Abstract
The purpose of this study was to evaluate the safety and effect of iopanoic acid in 13 cats with hyperthyroidism induced by daily subcutaneous administration of 25 μg/kg levothyroxine for a period of 42 days. On day 28 of levothyroxine administration, cats were randomly allocated to receive treatment twice daily with a placebo (control group; n=4), 50 mg iopanoic acid (low dose group; n=5), or 100 mg iopanoic acid (high dose group; n=4) for 14 days. Compared to the control group, T3 concentrations were significantly decreased in both the low dose and high dose groups on days 35 and 42. T3 concentrations in the low dose and high dose groups at days 35 and 42 were not different from day −8. The effect of iopanoic acid on clinical signs of hyperthyroidism was less apparent. Further clinical studies evaluating the long-term effect in cats with spontaneous hyperthyroidism are warranted.
Methimazole is the mainstay of medical treatment for hyperthyroidism in cats. While effective in most cases, methimazole administration is associated with side effects in up to 18% of treated cats. 1–4 The most common side effects are vomiting and anorexia, but more serious side effects necessitating discontinuation of the drug occur in up to 10% of cats. 1–4 These include self-induced excoriation, hepatotoxicity, thrombocytopenia, and agranulocytosis. In cats that cannot undergo surgical thyroidectomy or radioiodine therapy, there are limited options.
In humans, oral cholecystographic agents such as ipodate and iopanoic acid have been used to treat hyperthyroidism because of their rapid action and excellent safety record. 5–7 The principal effect of oral cholecystographic agents is inhibition of deiodinases that are responsible for the peripheral conversion of thyroxine (T4) to 3,5,3′-triiodothyronine (T3). 6 These effects are likely related to the chemical structure of the oral cholecystographic agents as these actions cannot be accounted for solely by iodine release. 6
Ipodate is the only oral cholecystographic agent that has been evaluated for the treatment of hyperthyroidism in cats. Administration of ipodate to cats with experimentally induced hyperthyroidism caused a significant reduction in serum T3 concentration and an increase in body weight (BW). 8 Ipodate treatment of cats with spontaneous hyperthyroidism resulted in effective control of the disease in 66% of cases. 9 Ipodate production was discontinued by the manufacturer in 2001. Iopanoic acid is a similar oral cholecystographic agent and has replaced ipodate for use in people. 5,6
The authors are unaware of published studies evaluating this agent in cats. The purpose of the pilot study reported here was to evaluate the safety and effect of two dosages of iopanoic acid in cats administered levothyroxine to induce a hyperthyroid state.
Materials and Methods
Animals
Fifteen healthy, adult, female spayed, domestic shorthair cats were used in the study. The cats were part of an established research colony and were determined to be healthy based on physical examination, complete blood count (CBC), biochemistry, and serum T4 concentration. The study was approved by the Virginia Tech Animal Care and Use Committee. All cats were housed in individual cages.
Experimental design
All cats were fed a commercially available dry adult maintenance diet (Science Diet Feline Adult Original dry food, Hill's Pet Nutrition, KS, USA). Maintenance energy requirement (MER) was calculated for each cat using the following formula: MER=1.2×70 (wtkg0.75). Each cat was fed its MER for 1 week prior to the study and during the first week of the study. For weeks 2–6 of the study, each cat was fed twice its initial MER to provide ample food for intake monitoring in anticipation of polyphagia induced by hyperthyroidism. Food was weighed prior to feeding and 24 h later to determine daily and weekly food intake. Cats were fasted for 12 h before blood collection. On day 42, fasting was continued until the 8 h sample was collected.
All cats were treated with levothyroxine for 42 days starting on day 0 in order to induce a hyperthyroid state. Initially levothyroxine (L-thyroxine Na salt, Sigma Aldrich, MO, USA, diluted to 500 μg/ml with 1 mM NaOH) was administered at a dose of 50 μg/kg once a day subcutaneously based on previous studies. 8,10,11 Severe hyperthyroidism manifested by complete anorexia developed in one cat on day 10 (T4 761 nmol/l, reference range 26–49 nmol/l). Subsequently, levothyroxine was discontinued in all cats on day 11 and administered at 25 μg/kg from day 12 to day 42.
