrate diet and acarbose for 4 months. 14 Insulin was discontinued in 11/18 cats. Finally, a study evaluating diet over 4 months in 63 diabetic cats receiving a variety of insulin types showed that more cats eating a low carbohydrate, low fiber diet (68%) were able to discontinue insulin use compared to those receiving a moderate carbohydrate, high fiber diet (41%). 15
Non-insulin-dependent DM is characterized by decreased insulin secretion and insulin resistance, and may account for up to 80–95% of feline DM cases. 16 Weight loss remains an important part of managing the diabetic cat by reversing insulin resistance. A recent study examined the effect of a high carbohydrate, low protein diet and an LCHP diet on glucose and fat metabolism in 12 lean and 16 obese cats before and after weight loss. 17 The authors concluded that the LCHP diet helped to maintain normal insulin sensitivity of fat metabolism in obese cats and facilitated the loss of fat during weight loss.
Thus far, published studies evaluating the efficacy of once daily insulin glargine compared to other insulins have used an LCHP diet. 3 Conversely, published studies evaluating diet have not exclusively used insulin glargine. 13–15 Therefore, the effects of dietary modification in addition to twice daily insulin glargine are not known. The purpose of this study was to compare glycemic control in cats with naturally occurring DM using a prescription LCHP diet and an over-the-counter control diet in cats with DM receiving twice daily insulin glargine.
Materials and Methods
Subjects
All cats with newly diagnosed or poorly controlled naturally acquired DM that were patients at the Foster Hospital for Small Animals at Tufts Cummings School of Veterinary Medicine were eligible for inclusion of the study. DM was diagnosed based on detection of consistent clinical signs (eg, polyuria, polydipsia, weight loss, polyphagia), and the presence of hyperglycemia (blood glucose >250 mg/dl), glucosuria, and/or fructosamine concentrations >350 umol/l despite treatment with insulin. Cats were excluded from the study if they were already being fed a LCHP diet, if they were receiving insulin glargine or corticosteroids, or if they had significant concurrent illness. The Tufts Institutional Animal Care and Use Committee approved the study, and all owners signed an informed consent form before enrolling cats in the study.
Experimental design
A prospective, randomized, double-masked design was used. At the initial visit (day 0), a physical examination [including body weight and body condition score (BCS); on a 1–9 scale, where 1=emaciated, 5=ideal, and 9=obese 18 ] was performed, and a complete blood count (CBC), serum biochemistry profile, serum thyroxine concentration, serum fructosamine concentration, and urinalysis and urine culture were obtained.
Glargine was administered to all cats at a starting dosage of 0.25 U/kg SQ q12 h. Both newly diagnosed and poorly controlled diabetic cats were randomized by a computer-generated random number list to receive either an LCHP diet (LCHP; DM Dietetic Management Feline Formula, canned and dry, Purina Veterinary Diets, Nestlé Purina PetCare, St Louis, MO) or a control diet. A dry (Pro Plan Adult Cat Total Care Chicken and Rice Formula, Nestlé Purina PetCare) and canned (Friskies Special Diet – Turkey and Giblets Dinner for Adult Cats, Nestlé Purina PetCare) control diets were selected to represent commonly available, over-the-counter, feline adult maintenance diets (see Appendix). Cats were fed either dry or canned food, or a combination of the two, based on their apparent individual preference. Cats ate the assigned diets exclusively throughout the study period. All investigators and owners were masked to the type of diet being fed to the cats.
Re-evaluations were performed on all cats at weeks 1, 2, 4, 6, and 10 and included an assessment of clinical signs, physical examination findings including body weight and BCS, a 12-hour blood glucose curve, and serum fructosamine concentrations. Adjustments in insulin dosages were made as needed, with the goal of maintaining most blood glucose measurements between 100 and 300 mg/dl and a glucose nadir between 80 and 120 mg/dl. Blood glucose concentrations were measured by use of a hand-held portable blood glucose monitor (PBGM; Ascensia Elite XL Glucometer, Bayer Corporation, Mishawaka, IN). The same PBGM was used on all cats for each blood glucose curve measurement. The PBGM uses reflectance photometry to measure production of a colored chromogen by a glucose oxidase–peroxidase reaction. Detectable glucose concentrations ranged from 20 to 500 mg/dl.
