Abstract
Single-dose pharmacokinetics and genotoxicity of metronidazole in cats were evaluated. Cats received either 5 mg/kg metronidazole intravenously, or 20 mg/kg metronidazole benzoate (12.4 mg/kg metronidazole base) orally in a single dose. Serial plasma samples were collected and assayed for metronidazole using high pressure liquid chromatography (HPLC). Genotoxicity was assessed in vitro in feline peripheral blood mononuclear cells (PBMC) and a feline T-cell lymphoma line incubated with metronidazole, and in vivo in PBMC collected before, during and 7 days after oral metronidazole, by use of the COMET assay. Systemic absorption of metronidazole was variable (mean=65±28%) with a peak of 8.84±5.4 μg/ml at 3.6±2.9 h. The terminal half-life was 5.34 h from the intravenous dose and 5.16 h from the oral dose. Systemic clearance was low (mean=91.57 ml/h/kg [1.53 ml/kg/min]), and the apparent volume of distribution (steady state) was 0.650±0.254 l/kg. Genotoxicity was detected at all concentrations of metronidazole in feline PBMC and the T-cell lymphoma line in vitro. Genotoxicity was also observed in PBMC collected from cats after 7 days of oral metronidazole but resolved within 6 days of discontinuing metronidazole.
Metronidazole is a commonly used antibiotic in cats, effective in treating anaerobic bacterial and protozoal infections. It is most commonly used to treat anaerobic infections and giardiasis with good clinical results. 1–3 Additionally, it is also used at lower doses in the treatment of feline inflammatory bowel disease. 4–6 However, adverse effects have been reported from its administration to cats. 5,7,8
Metronidazole kills target organisms by inducing formation of reactive intermediates within these organisms, resulting in disruption of DNA. 9 This disruptive effect is not restricted to microorganisms, and both carcinogenic and mutagenic effects have been documented in experimental animals 10 and metronidazole disrupts DNA in human blood lymphocytes. 11–13 The importance of these disruptions is unclear – in humans, damage was not evident within 6 days of discontinuation of the drug. No information about genotoxicity in cats is available.
Metronidazole is a weak base that is moderately lipophilic with a low molecular weight compared to other drugs (MW=171), which facilitates penetration across membranes and allows almost complete systemic absorption. Metronidazole is not registered for animal use, but is available as the base in capsules (375 mg), tablets (250 and 500 mg), or for injection as metronidazole hydrochloride in 500 mg vials. Metronidazole base is notoriously unpalatable for cats, but tablet or capsule size requires that they must be broken or opened for administration to cats, exposing the base. A more popular palatable (but not approved by the Food and Drug Administration) alternative is metronidazole benzoate, a metronidazole ester of benzoic acid, which can be synthesized by compounding pharmacists (Fig 1). Metronidazole base is well absorbed in people (>80%) and in dogs, 14 but, despite its popularity in cats, no pharmacokinetic studies exist for either the base or the benzoate form to guide dosing or to establish safe and effective therapeutic margins. Importantly, metabolism of metronidazole in humans involves hepatic metabolism and glucuronidation. As cats differ from other animals in the ability to glucuronidate compounds, commonly used doses may predispose to toxicity.
Fig 1.
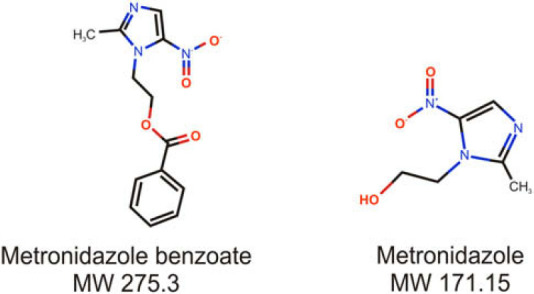
Chemical structure of metronidazole and metronidazole benzoate.
This study sought to (A) determine the pharmacokinetics of metronidazole administered intravenously, and after oral administration of metronidazole benzoate and (B) examine the genotoxicity of metronidazole via DNA disruption in feline peripheral blood mononuclear cells (PBMC) and a feline lymphoma cell line.
Materials and Methods
Cats
The protocols used in this study were approved by the Institutional Animal Care and Use Committee of Cornell University. Five purpose-bred young adult research cats were used (7.48 kg±1.7 body weight) for the pharmacokinetic portion of the study. Three of these cats were used for the lymphocyte genotoxicity portion of the study. Cats were housed in the institutional laboratory animal facility and all cats were adopted out at the completion of the study.