On day 28, 13 cats were randomly assigned to a control group (n=4), a low dose group (n=5), or a high dose group (n=4). Two cats, including the cat with severe hyperthyroidism, were removed from the study prior to randomization due to anorexia of greater than 3 days duration. The low dose group received a capsule containing 50 mg of iopanoic acid (Island Pharmacy Services, WI, USA) and 70 mg of lactose orally (PO) every 12 h, the high dose group received a capsule containing 100 mg of iopanoic acid and 20 mg of lactose PO every 12 h, and the control group received a capsule containing 120 mg of lactose PO every 12 h. Treatment was administered for 14 days. All capsules were the same size, shape, and color. All capsules were administered by one of the investigators (AEG) and both investigators were blinded to the treatment groups.
On days −8 (heart rate (HR) only), 0 (BW only), 28, 35, and 42 of the study, HR and BW were recorded. Blood was collected by jugular venepuncture on days −8, 28, 35, and 42 for CBC, biochemistry, and serum T4, T3, and 3,3′,5′−triiodothyronine (reverse T3; rT3) concentrations. All samples were collected 24 h after levothyroxine administration and 12 h after iopanoic acid or placebo administration. Additionally, on day 42 blood samples were collected 4 and 8 h after administration of levothyroxine and iopanoic acid or placebo for measurement of T4, T3, and rT3 concentrations. Samples were analyzed on the day of collection for CBC and biochemistries. Samples for T4, T3, and rT3 concentrations were allowed to clot for 30 min before centrifugation at 1000×g for 20 min. The serum was then harvested and frozen at −70°C until assayed.
Sample analysis
CBCs (Cell-Dyn 3700, Abbott Diagnostics, IL, USA) and plasma biochemistry (Olympus AU400 automated chemistry analyzer, Olympus America, NY, USA) were performed by the Virginia-Maryland Regional College of Veterinary Medicine Veterinary Teaching Hospital clinical pathology laboratory. Biochemical analyses included glucose, blood urea nitrogen (BUN), creatinine, phosphorus, calcium, total protein, albumin, globulin, alanine aminotransferase (ALT), alkaline phosphatase (ALKP), total bilirubin, cholesterol, sodium, potassium, chloride, total CO2, and anion gap.
Serum T4 (Coat-A-Count Total T4, Diagnostic Products Corporation, CA, USA), T3 (Coat-A-Count Total T3, Diagnostic Products Corporation, CA, USA), and rT3 (Reverse T3, Adaltis Italia SpA, Bologna, Italy) concentrations were determined using commercially available radioimmunoassay kits. All assays were performed in duplicate. Any result that initially exceeded the highest standard was diluted using the ‘0’ standard and re-analyzed. The T4 assay had been previously validated for use with feline serum. 12 Assay sensitivity was estimated at 5.0 nmol/l, the point of 95% of total binding on the standard curve. Intra-assay coefficients of variation (CVs) for two feline pooled sera were 7.8% (mean, 29.8 nmol/l, n=10) and 14.4% (mean, 136.2 nmol/l, n=10). Dilutional parallelism was evaluated in the T3 assay by determining recovery of expected concentration when aliquots of one serum sample (3.15 nmol/l) were serially diluted with the ‘0’ standard. When serum was diluted 75%, 50%, 25%, and 12.5% to final volume before addition to the assay, 103%, 96%, 96%, and 92%, respectively, of expected concentrations were recovered. Assay sensitivity was estimated at 0.15 nmol/l, the point of 95% of total binding on the standard curve. Intra-assay CVs for two feline pooled sera were 16.9% (mean, 0.65 nmol/l, n=8) and 5.2% (mean, 3.05 nmol/l, n=10). Dilutional parallelism was evaluated in the rT3 assay by determining recovery of expected concentration when aliquots of one serum sample (0.39 ng/ml) were serially diluted with the ‘0’ standard. When serum was diluted 75%, 50%, 25%, and 12.5% to final volume before addition to the assay, 100%, 96%, 101%, and 95%, respectively, of expected concentrations were recovered. Assay sensitivity was estimated at 0.04 ng/ml, the point of 95% of total binding on the standard curve. Intra-assay CVs for two feline pooled sera were 5.1% (mean, 0.16 ng/ml, n=4) and 6.8% (mean, 0.71 ng/ml, n=4).
Statistical analysis
Data are reported as mean±standard error of the mean (SEM). Serum T3, T4, and rT3 concentrations and HR and BW measurements between baseline values (day −8 or 0) and day 28 for all cats were compared using Student's t-test. Differences in these parameters and weekly food intake between groups and between time periods were compared using the GLIMMIX procedure for repeated measures ANOVA with a Tukey-Kramer adjustment using commercial statistical software (SAS statistical software, version 9.1.3, SAS Institute, NC, USA). For all measurements, group means and SEMs for each time period were determined using separate spreadsheet and analysis software (Microsoft Excel 2002, Microsoft Corporation, WA, USA). Using the same software, differences in ALT, ALKP, creatinine, and phosphorus between groups and between time periods were compared using Student's t-test. For all comparisons, P<0.05 was considered significant.