For the purposes of this study, effective glycemic control was defined as a resolution of clinical signs (based on the owner's subjective evaluation), average (over 12 h) blood glucose concentration <300 mg/dl, blood glucose nadir >100 mg/dl, and a serum fructosamine concentration <350 mmol/l. Complete remission was defined as glycemic control that required discontinuation of exogenous insulin administration.
Statistical analysis
Sample size was calculated based on the results from our group's previous study. 3 Data were examined graphically before analysis. Most data were not normally distributed so results are reported as median (range). The difference in the percentage of cats with effective glycemic control between the two diet groups was analyzed using the χ2 analysis. The change over time for glucose and fructosamine between diet groups was assessed using analysis of variance with repeated measures. Statistical analyses were performed with commercial statistical software (Systat 11.0, SPSS, Chicago, IL). A value of P<0.05 was considered statistically significant.
Results
Thirteen cats were enrolled in the study. One newly diagnosed diabetic cat fed the prescription LCHP diet died during the study due to complications from diabetic ketoacidosis and hepatic failure. Therefore, 12 cats completed the 10-week study (LCHP: n=6; control: n=6). All subsequent results will be for these 12 cats only. Breeds included domestic shorthair (n=9) and domestic longhair (n=3). Most cats enrolled were castrated males, although the control group included two spayed females (Table 1). Age, sex, breed, body weight, and BCS were not significantly different between the two diet groups (Table 1).
Table 1.
Baseline characteristics of cats randomized to the LCHP diet group (LCHP: n=6) and to the control group (control: n=6)
| LCHP diet | Control diet | P value | |
|---|---|---|---|
| Age (yr) | 9 (5–11) | 12 (6–15) | 0.06 |
| Gender | Six castrated males | Four castrated males | 0.12 |
| Two spayed females | |||
| Weight (kg) | 6.9 (3.3–8.1) | 5.4 (4.2–8.6) | 0.63 |
| BCS | 7 (3–9) | 8 (5–8) | 0.87 |
| Glucose (mg/dl) | 332 (100–488) | 369 (284–529) | 0.30 |
| Fructosamine (umol/l) | 432 (363–497) | 510 (311–547) | 0.11 |
Reference ranges: glucose 70–120 mg/dl; fructosamine 191–349 umol/l. Data are presented as median (range).
Six cats were newly diagnosed, untreated diabetics (LCHP: n=2; control: n=4), and six cats were being treated with insulin with poor glycemic control (LCHP: n=4; control: n=2). Median fructosamine concentrations were 498 umol/l (range, 445–547 umol/l) in the newly diagnosed, untreated diabetics and 400 umol/l (range, 311–518 umol/l) in the cats with poor glycemic control (reference range=191–349 umol/l; P=0.26). Diabetes was considered poorly controlled based on persistent clinical signs, hyperglycemia, and glucosuria. There were no statistically significant differences between the two diet groups for baseline glucose or fructosamine concentrations (Table 1). Types of insulin being used before enrollment in the study included recombinant human Neutral Protamine Hagedorn insulin (NPH) (n=2) and Protamine Zinc Insulin (PZI) (n= 4). Types of diets previously fed before enrollment included high fiber diets (n=3) and adult maintenance feline diets (n=9).
Abnormalities identified in the 12 cats at the time of entry into the study based on physical examination included obesity (BCS>7/9; n=7), dry hair coat and hyperkeratosis (n=4), cardiac murmur (n=3), periodontal disease (n=2), bilateral corneal ulceration (n=1), a thin appearance (BCS<4/9; n=1), and a plantigrade stance (n=1). Echocardiograms were not performed on the cats with cardiac murmurs. Serum thyroxine concentrations were within the reference range for all cats.