Single-dose pharmacokinetic protocol
Metronidazole administration
Injectable metronidazole USP (metronidazole 5 mg/ml, Braun Medical, Irvine, CA) was administered intravenously at a dose of 5 mg/kg by slow bolus via a peripheral catheter through the cephalic vein in four cats. One cat received 3.5 mg/kg. Metronidazole benzoate was administered orally at a dose of 20 mg/kg (equivalent to 12.4 mg/kg of metronidazole base), after withholding food from the cats for 12 h. A washout period of 7 days was allowed between the intravenous and oral dosing for the pharmacokinetic study. Intravenous dosing was performed first, followed by oral dosing, in all cats.
Sample collection
Blood samples (1.5 ml each) were collected via a saphenous catheter, prior to, and 0.25 (intravenous only), 0.5, 1, 2, 4, 8, 12, and 24 h after drug administration by either route. Cumulative blood collection volumes were within published guidelines. 15 Blood was immediately transferred to tubes containing lithium heparin and centrifuged to harvest plasma. Plasma was frozen at −80°C temperature and shipped frozen to North Carolina State University College of Veterinary Medicine for metronidazole assay.
Metronidazole assay
Feline plasma samples were analyzed by high pressure liquid chromatography (HPLC) using a method developed in the laboratory of one of the authors (MP). A reference standard of metronidazole was obtained from the United States Pharmacopeia (USP, Rockville, Maryland, USA). Metronidazole was dissolved in distilled water to make up a 1 mg/ml stock solution. From this stock solution, further dilutions were made in distilled water to make up fortifying solutions for plasma and to make up fortifying solutions for the calibration curve. The stock solution was kept at 4°C in a tightly sealed dark vial. Metronidazole solutions were added to blank (control) plasma, to make up nine calibration standards (range 0.00–30 μg/ml).
The mobile phase for HPLC analysis consisted of 0.05 M sodium acetate buffer (82%) and acetonitrile (18%). Tetrabutylammonium hydroxide (4 ml) was added to each liter of mobile phase to improve peak shape. The buffer pH was adjusted with glacial acetic acid to a pH of 5.1. Fresh mobile phase was prepared for each day's run. The HPLC system consisted of a quaternary solvent delivery system (Agilent Technologies, Wilmington, DE, USA) at a flow rate of 1 ml/min, an autosampler (1100 Series Autosampler, Agilent Technologies, Wilmington, DE, USA), and ultraviolet detector set at a wavelength of 320 nm (1100 Series Autosampler, Agilent Technologies, Wilmington, DE, USA). The chromatograms were integrated with a computer program (1100 Series Chemstation software, Agilent Technologies, Wilmington, DE, USA). The column was a reverse-phase, 4.6 mm×15 cm C8 column (Zorbax SB-C18, MAC-MOD Analytical, Chadds Ford, PA, USA) kept at a constant temperature of 37°C.
All plasma samples, calibration samples, and blank (control) plasma samples were prepared identically. Solid phase extraction cartridges (Oasis cartridges, Waters Associates, Milford, MA, USA) were conditioned with 1 ml methanol followed by 1 ml distilled water. Each plasma sample was added to a conditioned cartridge, followed by a wash step of 1 ml distilled water:methanol (95:5). The drug was eluted with 1 ml 100% methanol and collected in clean glass tubes. The tubes were evaporated at 40°C for 15 min. Each tube was then reconstituted with 200 μl of mobile phase and vortexed. Fifty microliters of each tube was then injected into the HPLC system. Retention time for peak of interest was 2.5–3.5 min. A fresh set of calibration and blank samples were prepared for each day's run. All calibration curves were linear with a R2 value of 0.99 or higher. The limit of quantification for metronidazole in feline plasma was 0.05 μg/ml, which was determined from the lowest point on a linear calibration curve that produced an appropriate signal-to-noise ratio. The laboratory used guidelines published by the United States Pharmacopeia (USP Chapter 621, United States Pharmacopeia, Rockville Maryland).
Pharmacokinetic calculations
Plasma drug concentrations were plotted on linear and semi-logarithmic graph for analysis. Analysis of curves and pharmacokinetic modeling was performed using a commercial pharmacokinetic program (WinNonlin Version 5.0.1, Pharsight Corporation, Mountain View, California, USA). A compartmental analysis was performed initially, but it was obvious that there was no consistent compartmental model that would be suitable for all cats. For some cats, analysis of the of the intravenous curve resulted in a highly uncertain estimate for the distribution rate constant, therefore, a non-compartmental analysis (NCA) that does not assume any compartmental structure was used for the analysis.