Results
Animals
The mean age of the 13 cats in the study was 5.4±0.7 years. There was no difference in mean age between groups. Twelve cats completed the study. One cat in the control group was removed on day 36 due to the development of heart failure. This cat was included in the analysis through day 35. In addition, one cat in the low dose group developed fever and inappetence on day 36 and was not included in the food intake analysis, but was able to complete the study. The cause of the cat's illness was not determined despite extensive diagnostic testing that included multiple physical examinations, CBC, plasma biochemistry, thoracic radiographs, and abdominal ultrasound and treatment with clindamycin. The illness persisted for 7 days after cessation of iopanoic acid administration until prednisolone was administered beginning day 49. No recurrence of the illness was noted 12 weeks after discontinuation of the 2-week course of prednisolone treatment.
Thyroid hormone concentrations
There was no difference between groups at day −8 or at day 28 for mean T4, T3, or rT3 concentrations. Compared to day −8, there was a significant increase at day 28 for T4 (P<0.001), T3 (P<0.001), and rT3 (P<0.001) concentrations in all groups.
Compared to the control group, mean T4 concentrations were higher at day 35 for the low and high dose groups (P=0.008 and P=0.012, respectively), and at day 42 for the high dose group (P=0.0011), but not the low dose group (P=0.053) (Fig 1). There was no difference in mean T4 concentrations between the low dose and high dose groups at any time period. For the control group there was no difference in mean T4 concentrations between days 28, 35, and 42.
Fig 1.
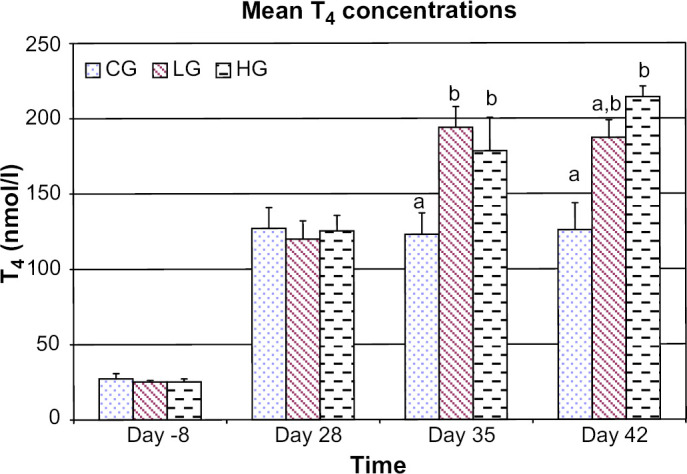
Mean T4 concentrations for control group (CG), low dose group (LG), and high dose group (HG) at days −8, 28, 35, and 42. For each time period bars with different letters are significantly different (P<0.05). Error bars denote SEM.
Compared to the control group, mean T3 concentrations were lower in both the low dose and high dose groups on days 35 (P<0.001 and P=0.003, respectively) and 42 (P<0.001 and P<0.001, respectively) (Fig 2). Compared to day −8, there was no difference for mean T3 concentrations in the low dose and high dose groups at days 35 and 42. There was no difference in mean T3 concentrations between the low and high dose groups at any time period. There was no difference in mean rT3 concentrations between groups at any time period (Fig 3).
Fig 2.
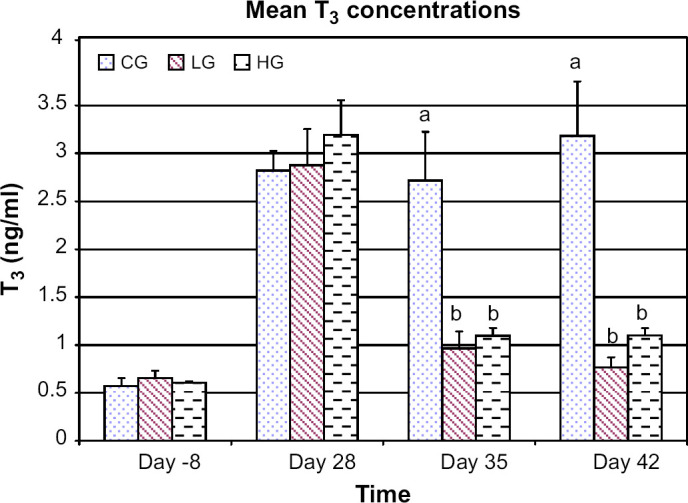
Mean T3 concentrations for control group (CG), low dose group (LG), and high dose group (HG) at days −8, 28, 35, and 42. For each time period bars with different letters are significantly different (P<0.05). Error bars denote SEM.