Abnormalities identified from the biochemistry profile included hyperglycemia (n=11), hypercholesterolemia (n=6), increased creatinine kinase (n=5), increased aspartate aminotransferase (n=4), increased triglycerides (n=3), lymphopenia (n=2), increased BUN (n=2), increased creatinine (n=1), increased alanine aminotranferase (n=1), and increased alkaline phosphatase (n=1). Abnormalities identified from the CBC included a normocytic normochromic anemia (n=1), monocytosis (n=1), and basophilia (n=1). All cats had glucosuria but no cats had ketonuria at the time of enrollment into the study.
Cats varied in apparent food preference, but there were no differences between the two groups: LCHP group – four ate equal proportions of canned and dry, one ate dry only, and one ate canned only; control group – all cats ate equal proportions of both the canned and dry food. Median body weight increased for both groups over the course of the study [LCHP group: +0.4 kg (range, −1.0 to +1.6 kg); control group: +0.6 kg (range, +0.1 to +1.0 kg)]. All cats had subjective improvement in their clinical signs over the course of the study period. Fructosamine concentrations were higher in the control group compared to the LCHP group at each time point, but this was significant only at week 6 (baseline: P=0.11; week 1: P=0.06; week 2: P=0.06; week 4: P=0.13; week 6: P=0.02; week 10: P=0.30). However, over the entire course of the study, there was a statistically significantly greater decrease in fructosamine concentrations for the LCHP group compared to the control group (Fig 1; P=0.01). There were no significant differences between diet groups over time in clinical signs (P=0.30), insulin doses (P=0.34), body weight (P=0.62), or peak (P=0.18) and nadir (P=0.86) glucose concentrations between diet groups.
Fig 1.
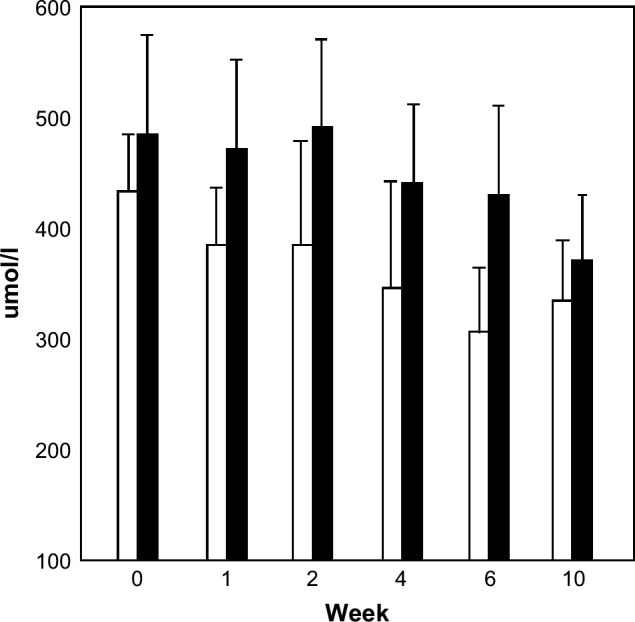
Fructosamine concentrations at baseline (week 0) and weeks 1, 2, 4, 6, and 10 in cats with DM treated with insulin glargine and randomized to receive either a LCHP diet (open bars) or a control diet (closed bars). Over the entire course of the study, there was a significantly greater decrease in fructosamine concentrations for the LCHP group compared to the control group (P=0.01).