For the NCA the area under the plasma concentration vs time curve (AUC) from time 0 to the last measured concentration (defined by the sensitivity of the assay) was calculated using the log-linear trapezoidal method. The AUC from time 0 to infinity was calculated by adding the terminal portion of the curve, estimated from the relationship Cn/λZ, to the AUC0–Cn, where λZ is the terminal slope of the curve, and Cn is the last measured concentration point. Values for the maximum plasma concentration after dosing (CMAX) and time to maximum plasma concentration (TMAX) were taken directly from the data. Half-lives were calculated from the terminal slope: T½=ln 2.0/(terminal rate constant), where ln 2.0 is the natural logarithm of 2.0. For the intravenous dose, apparent volume of distribution using the area method (VDAREA), apparent volume of distribution at steady state (VDSS) and systemic clearance (CL) were calculated according to previously described methods. 16,17 These values were also reported for the oral dose, but were listed as ‘per fraction absorbed’ (VD/F, CL/F).
Isolation of feline PBMC
PBMC were isolated immediately after blood collection according to a previously described method, 18 yielding approximately one million cells from the 3 ml collected, with viability of >90% when evaluated with trypan blue and ethidium homodimer-1 and calcein Am (Molecular probes). Briefly, ethylenediaminetetraacetic acid (EDTA) whole blood (3 ml) was diluted 1:1 with 0.1 M Ca2+-free and Mg2+-free phosphate buffered saline (PBS), pH 7.2 and mixed thoroughly. The diluted blood sample was carefully layered over an equal volume of Histopaque 1.077 (Pharmacia) and centrifuged at 400×g for 30 min at room temperature. The leukocyte band at the interface between the diluted plasma and Histopaque 1.077 was harvested by aspiration with a Pasteur pipette and transferred to a new conical centrifuge tube. The cells were washed twice in 10 ml of 0.1 M PBS and centrifuged at 250×g for 5 min to remove contaminating platelets and residual Histopaque. Cell pellets were then resuspended in 1 ml of 0.1 M Ca2+-free and Mg2+-free PBS and cells were counted by hemocytometer. Lymphocytes used in in vitro experiments (from blood collected on day 0) were then incubated with metronidazole as described below.
Evaluation of metronidazole cytotoxicity and genotoxicity in vitro
PBMC harvested from three healthy cats and a T-cell lymphoma cell line (feline lymphoma cell line 3201, kindly provided by Colin Parrish, Cornell University) were incubated with 0, 5, 50 and 100 μg/ml metronidazole USP for 1 h at room temperature, and 100 cells per slide were examined at each concentration of metronidazole.
Potential cytotoxic effects of metronidazole at these concentrations were evaluated by staining on PBMC and lymphoma cells with trypan blue, and ethidium homodimer-1, which detects non-viable cells (E1169, Molecular probes, Invitrogen, Carlsbad CA), and calcein AM, which detects viable cells (C1430, Molecular probes, Invitrogen, Carlsbad, CA). Positive and negative controls for cytotoxicity were performed in parallel by incubating cells with 70% methanol and 100 μm H2O2, respectively.
Genotoxicity was evaluated using the COMET assay. 19 PBMC or T- lymphoma cells (50 μl of 1×105 cells suspended in 1 ml 0.1 M Ca2+-free and Mg2+-free PBS) were suspended in 500 μl Low Melting Point agarose to enable evaluation of 500–1000 cells/comet slide (75 μl/slide). Positive control PBMC/lymphocytes were incubated with 100 μM H2O2 for 20 min at 4°C before adding to agarose. Untreated control PBMC/lymphocytes were also included in every run. Prepared slides were immersed in pre-chilled lysis solution followed by alkaline lysis solution, then subjected to horizontal electrophoresis (1 V/cm, ie, 15 V, at 300 mA) in Tris/Borate/EDTA buffer at 4°C, dried, and silver stained (performed according to the manufacturers instructions – Trevigen, Gaithersburg, MD). Silver stained slides were evaluated for qualitative damage by selecting at least 100 randomly chosen cells/sample.
Genotoxicity studies on PBMC were run in duplicate for each sample while T-cell lymphoma cell line, experiments were run in triplicate on two separate occasions.
Evaluation of metronidazole genotoxicity in vivo
Three cats were administered metronidazole benzoate orally every 12 h at a dose of 20 mg/kg (equivalent to 12.4 mg/kg metronidazole) for 7 days. Blood samples (5 ml) were collected into tubes containing EDTA via a saphenous catheter on day 0, day 7 and day 14, with the drug being administered from day 1 to day 7. PBMC were isolated from EDTA samples and the COMET assay was performed as described above.