Fig 3.
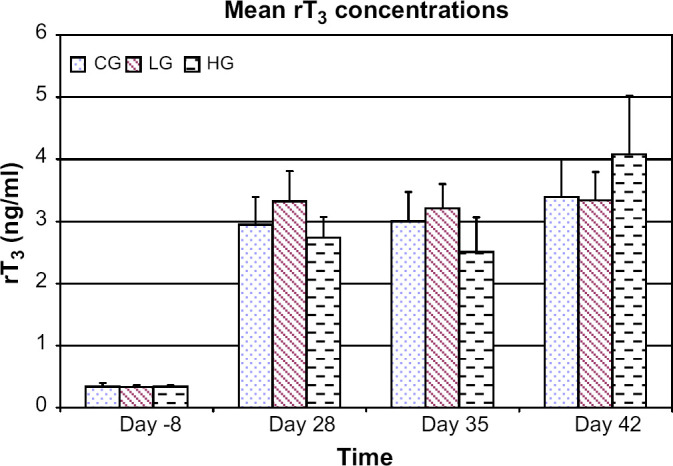
Mean rT3 concentrations for control group (CG), low dose group (LG), and high dose group (HG) at days −8, 28, 35, and 42. Error bars denote SEM.
On day 42, T3, T4, and rT3 concentrations were measured prior to T4 injection and iopanoic acid or placebo dosing (0 h), and at 4 and 8 h after dosing. When compared to 0 h, mean T3 concentrations in the control group were increased at 4 h (P=0.009) but not at 8 h while no difference was noted in either treatment group (Fig 4). When compared to 0 h, mean T4 concentrations were increased in the control and high dose groups at 4 h (P=0.0009 and P=0.040, respectively) and in the low dose group at 4 and 8 h (P=0.002 and P=0.011, respectively). When compared to 0 h, mean rT3 concentrations in the control group were increased at 8 h (P=0.006).
Fig 4.
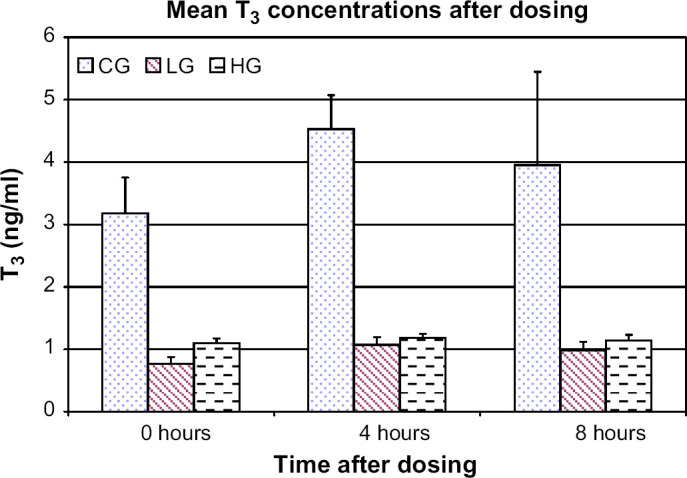
Mean T3 concentrations for control group (CG), low dose group (LG), and high dose group (HG) on day 42 prior to administration of T4, iopanoic acid, or placebo (0 h) and at 4 and 8 h after administration. Error bars denote SEM.
CBC and biochemistry analyses
There were no clinically relevant changes in CBCs for any group at any time period. In addition there were no clinically relevant changes in BUN, glucose, calcium, albumin, globulins, total bilirubin, cholesterol, sodium, potassium, chloride, total CO2, or anion gap.
There was no difference in mean ALT activity between groups at days −8 or 28 (Table 1). On day 28, one cat in the control group had an ALT of 474 U/l, but on day 35 it was 250 U/l. Mean ALT activity of the control group at day 28 excluding this cat was 127.0±20.8 U/l while it was 213.8±88.0 U/l when the cat was included. For days 35 and 42 mean ALT activity in the control group was higher compared to the low dose (P<0.01 and P=0.03, respectively) and high dose (P<0.01 and P=0.03, respectively) groups.
Table 1.