Two of the 12 cats achieved complete remission by the end of the 10-week study period and no longer required exogenous insulin administration. Of the two cats in complete remission (LCHP group: n=1; control group: n=1), one was a newly diagnosed diabetic and one a poorly controlled diabetic. Time to complete remission ranged from 4 to 10 weeks. The remaining 10 cats still receiving exogenous insulin at the end of the study achieved effective glycemic control (five in each diet group). Median daily insulin dose increased for both groups over the course of the study [LCHP group: +5 U (range, −4 to +8 U); control group: +5 U (range, 0 to +12 U)]. All cats had their insulin dose adjusted on at least two visits, with a median number of insulin dose changes of four [five changes in insulin dose (n=5), four changes in insulin dose (n=3), three changes in insulin dose (n=1), two changes in insulin dose (n=3)].
Discussion
In the study reported here, diet had a modest effect on glucose control, as assessed by serum fructosamine concentrations, in cats with naturally occurring DM treated with twice daily insulin glargine. Those fed the veterinary LCHP diet had significantly lower serum fructosamine concentrations over the course of the study when compared to those cats fed the control diet. The clinical significance of this effect on fructosamine remains to be determined as all cats in both diet groups achieved effective glycemic control.
The control diets were selected to represent typical over-the-counter adult maintenance cat foods that are commonly fed to cats. Although there is variability depending upon the individual brand, canned forms of these diets are commonly low in carbohydrates while most dry forms are higher in carbohydrates. As most cats in the current study ate a combination of canned and dry food, and exact food intake was not quantified, most cats in the control group likely had similar carbohydrate intake (mean carbohydrate content for canned and dry forms=4.5 g/100 kcal) compared to the LCHP group eating an intermediate level of carbohydrate (mean carbohydrate content for canned and dry forms=3.0 g/100 kcal). Therefore, this study was not able to compare the effects of high versus low dietary carbohydrate intake but did compare the use of a veterinary LCHP therapeutic diet to over-the-counter adult maintenance foods commonly fed to cats. The authors believe these results provide useful information that with twice daily insulin glargine and careful monitoring, successful glycemic control can be achieved in most cats. While a veterinary LCHP diet may have some benefits (eg, reduction in fructosamine levels) and is typically the first choice dietary recommendation in most cats with DM, other dietary options can be offered when financial constraints are present or when cats will not readily eat a veterinary diet. Therefore, it is useful to be able to offer other options to owners in these situations. Many canned over-the-counter diets are relatively low in carbohydrate content (ie, <5.0 g/100 kcal) but it is important to obtain information from the manufacturer on specific brands and flavors to ensure that the goal nutrient composition is being met for a cat with DM. Most dry over-the-counter diets are higher in carbohydrate content so if a dry veterinary LCHP diet is not an option, it can be more difficult to identify a good quality dry food that has a low carbohydrate content.
All cats in the study had improvements in clinical signs, including the cats in the previously diagnosed, poorly controlled diabetic group. This response is likely due to a combination of twice daily glargine administration and frequent monitoring and testing of all the subjects in the study. None of the cats included in the study had been treated with insulin glargine before enrollment, and this could be a contributing factor in the reported improvement in clinical signs. The resolution of clinical signs in all of the cats in this study is more likely attributable to the close monitoring of the subjects, underscoring the importance of this aspect in managing the diabetic cat. Most diabetic cats are not so closely monitored and this likely contributed to the perception of improvement in clinical signs as reported by owners in this study.
All cats accepted both diets well and most cats gained weight over the course of the study. The weight gain was attributed to improved glycemic control, as many cats had lost weight before enrolling in the study. However, eight cats were overweight or obese at the start of the study (BCS>five) and 11 were overweight or obese at the end of the study. A better complete remission rate may have been achieved in this study if optimal body condition had been achieved over the course of the study as obesity is associated with decreased insulin sensitivity. 19
Cats in the current study did not exhibit the high remission rates that have been reported in some previous studies. For example, remission rates in previously published studies have ranged from 33% to 61% while our remission rate was 17%. 13–15,20 Reasons for the lower remission rate in the current study compared to other recent studies may include a large proportion of overweight and obese cats in the current study, weight gain that occurred in most cats over the course of the study, the inclusion of previously diagnosed, poorly controlled diabetic cats, and the relatively short study duration. Previous studies have ranged from 12 to 16 weeks, providing a longer period in which to achieve remission. 13–15, 20 Additionally, there may be a component of the genetic make-up of the cats themselves that influenced the remission rate in this study. All cats were from New England, and most people obtain cats from relatively near where they live. Whether there is something intrinsic in the population of cats in the New England area that makes them more insulin dependent remains to be proven, and further studies would need to be done to assess the significance of this issue.