Statistical analysis
In vitro genotoxicity data were analyzed by two-way analysis of variance (ANOVA), with ‘drug’ and ‘cat’ or ‘drug’ and ‘cell line’ as factors using commercial statistical software (SAS). Drug×cat (or drug×cell line) interactions were considered. In vivo genotoxicity data were analyzed by two-way ANOVA with ‘day’ and ‘cat’ as factors. Results were considered different at P<0.05.
Results
Single-dose pharmacokinetics
Mean plasma metronidazole concentrations are reported in Table 1, with accompanying graph of plasma concentration vs time shown in Fig 2. Pharmacokinetic parameters are reported in Table 2. Systemic absorption of metronidazole was variable (mean=65±28%) with a peak of 8.84±5.4 μg/ml at 3.6±2.9 h. The terminal half-life was 5.34 h from the intravenous dose and 5.16 h from the oral dose. Systemic clearance was low (mean=91.57 ml/h/kg [1.53 ml/kg/min]), and the apparent volume of distribution (steady state) was 0.650±0.254 l/kg.
Table 1.
Plasma metronidazole concentrations after oral and intravenous administration of metronidazole to cats
| Mean±SD | Median (range) | |
|---|---|---|
| Oral dose (12.4 mg/kg) | ||
| Weight (kg) | 7.5±1.7 | 6.8 (6.4, 10.5) |
| Time (h) | MTZ (μg/ml, mean±SD) | |
| 0.5 | 3.3±1.2 | 3.5 (1.7, 5.0) |
| 1 | 5.5±1.3 | 6.3 (3.6, 6.5) |
| 2 | 7.6±3.4 | 6.2 (4.8, 12.8) |
| 4 | 8.1±6.0 | 5.2 (3.5, 17.8) |
| 8 | 6.1±4.5 | 5.2 (2.1, 13.2) |
| 12 | 3.9±2.9 | 3.5 (1.2, 8.4) |
| 24 | 0.8±0.7 | 0.6 (0.2, 2.0) |
| Intravenous dose (5 mg/kg) | ||
| Weight (kg) | 7.5±1.7 | 6.8 (6.4, 10.5) |
| Time (h) | MTZ (μg/ml, mean±SD) | |
| 0.25 | 8.2±1.3 | 8.5 (6.4, 9.4) |
| 0.5 | 7.4±1.9 | 8.0 (4.4, 9.5) |
| 1 | 6.7±1.8 | 6.8 (3.9, 8.7) |
| 2 | 6.5±1.0 | 6.4 (0, 7.7) |
| 4 | 4.4±1.4 | 4.8 (2.1, 5.6) |
| 8 | 2.8±1.0 | 3.0 (1.1, 3.7) |
| 12 | 1.7±0.6 | 1.8 (0.6, 2.4) |
Fig 2.
Plasma concentrations (mean±SD) of metronidazole in five cats after administration of metronidazole intravenously (5 mg/kg) or metronidazole benzoate oral (20 mg/kg, 12.4 mg/kg as the base). Top panel is a linear axis, bottom panel is a semi-logarithmic axis.
Table 2.
Pharmacokinetic parameters in cats after administration of metronidazole intravenously (5 mg/kg) or metronidazole benzoate orally (12.4 mg/kg)
| Parameter | Cat | Mean±SD | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Intravenous administration (5 mg/kg) * | ||||||
| Terminal rate constant | 1 | 0 | 1 | 1 | 1 | 1±0.0 |
| Terminal half-life (h) | 5.7 | 4.2 | 5.8 | 5.5 | 5.6 | 5.3±0.7 |
| C0 (μg/ml) | 9.4 | 4.9 | 6.4 | 10.2 | 8.0 | 7.8±2.1 |
| AUC (0 to infinity) (hμg/ml) | 77.9 | 27.1 | 59.4 | 65.1 | 72.6 | 60.4±19.9 |
| AUC (% extrapolated) | 23.5 | 13.5 | 23.9 | 21.4 | 22.0 | 20.9±4.2 |
| VD (AREA) (ml/kg) | 527.4 | 1108.4 | 706.1 | 603.4 | 390.8 | 667.2±272.1 |
| Clearance (ml/h/kg) | 64.2 | 184.2 | 84.2 | 76.8 | 48.4 | 91.6±53.6 |
| MRT (h) | 8.1 | 5.7 | 8.3 | 7.7 | 7.9 | 7.6±1.0 |
| VD (SS) (ml/kg) | 522.0 | 1057.4 | 698.3 | 588.3 | 384.4 | 650.1±254.5 |
| Oral administration (12.4 mg/kg) | ||||||
| Terminal rate constant | 1 | 0.2 | 1 | 1 | 1 | 1±0.0 |
| Terminal half-life (h) | 4.9 | 4.6 | 5.1 | 5.3 | 5.9 | 5.2±0.5 |
| TMAX (h) | 8.0 | 1.0 | 4.0 | 1.0 | 4.0 | 3.6±2.9 |
| CMAX (μg/ml) | 5.2 | 6.3 | 10.1 | 4.9 | 17.8 | 8.8±5.4 |
| AUC (0 to infinity) (hμg/ml) | 83.2 | 54.0 | 126.2 | 44.7 | 229.0 | 107.4±75.0 |
| AUC (% extrapolated) | 4.8 | 2.8 | 4.5 | 4.5 | 7.6 | 4.8±1.7 |
| VD/F (ml/kg) | 1041.8 | 1530.7 | 724.2 | 2135.3 | 462.0 | 1178.8±666.7 |
| CL/F (ml/h/kg) | 149.0 | 229.4 | 98.3 | 277.5 | 54.2 | 161.7±91.9 |