Mean ALT by group *
| Control | Low dose | High dose | |
|---|---|---|---|
| Day −8 | 48.3±4.6 | 46.6±4.3 | 53.5±10.1 |
| Day 28 | 213.8±88.0 | 82.4±20.5 | 95.5±10.3 |
| Day 35 | 200.5±22.7a | 62.6±17.0b | 80.0±9.4b |
| Day 42 | 220.3±37.3a | 57.2±9.5b | 78.8±7.0b |
a,b Means with different letters are significantly different (P<0.05) within each row.
ALT in U/l as mean±SEM. Reference interval 25–114 U/l.
The mean ALKP activity in the high dose group at day −8 was higher compared to the low dose group (P=0.03), but was not different from the control group (Table 2). There was no difference between groups at days 28, 35, or 42. Mean creatinine concentrations were decreased (P<0.05) and mean phosphorus concentrations were increased (P<0.05) in all groups at days 28, 35, and 42 compared to day −8. There was no difference between groups at any time period for either variable.
Table 2.
Mean ALKP by group *
| Control | Low dose | High dose | |
|---|---|---|---|
| Day −8 | 34.0±9.4a,b | 39.6±3.9a | 52.0±4.0b |
| Day 28 | 91.0±14.2 | 76.0±8.5 | 87.8±12.6 |
| Day 35 | 87.0±9.4 | 70.8±11.3 | 91.0±13.7 |
| Day 42 | 98.7±19.8 | 70.0±15.8 | 100.3±14.2 |
Means with different letters are significantly different (P<0.05) within each row.
ALKP in U/l as mean±SEM. Reference interval 11–45 U/l.
Body weight, food intake, and heart rate
There was no significant difference in mean BW or mean weekly food intake between groups or between time periods. Compared to day −8, there was a significant increase in mean HR at day 28 for the low (204.4±5.3 beats/min vs 250.8±6.8, P=0.002) and high dose groups (207±2.4 vs 257±3.3, P=0.001). There was a decrease in mean HR in the low dose group on day 35 (210.8±11) compared with days 28 (P=0.012) and 42 (P=0.044), but there were no other differences in mean HR between groups or time periods.
Discussion
The decrease in T3 noted in the present study was consistent with the primary mechanism of action of iopanoic acid, namely inhibition of conversion of T4 to T3. Because T4 is essentially a prohormone for T3, as thyroid hormone receptors have 10 times higher affinity for T3 than T4, the reduction in T3 would be expected to alleviate the hyperthyroid state despite continued elevation of serum T4 concentration. Serum T3 concentrations during treatment with iopanoic acid were not different from concentrations measured prior to the induction of hyperthyroidism. Therefore, it is expected that the reduction in T3 would result in improvement or resolution of clinical signs of hyperthyroidism in cats with spontaneous disease if the response were similar. The magnitude of the decrease in T3 noted during iopanoic acid administration in this study was similar to that found in cats treated with calcium ipodate using a similar model of hyperthyroidism. 8 Because ipodate is effective in treatment of some cats with spontaneous hyperthyroidism, it seems likely that iopanoic acid would have a similar efficacy. 9
Iopanoic acid lowers T3 concentrations primarily by inhibition of 5′-deiodinase, thus decreasing conversion of T4 to T3. Inhibition of deiodinase also reduces degradation of rT3, resulting in an increase in serum rT3, although it is unknown if this occurs in the cat. 13 The increase in rT3 may further inhibit conversion of T4 to T3. 14,15 In addition, oral cholecystographic agents inhibit cellular uptake of T4 and T3, thus reducing T4 available for conversion to T3 and T3 available to bind to thyroid hormone receptors. 16–18 In the present study T3 concentrations were reduced within 1 week of initiating treatment which is consistent with the rapid decrease seen in human patients treated with oral cholecystographic agents. 15
The increase in serum T4 concentration during iopanoic acid administration was most likely due to decreased degradation of T4 by inhibition of 5′-deiodinase 15 and reduced transport of T4 into cells. 16–18 Increases in T4 concentrations have also been noted in human patients treated with oral cholecystographic agents. For this reason serum T3 rather than T4 concentration should be measured in cats treated with iopanoic acid to determine the effect of treatment.