In addition to the previously mentioned diet issues, this study has a number of limitations. One is the small sample size which makes the possibility of a type II statistical error an important consideration. In addition, this study only extended over a 10-week period and higher remission rates may have been achieved with longer study duration. The test diet in this study was both high in protein and low in carbohydrate. Because of the composition of the test diet in the current study, the question remains if the benefit in this dietary modification is attributable to the combination of the two, or if the benefit lies in the high protein content or the low carbohydrate content of the diet. Future studies, including those in which exact food intake is measured, are needed to examine this issue.
In conclusion, good glycemic control of the diabetic cat is possible using twice daily insulin glargine and frequent monitoring in conjunction. An LCHP diet may provide some additional benefits but further evaluation of diet is warranted.
Acknowledgement
Supported by grant from Nestlé Purina PetCare Research.
Appendix
Composition of the two diets fed to cats with DM
| Nutrient | LCHP diet | Control diet | ||
|---|---|---|---|---|
| Dry | Canned | Dry | Canned | |
| ME-calculated (kcal/kg) | 3840 | 990 | 3960 | 1140 |
| Protein (g/100 kcal) | 13.88 | 12.52 | 9.09 | 10.61 |
| Fat (g/100 kcal) | 4.66 | 5.51 | 4.77 | 6.89 |
| Carbohydrate (g/100 kcal) | 3.34 | 2.59 | 7.92 | 1.13 |
| Crude fiber (g/100 kcal) | 0.33 | 0.56 | 0.13 | 0.18 |
ME=metabolizable energy and NFE=nitrogen-free extract (estimate of carbohydrate). Main diet ingredients (taurine, vitamin and mineral sources are not included): LCHP diet (dry): Poultry by-product meal, soy protein isolate, corn gluten meal, soy flakes, animal fat preserved with mixed-tocopherols, and corn starch; LCHP diet (canned): Liver, water, beef, corn gluten meal, trout, fish meal, animal fat preserved with mixed-tocopherols, wheat flour, soy protein isolate, powdered cellulose, and carrageenan; Control diet (dry): Chicken, brewers rice, corn gluten meal, poultry by-product meal, wheat flour, beef tallow preserved with mixed-tocopherols, whole grain corn, sodium caseinate, fish meal, and egg product; Control diet (canned): Meat by-products, water, turkey, poultry by-products, poultry giblets, brewers rice, guar gum, and carrageenan.