| MRT (h) | 9.5 | 7.1 | 8.8 | 7.7 | 10.3 | 8.7±1.3 |
| Systemic absorption (%F) | 43.1 | 80.3 | 85.7 | 27.7 | 89.0 | 65.2±27.9 |
C0=initial plasma concentration after intravenous dose (y-axis intercept); AUC=area under the curve; AUC (% extrapolated)=portion of the AUC estimated from the terminal slope; VD=apparent volume of distribution; CL=clearance, MRT=mean residence time; F=fraction of total dose absorbed systemically.
Cat 5 received an intravenous dose of 3.5 mg/kg.
In vitro genotoxicity study
We observed no cytotoxicity of metronidazole at any dose (Fig 3). Dose-dependent genotoxicity was apparent in both the fresh PBMC and the T-cell lymphoma cell line at all three tested concentrations of metronidazole (P<0.05) (Fig 4).
Fig 3.
Lack of cytotoxicity of metronidazole (MTZ) in vitro on freshly harvested PBMC. (A) Percent viability (mean±SD) of PMBC incubated in MTZ and control solutions (H2O2=hydrogen peroxide negative control, MtOH=methanol positive control). (B) Fluorescent micrograph of PBMC after incubation with 100 μg/ml MTZ. Viable cells fluoresce green, non-viable cells fluoresce red. Only two non-viable cells can be seen in this image (upper left and lower right corners, sub-panels). 20× magnification.
Fig 4.
Metronidazole (MTZ) genotoxicity on PBMC and feline lymphoma cells. (A) MTZ induced DNA damage in both PBMC and 3201 lymphoma cells, as assessed by COMET assay, at all concentrations tested. c=Number of comets, v=number of viable cells. (B) Silver stain of gel electrophoresis of control PBMC (no drug exposure). (C) Silver stain of gel electrophoresis of PBMC exposed to 100 μg/ml MTZ. (D) Silver stain of gel electrophoresis of PBMC exposed to H2O2 (positive control). Comet tails can be clearly seen in panels (C) and (D). 60× magnification.
In vivo genotoxicity study
Similarly, we observed genotoxicity in all cats after 7 days of metronidazole benzoate administration at 20 mg/kg PO q12 h (12.4 mg/kg metronidazole) (P<0.05) (Fig 5). However, genotoxicity was no longer apparent 7 days after discontinuing the drug.
Fig 5.
Metronidazole PBMC genotoxicity is reversible within 7 days of discontinuing metronidazole. Mean percentage of PBMC comets for three cats administered MTZ from day 0 to day 7. COMET assays were performed on day 0, day 7 and day 14. c=Number of comets, v=number of viable cells. *Different from other days at P<0.05.
Discussion
We have determined the single-dose pharmacokinetics of both intravenously administered metronidazole, and orally administered metronidazole benzoate. Additionally, we have demonstrated that standard oral dosing of cats with metronidazole for 7 days disrupts DNA (genotoxicity) in PBMC as well as in PBMC and feline T-cell lymphoma cells incubated with metronidazole at concentrations within the therapeutic range (0.4–8 μg/ml). This genotoxicity appears to resolve within 7 days of discontinuing drug administration. Additionally, metronidazole does not appear to be cytotoxic to lymphocytes at the concentrations examined.
In this study, the time at which the peak plasma concentration occurred after oral administration was highly variable between cats – two cats had peak concentrations 1 h after administration, two had peak concentrations 4 h after administration, and one had peak concentration 8 h after administration. These values were somewhat lower than peak concentrations in horses and dogs, which ranged from 12.9 to 22 μg/ml at oral doses ranging from 15 to 25 mg/kg. 14,20–23 In people, the peak concentration during steady state after multiple doses ranges between 18 and 25 μg/ml.