An increase in serum rT3 would be expected in response to decreased type I iodothyronine deiodinase activity as this enzyme is the major catalyst for deiodination of rT3 in most species. In the cat, however, type I 5′-deiodinase in the liver and kidney have extremely low activity utilizing rT3 as a substrate, thus rT3 concentrations would be minimally affected by iopanoic acid as noted in the present study. 13 In addition, enhanced excretion by alternative metabolic pathways for the degradation of rT3, such as sulfation, deamination, or decarboxylation that are not inhibited by oral cholecystographic agents may be present. Wu et al 19 demonstrated that treatment with ipodate in humans resulted in increases in rT3 sulfate. In addition, iopanoic acid administration in humans has been shown to increase serum and urine concentrations of triiodothyroacetic acid. 20 If the increase in triiodothyroacetic acid is a result of increased production rather than decreased degradation, it is possible that this alternate pathway of T4 metabolism accounts in part for the decrease in T3 and lack of increase in rT3 noted in cats treated with iopanoic acid.
While the mean serum T3 concentration decreased significantly in cats treated with both dosages of iopanoic acid, clinical signs of hyperthyroidism induced by levothyroxine administration were not as convincingly affected. Of the three clinical parameters evaluated in the current study, HR was the only measurement in which improvement was noted during iopanoic acid administration, and that effect was inconsistent. Failure to note consistent and significant differences in HR, BW, and food intake during the study in any group may have been a result of the model used, short duration of the study, or the small number of animals studied. Cats were fed twice MER because they consumed all food when fed MER prior to instituting levothyroxine administration. The investigators felt that offering all cats more food would allow for the opportunity to assess any increase in food intake anticipated after inducing hyperthyroidism, but this may have masked any change in BW that would have occurred secondary to hyperthyroidism. Treatment duration was only 2 weeks, but a decrease in T3 after ipodate or iopanoic acid administration has been documented after a single dose in humans. 15,21 Hence, a clinical response would have been expected after 2 weeks of treatment. Alternatively, the lack of consistent response to treatment may have been due to persistence of the hyperthyroid state regardless of treatment. However, ipodate administration to cats using a similar model for 4 weeks resulted in a proportionately smaller reduction in T3 yet BW and heart weight to BW ratios were lower in treated cats, indicating reduction of thyrotoxicosis. 8
The biochemical abnormalities noted in this study are typical of those described in cats with spontaneous hyperthyroidism. The mean ALKP activity was increased above the reference range in all groups after the induction of hyperthyroidism and remained elevated even in iopanoic acid treatment groups. The increased ALKP activity in hyperthyroid cats is predominately the result of increased bone isoenzyme activity. 22–24 The persistence of the increase in ALKP activity in cats administered iopanoic acid likely indicates that the hyperthyroid state was not completely controlled. Previous studies in rat models have shown that either T4 or T3 can affect bone metabolism resulting in increased bone ALKP isoenzyme activity and bone turnover. 25,26 Therefore, the persistently increased ALKP activity seen in this study may be due to the increased T4 concentrations as T3 concentrations returned to normal in the low and high dose treatment groups. Because a concurrent increase in ALT activity was noted during the hyperthyroid state, some of the ALKP activity may have originated from the liver. The mean ALT activity was significantly lower in the high and low dose groups at days 35 and 42 compared to the control group. This suggests that iopanoic acid was partially effective in reversing the clinicopathologic abnormalities associated with hyperthyroidism.
One cat in the low dose group developed a fever and inappetence on day 36. The fever continued despite cessation of the iopanoic acid on day 42. A cause of the fever was not determined despite a thorough investigation. Although a reaction to the iopanoic acid cannot be excluded, the lack of other clinical signs and continuation of the fever for 1 week after stopping therapy would suggest that this is unlikely. No other cats developed fever or any other signs of illness unrelated to hyperthyroidism. When used at higher doses for cholecystography in people, adverse effects of iopanoic acid have included gastrointestinal complaints, renal disease, and rarely thrombocytopenia. 6,27 However, adverse effects have not been noted when iopanoic acid has been used for the treatment of hyperthyroidism in people. 6,7,28,29 The results of this study support the safety of iopanoic acid administration in hyperthyroid cats. However, the small number of cats evaluated and the short duration of treatment necessitates evaluation in a larger population of cats with spontaneous hyperthyroidism to confirm this finding.
The model of hyperthyroidism used in the present study has been used effectively by others to study the effects of hyperthyroidism on glucose homeostasis and renal function as well as the efficacy of ipodate for the treatment of hyperthyroidism. 8,10,11 However, spontaneous hyperthyroidism probably develops gradually over a period of many months and results in the secretion of excessive amounts of both T4 and T3. The resultant clinical signs of this chronic disorder, including polyphagia, vomiting, diarrhea, weight loss, and tachycardia, were largely absent in the cats of the current study. 30,31 This indicates that although our model was sufficient to induce hyperthyroidism based on elevated T3, T4, and rT3 concentrations and increased HR, the duration of thyroid hormone elevation was insufficient to allow other clinical signs to develop. The model was chosen because the primary mechanism of action of oral cholecystographic agents is inhibition of peripheral conversion of T4 to T3. However, the model does not account for other actions that these agents may have in spontaneous hyperthyroidism such as inhibition of thyroid hormone release from the thyroid gland.