References
- 1.Heinemann L., Linkeschova R., Rave K., Hompesch B., Sedlak M., Heise T. Time action profile of the long acting insulin analogue insulin glargine (HOE901) in comparison with those of NPH insulin and placebo, Diabetes Care 23, 2000, 644–649. [DOI] [PubMed] [Google Scholar]
- 2.Marshall R.D., Rand J.S. Comparison of the pharmacokinetics and pharmacodynamics of glargine, protamine zinc, and porcine lente insulins in normal cats [abstract], J Vet Intern Med 16, 2002, 358. [Google Scholar]
- 3.Weaver K.E., Rozanski E.A., Mahony O.M., Chan D.L., Freeman L.M. Use of glargine and lente insulins in cats with diabetes mellitus, J Vet Intern Med 20, 2006, 234–238. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins D.J.A., Ghafari H., Wolover T.M.S. Relationship between the rate of digestion of foods and postprandial glycaemia, Diabetologia 22, 1982, 450–455. [DOI] [PubMed] [Google Scholar]
- 5.Brand J.C., Nicholson P.L., Thorburn P.W., Truswell A.S. Food processing and the glycaemic index, Am J Clin Nutr 42, 1985, 1192–1196. [DOI] [PubMed] [Google Scholar]
- 6.Holt S., Brand J., Soveny C., Hansky J. Relationship of satiety to postprandial glycemic, insulin and cholecystokinin responses, Appetite 18, 1992, 129–141. [DOI] [PubMed] [Google Scholar]
- 7.Nelson R.W. Dietary management of diabetes mellitus, J Small Anim Pract 33, 1992, 213–217. [Google Scholar]
- 8.Ihle S.L. Nutritional therapy for diabetes mellitus, Vet Clin North Am Small Anim Pract 25, 1995, 585–597. [DOI] [PubMed] [Google Scholar]
- 9.Kimmel S.E., Michel K.E., Hess R.S., Ward C.R. Effects of insoluble and soluble dietary fiber on glycemic control in dogs with naturally occurring insulin-dependent diabetes mellitus, J Am Vet Med Assoc 216, 2000, 1076–1081. [DOI] [PubMed] [Google Scholar]
- 10.Nelson R.W., Scott-Moncrieff J.C., Feldman E.C., et al. Effect of dietary insoluble fiber on control of glycemia in cats with naturally acquired diabetes mellitus, J Am Vet Med Assoc 216, 2000, 1082–1088. [DOI] [PubMed] [Google Scholar]
- 11.Westman E.C., Feinman R.D., Mavropoulos J.C., et al. Low-carbohydrate nutrition and metabolism, Am J Clin Nutr 86, 2007, 276–284. [DOI] [PubMed] [Google Scholar]
- 12.Kirk C.A. Feline diabetes mellitus: Low carbohydrates versus high fiber?, Vet Clin North Am Small Anim Pract 36, 2006, 1297–1306. [DOI] [PubMed] [Google Scholar]
- 13.Frank G., Anderson W., Pazak H., Hodgkins E., Ballam J., Laflamme D. Use of a high-protein diet in the management of feline diabetes, Vet Ther 2, 2001, 238–246. [PubMed] [Google Scholar]
- 14.Mazzaferro E.M., Greco D.S., Turner A.S., Fettman M.J. Treatment of feline diabetes mellitus using an alpha-glucosidase inhibitor and a low-carbohydrate diet, J Feline Med Surg 5, 2003, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett N., Greco D.S., Peterson M.E., Kirk C., Mathes M., Fettman M.J. Comparison of a low carbohydrate-low fiber diet and a moderate carbohydrate-high fiber diet in the management of feline diabetes mellitus, J Feline Med Surg 8, 2006, 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rand J.S. Current understanding of feline diabetes: Part 1, pathogenesis, J Feline Med Surg 1, 1999, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoenig M., Thomaseth K., Waldron M., Ferguson D.C. Insulin sensitivity, fat distribution, and adipocytokine response to different diets in lean and obese cats before and after weight loss, Am J Physiol Regul Integr Comp Physiol 292, 2007, 227–234. [DOI] [PubMed] [Google Scholar]
- 18.Laflamme D. Development and validation of a body condition score system for cats: A clinical tool, Feline Pract 25, 1997, 13–18. [Google Scholar]
- 19.Hoenig M., Thomaseth K., Brandao J., Waldron M., Ferguson D.C. Assessment and mathematical modeling of glucose turnover and insulin sensitivity in lean and obese cats, Domest Anim Endocrinol 31, 2006, 373–389. [DOI] [PubMed] [Google Scholar]
- 20.Alt N., Kley S., Tschuor F., Zapf J., Reusch C.E. Evaluation of IGF-1 levels in cats with transient and permanent diabetes mellitus, Res Vet Sci 83, 2007, 331–335. [DOI] [PubMed] [Google Scholar]