Terminal half-life (5.2±0.5 h) was not statistically longer than what has been reported for dogs (4.5±0.9 h), 14 but was longer than that reported for horses (approximately 3–4 h). 20–23 Mean systemic clearance in cats was slower than in dogs (1.53 ml/kg/min vs 2.49 ml/kg/min), and also slower than reported for horses. Despite the smaller size of cats compared to dogs and horses, the longer terminal half-life and slower clearance suggest that metronidazole pharmacokinetics might not scale allometrically across species. This might be caused by a decreased ability of cats to metabolize metronidazole by glucuronidation as effectively as other species. Whether multiple dosing would result in higher plasma concentrations is debatable, as the terminal half-life is likely too short to substantially alter steady-state pharmacokinetics. However, this requires additional investigation.
Oral metronidazole is well absorbed after oral administration. In this study the median systemic absorption of metronidazole benzoate after oral administration was 80% among the five cats, but was extremely variable (range 28–89%). This median is similar to that for other species: in humans bioavailability is usually >80%; in dogs, it ranges from 60% to 100% 14 and in horses it ranges from 85% to 97%. 20,22 We expected lower systemic absorption of metronidazole benzoate because it is more lipophilic than metronidazole base, but this is not supported by our data.
For most anaerobic Gram-negative bacteria, the minimum inhibitory concentration (MIC90) of metronidazole ranges from 0.25 to 4 μg/ml, but Clostridium species have been reported to have MIC90 values up to 8 μg/ml. 24,25 In this study, concentrations following the single oral dose remained above 3.5 μg/ml in 3/5 cats for at least 12 h and above 0.5 μg/ml in 3/5 cats for at least 24 h. However, two cats had 12 h concentrations <1.6 μg/ml and <0.3 μg/ml at 24 h. As stated earlier, whether steady-state concentrations in cats would be higher than those reported here, providing serum concentrations above the MIC90 of most anaerobic bacteria, remains to be demonstrated.
Genotoxicity, as measured by DNA disruption in PBMC obtained from cats administered metronidazole for 7 days and a feline lymphocyte cell line incubated with metronidazole, was observed in all cats and all lymphocyte experiments. This is similar to observations in people treated with metronidazole. 11–13 The DNA damage resolved within 6 days of discontinuing metronidazole, also similar to findings in humans, 11,12 suggesting that DNA repair mechanisms correct the disruption induced by metronidazole. However, experimental studies in rodents have demonstrated both carcinogenic and mutagenic effects of metronidazole. 10 Lymphoma is the most common hematological neoplasia in cats 26 and cats have a propensity to develop intestinal T-cell lymphoma. 27 It is interesting to speculate that chronic metronidazole therapy may damage lymphoid DNA, which, if unable to be effectively repaired by a defective repair system, could result in development of lymphoma. This may be particularly relevant to cats receiving chronic metronidazole to treat inflammatory bowel disease. Currently, no evidence exists that chronic metronidazole therapy results in lymphoma formation in cats, and additional associative studies are needed to show a correlation between chronic metronidazole exposure and subsequent development of lymphoma.
No clinical adverse effects were observed after the administration of either single or multiple doses to these cats. Metronidazole has been reported to cause neurologic effects in humans and other mammals, including cats. 7,28–30 Neurotoxicity appears to be associated with oral doses greater than 60 mg/kg for several months, and results from inhibition γ-aminobutyric acid (GABA). 7,29,30 Thus, it is unlikely that 20 mg/kg oral metronidazole benzoate will induce neurotoxicity in the majority of cats.
Additionally, there has been concern expressed about administering benzoic acid-containing medications and feed additives to cats, 31 because feed additives and medications that contain benzoate (benzoic acid) may accumulate in cats, and cats are susceptible to benzoate poisoning. 32,33 Toxicosis may be seen as ataxia hyperesthesia, fasciculations, behavior changes, seizures, and even death. Studies by Bedford and Clarke 34 concluded that benzoate doses of 450 mg/kg as a single dose, or 200 mg/kg fed daily are toxic to cats. In this study, metronidazole benzoate was administered at a dose of 20 mg/kg, which is equivalent to 12.4 mg/kg metronidazole, and 7.6 mg/kg benzoate (Fig 1). Therefore, at these dosages, it is unlikely that administration of metronidazole benzoate will produce toxicosis in cats.