In conclusion, this pilot study demonstrates that iopanoic acid administration markedly reduces T3 levels in cats made thyrotoxic by administration of levothyroxine, and that it appears to be safe. A clinical effect was not evident in this study, but may have been due to the short duration of therapy or the experimental model. Based on the results of this study and previous studies with ipodate, further work to evaluate the long-term effect and safety of iopanoic in cats with spontaneous hyperthyroidism is warranted to determine its clinical utility.
Acknowledgments
This study was funded by a grant from the Veterinary Memorial Fund. The authors would like to acknowledge Dr Stephen Werre for his statistical assistance and Drs WE Monroe and David C Grant for their assistance with this study.
References
- 1.Peterson M.E., Kintzer P.P., Hurvitz A.I. Methimazole treatment of 262 cats with hyperthyroidism, J Vet Int Med 2, 1988, 150–157. [DOI] [PubMed] [Google Scholar]
- 2.Trepanier L.A., Hoffman S.B., Kroll M., Rodan I., Challoner L. Efficacy and safety of once versus twice daily administration of methimazole in cats with hyperthyroidism, J Am Vet Med Assoc 222, 2003, 954–958. [DOI] [PubMed] [Google Scholar]
- 3.Sartor L.L., Trepanier L.A., Kroll M.M., Rodan I., Challoner L. Efficacy and safety of transdermal methimazole in the treatment of cats with hyperthyroidism, J Vet Int Med 18, 2004, 651–655. [DOI] [PubMed] [Google Scholar]
- 4.Lecuyer M., Prini S., Dunn M.E., Doucet M.Y. Clinical efficacy and safety of transdermal methimazole in the treatment of feline hyperthyroidism, Can Vet J 47, 2006, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bal C., Nair N. The therapeutic efficacy of oral cholecystographic agent (iopanoic acid) in the management of hyperthyroidism, J Nuclear Med 31, 1990, 1180–1182. [PubMed] [Google Scholar]
- 6.Braga M., Cooper D.S. Clinical review 129: Oral cholecystographic agents and the thyroid, J Clin Endocrinol Metab 86, 2001, 1853–1860. [DOI] [PubMed] [Google Scholar]
- 7.Panzer C., Beazley R., Braverman L. Rapid preoperative preparation for severe hyperthyroid Graves' disease, J Clin Endocrinol Metab 89, 2004, 2142–2144. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson DC, Jacobs GJ, Hoenig M. Ipodate as an alternative medical treatment for feline hyperthyroidism: Preliminary results in experimentally-induced disease. ACVIM Proceedings, 6th Annual ACVIM Forum, Washington, DC, 1988.
- 9.Murray L.A.S., Peterson M.E. Ipodate treatment of hyperthyroidism in cats, J Am Vet Med Assoc 211, 1997, 63–67. [PubMed] [Google Scholar]
- 10.Hoenig M., Ferguson D.C. Impairment of glucose tolerance in hyperthyroid cats, J Endocrinol 121, 1989, 249–251. [DOI] [PubMed] [Google Scholar]
- 11.Adams W.H., Daniel G.B., Legendre A.M. Investigation of the effects of hyperthyroidism on renal function in the cat, Can J Vet Res 61, 1997, 53–56. [PMC free article] [PubMed] [Google Scholar]
- 12.Kemppainen R.J., Birchfield J.R. Measurement of total thyroxine concentration in serum from dogs and cats by use of various methods, Am J Vet Res 67, 2006, 259–265. [DOI] [PubMed] [Google Scholar]
- 13.Foster D.J., Thoday K.L. Tissue sources of serum alkaline phosphatase in 34 hyperthyroid cats: A qualitative and quantitative study, Res Vet Sci 68, 2000, 89–94. [DOI] [PubMed] [Google Scholar]
- 14.Larson F.C., Albright E.C. Inhibition of l-thyroxine monodeiodination by thyroxine analogs, J Clin Invest 40, 1961, 1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu S.Y., Chopra I.J., Solomon D.H., Bennett L.R. Changes in circulating iodothyronines in euthyroid and hyperthyroid subjects given ipodate (Oragrafin), an agent for oral cholecystography, J Clin Endocrinol Metab 46, 1978, 691–697. [DOI] [PubMed] [Google Scholar]