In conclusion, we have provided important pharmacokinetic data for single-dose intravenous and oral metronidazole. Our data suggest that 20 mg/kg metronidazole benzoate orally provides serum concentrations in excess of the MIC90 for most anaerobic organisms for at least 12 h in the majority of cats. However, the same oral dose, given for 7 days, induces apparently reversible DNA damage in lymphocytes, but is unlikely to produce clinical signs of acute toxicity. Whether chronic therapy with metronidazole predisposes cats to lymphoid neoplasia is not known.
Acknowledgements
This study was funded by a grant from the Cornell Feline Health Center. Ivana Sekis was a participant in the Cornell Leadership Program and was supported by a grant from Merck. We would like to thank Francis Davis, Susan Klaessig, Angela Struble and Devin Morse at Cornell University for technical assistance on this project, Colin Parrish for providing the feline T-cell lymphoma line and Ms Delta Dise at North Carolina State University for the HPLC analysis of metronidazole. We would like to dedicate this study to the late Dr James Richards, Director of the Cornell Feline Health Center.
References
- 1.Jang S.S., Breher J.E., Dabaco L.A., Hirsh D.C. Organisms isolated from dogs and cats with anaerobic infections and susceptibility to selected antimicrobial agents, J Am Vet Med Assoc 210 (11), 1997, 1610–1614. [PubMed] [Google Scholar]
- 2.Scorza A.V., Lappin M.R. Metronidazole for the treatment of feline giardiasis, J Feline Med Surg 6 (3), 2004, 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane E., Lobetti R., Burroughs R. Treatment with omeprazole, metronidazole, and amoxicillin in captive South African cheetahs (Acinonyx jubatus) with spiral bacteria infection and gastritis, J Zoo Wildlife Med 35 (1), 2004, 15–19. [DOI] [PubMed] [Google Scholar]
- 4.Zimmer J.F. Treatment of feline giardiasis with metronidazole, Cornell Vet 77, 1987, 383–388. [PubMed] [Google Scholar]
- 5.Johnston K.L., Lamport A.I., Ballèvre O.P., Batt R.M. Effects of oral administration of metronidazole on small intestinal bacteria and nutrients of cats, Am J Vet Res 61 (9), 2000, 1106–1112. [DOI] [PubMed] [Google Scholar]
- 6.Jergens A.E. Feline inflammatory bowel disease—current perspectives on etiopathogenesis and therapy, J Feline Med Surg 4, 2002, 175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caylor K.B., Cassimatis M.K. Metronidazole neurotoxicosis in two cats, J Am Anim Hosp Assoc 37, 2001, 258–262. [DOI] [PubMed] [Google Scholar]
- 8.Olson E.J., Morales S.C., McVey A.S., Hayden D.W. Putative metronidazole neurotoxicosis in a cat, Vet Pathol 42 (5), 2005, 665–669. [DOI] [PubMed] [Google Scholar]
- 9.Sisson G., Jeong J.Y., Goodwin A., et al. Metronidazole activation is mutagenic and causes DNA fragmentation in Helicobacter pylori and in Escherichia coli containing a cloned H pylori RdxA(+) (Nitroreductase) gene, J Bacteriol 182 (18), 2000, 5091–5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mudry M.D., Martinez-Flores I., Palermo A.M., Carballo M.A., Egozcue J., Caldes M. Garcia. Embryolethality induced by metronidazole (MTZ) in Rattus norvegicus, Teratog Carcinog Mutagen 21, 2001, 197–205. [DOI] [PubMed] [Google Scholar]
- 11.Reitz M., Rumpf M., Knitza R. DNA single strand-breaks in lymphocytes after metronidazole therapy, Arzneimittelforschung 41 (2), 1991, 155–156. [PubMed] [Google Scholar]
- 12.Menéndez D., Rojas E., Herrera L.A., et al. DNA breakage due to metronidazole treatment, Mutat Res 478 (1–2), 2001, 153–158. [DOI] [PubMed] [Google Scholar]
- 13.Ferreiro G. Rodriguez, Badías L. Cancino, Lopez-Nigro M., et al. DNA single strand breaks in peripheral blood lymphocytes induced by three nitroimidazole derivatives, Toxicol Lett 132 (2), 2002, 109–115. [DOI] [PubMed] [Google Scholar]
- 14.Neff-Davis C.A., Davis L.E., Gillette E.L. Metronidazole: A method for its determination in biological fluids and its disposition kinetics in the dog, J Vet Pharmacol Ther 4, 1981, 121–127. [DOI] [PubMed] [Google Scholar]
- 15.Diehl K.H., Hull R., Morton D., et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes, J Appl Toxicol 21, 2001, 15–23. [DOI] [PubMed] [Google Scholar]
- 16.Gibaldi M., Perrier D. Absorption kinetics and bioavailability. Gibaldi, Perrier Pharmacokinetics, 2nd edn, 1982, Informa Healthcare: London, UK, 145. [Google Scholar]
- 17.Gabrielsson J., Weiner D. Pharmacokinetic concepts. Gabrielsson, Weiner Pharmacokinetic/pharmacodynamic Data Analysis: Concepts and Applications, 2nd edn, 2000, Taylor & Francis: London, UK, 45. [Google Scholar]
- 18.Toth T.E., Smith B., Pyle H. Simultaneous separation and purification of mononuclear and polymorphonuclear cells from the peripheral blood of cats, J Virol Methods 36, 1992, 185–195. [DOI] [PubMed] [Google Scholar]
- 19.Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells, Exp Cell Res 175 (1), 1988, 184–191. [DOI] [PubMed] [Google Scholar]
- 20.Sweeney R.W., Sweeney C.R., Soma L.R., Woodward C.B., Charlton C.A. Pharmacokinetics of metronidazole given to horses by intravenous and oral routes, Am J Vet Res 47, 1986, 1726–1729. [PubMed] [Google Scholar]
- 21.Baggot J.D., Wilson W.D., Hietala S. Clinical pharmacokinetics of metronidazole in horses, J Vet Pharmacol Ther 11, 1988, 417–420. [DOI] [PubMed] [Google Scholar]
- 22.Specht T.E., Brown M.P., Gronwall R.R., Rib W.J., Houston A.E. Pharmacokinetics of metronidazole and its concentration in body fluids and endometrial tissues of mares, Am J Vet Res 53, 1992, 1807–1812. [PubMed] [Google Scholar]
- 23.Steinman A., Gips M., Lavy E., Sinay I., Soback S. Pharmacokinetics of metronidazole in horses after intravenous, rectal, and oral administration, J Vet Pharmacol Ther 23, 2000, 353–357. [DOI] [PubMed] [Google Scholar]
- 24.Kinsey P.B., Hirsch D.C. Obligate anaerobes in clinical veterinary medicine: Susceptibility to antimicrobial agents, J Vet Pharmacol Ther 1, 1978, 63–68. [Google Scholar]
- 25.Lorian V. Susceptibility to antibiotics: Species incidence and trends. Lorian V. Antibiotics in laboratory medicine, 2nd edn, 1996, Williams & Wilkins: Baltimore, 900–1168. [Google Scholar]
- 26.Louwerens M., London C.A., Pedersen N.C., Lyons L.A. Feline lymphoma in the post-feline leukemia virus era, J Vet Int Med 19 (3), 2005, 329–335. [DOI] [PubMed] [Google Scholar]
- 27.Kiselow M.A., Rassnick K.M., McDonough S.P., et al. Outcome of cats with low-grade lymphocytic lymphoma: 41 cases (1995–2005), J Am Vet Med Assoc 232 (3), 2008, 405–410. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson E.R. Use of chemotherapeutics in reptile medicine. Jacobson E.R., Kollias G.V. Exotic animals: contemporary issues in small animal medicine, 1988, Churchill Livingstone: New York, NY, 35–48. [Google Scholar]
- 29.Dow S.W., LeCouteur R.A., Poss M.L., Beadleston D. Central nervous system toxicosis associated with metronidazole treatment of dogs: Five cases (1984–1987), J Am Vet Med Assoc 195, 1989, 365–368. [PubMed] [Google Scholar]
- 30.Evans J., Levesque D., Knowles K., Longshore R., Plummer S. Diazepam as a treatment for metronidazole toxicosis in dogs: A retrospective study of 21 cases, J Vet Int Med 17, 2003, 304–310. [DOI] [PubMed] [Google Scholar]
- 31.Davidson G. To benzoate or not to benzoate: Cats are the question, Int J Pharm Compound 5, 2001, 89–90. [PubMed] [Google Scholar]
- 32.Bedford P.G.C., Clarke M.A. Suspected benzoic acid poisoning in the cat, Vet Rec 89, 1971, 599–601. [DOI] [PubMed] [Google Scholar]
- 33.McCaughey H. Poisoning of cats with benzyl benzoate, Aust Vet J 44, 1968, 82. [DOI] [PubMed] [Google Scholar]
- 34.Bedford P.G.C., Clarke M.A. Experimental benzoic acid poisoning in the cat, Vet Rec 90, 1972, 53–58. [DOI] [PubMed] [Google Scholar]