- 16.DeGroot L.J., Rue P.A. Roentgenographic contrast agents inhibit triiodothyronine binding to nuclear receptors in vitro, J Clin Endocrinol Metab 49, 1979, 538–542. [DOI] [PubMed] [Google Scholar]
- 17.Felicetta J.V., Green W.L., Nelp W.B. Inhibition of hepatic binding of thyroxine by cholecystographic agents, J Clin Investig 65, 1980, 1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felicetta J.V., Czanko R., Huber-Smith M.J., McCann D.S. Cholecystographic agents and sulfobromophthalein inhibit the binding of l-thyroxine to plasma membranes of rat hepatocytes, Endocrinol 118, 1986, 2500–2504. [DOI] [PubMed] [Google Scholar]
- 19.Wu S.Y., Huang W.S., Polk D., Chen W.L., Reviczky A., Williams J., 3rd, Chopra I.J., Fisher D.A. The development of a radioimmunoassay for reverse triiodothyronine sulfate in human serum and amniotic fluid, J Clin Endocrinol Metab 76, 1993, 1625–1630. [DOI] [PubMed] [Google Scholar]
- 20.Siegrist-Kaiser C.A., Burger A.G. Modification on the side chain of thyroid hormones. Wu S.Y., Visser T.J. Thyroid Metabolism: Molecular Biology and Alternate Pathways, 1994, CRC Press: Boca Raton, 175–198. [Google Scholar]
- 21.Burgi H., Wimpfheimer C., Burger A., Zaunbauer W., Rosler H., Lemarchand-Beraud T. Changes of circulating thyroxine, triiodothyronine and reverse triiodothyronine after radiographic contrast agents, J Clin Endocrinol Metab 43, 1976, 1203–1210. [DOI] [PubMed] [Google Scholar]
- 22.Horney B.S., Farmer A.J., Honor D.J., MacKenzie A., Burton S. Agarose gel electrophoresis of alkaline phosphatase isoenzymes in the serum of hyperthyroid cats, Vet Clin Pathol 23, 1994, 98–102. [DOI] [PubMed] [Google Scholar]
- 23.Archer F.J., Taylor S.M. Alkaline phosphatase bone isoenzyme and osteocalcin in the serum of hyperthyroid cats, Can Vet J 37, 1996, 735–739. [PMC free article] [PubMed] [Google Scholar]
- 24.Foster D.J., Thoday K.L., Beckett G.J. Thyroid hormone deiodination in the domestic cat, J Molecul Endocrinol 24, 2000, 119–126. [DOI] [PubMed] [Google Scholar]
- 25.Mundy G.R., Shapiro J.L., Bandelin J.G., Canalis E.M., Raisz L.G. Direct stimulation of bone resorption by thyroid hormones, J Clin Investig 58, 1976, 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato K., Han D.C., Fujii Y., Tsushima T., Shizume K. Thyroid hormone stimulates alkaline phosphatase activity in cultured rat osteoblastic cells (ROS 17/2.8) through 3,5,3′-triiodo-l-thyronine nuclear receptors, Endocrinol 120, 1987, 1873–1881. [DOI] [PubMed] [Google Scholar]
- 27.Fontanilla J.C., Schneider A.B., Sarne D.H. The use of oral radiographic contrast agents in the management of hyperthyroidism, Thyroid 11, 2001, 561–567. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y.S., Tsou C.T., Lin W.H., Hershman J.M. Long term treatment of Graves' disease with iopanoic acid (Telepaque), J Clin Endocrinol Metab 65, 1987, 679–682. [DOI] [PubMed] [Google Scholar]
- 29.Baeza A., Aguayo J., Barria M., Pineda G. Rapid preoperative preparation in hyperthyroidism, Clin Endocrinol (Oxford) 35, 1991, 439–442. [DOI] [PubMed] [Google Scholar]
- 30.Peterson M.E., Kintzer P.P., Cavanagh P.G., Fox P.R., Ferguson D.C., Johnson G.F., Becker D.V. Feline hyperthyroidism: Pretreatment clinical and laboratory evaluation of 131 cases, J Am Vet Med Assoc 183, 1983, 103–110. [PubMed] [Google Scholar]
- 31.Thoday K.L., Mooney C.T. Historical, clinical and laboratory features of 126 hyperthyroid cats, Vet Rec 131, 1992, 257–264. [DOI] [PubMed] [Google Scholar]


