Abstract
This study reports the scintigraphy, histopathology, sole treatment with high-dose radioactive iodine and outcome of eight cases of feline thyroid carcinoma. Scintigraphic findings were variable and in 7/8 cases scintigraphic features could not reliably distinguish whether the thyroid tissue was malignant. Histopathology revealed typical criteria of malignancy in all cases, with mitotic activity described most frequently (7/8 cases), followed by infiltration of local tissues (4/8 cases). Cellular pleomorphism was infrequently observed. Single high-dose (1100 MBq I131) radioiodine therapy was successful in 6/8 cases, with complete resolution of hyperthyroidism, and was associated with prolonged survival times (181–2381 days). Sole treatment with high-dose radioiodine is a safe and effective treatment for functional thyroid carcinoma. The prognosis for feline thyroid carcinoma successfully treated with radioiodine is good, with extended survival times commonly achieved.
Thyroid carcinoma has been reported to be the cause of hyperthyroidism in 1–3% of hyperthyroid cats. 1,2 There are several reports of feline thyroid carcinoma in the veterinary literature, however, features of malignancy relating to scintigraphy and histopathology are variable between publications. 3–7 Specific scintigraphic characteristics that have been described as features of malignancy include multiple areas of hyperfunctional tissue and intrathoracic tissue, however, this interpretation is limited by the existence of ectopic thyroid tissue. 5,8 Distinguishing thyroid adenoma from carcinoma is critical, as successful management of the latter requires a different treatment strategy. High-dose radioiodine is commonly used in human thyroid carcinoma patients, in conjunction with surgery, to treat functional residual primary tumour and metastatic lesions. 9 In this report the term functional will only be used in relation to scintigraphic results, where functional tissue has been identified as tissue that has the capacity to take up a radionuclide, as defined in human medicine. 10 Scintigraphic assessment of thyroid functionality is the basis of determining a patient's suitability to receive radioiodine; non-functional tumours will not take up and organify radioiodine and, therefore, this modality of treatment is not considered appropriate. 11 Successful treatment of thyroid carcinomas with radioiodine requires ablative doses three- to 10-fold higher than those used for thyroid adenoma. 12 Two recent reports have described the successful treatment of canine thyroid carcinoma solely with high-dose radioiodine. However, there is limited information available regarding the sole treatment of feline patients with high-dose radioiodine. 5,10,11 Previously high-dose radioiodine has only been evaluated in conjunction with thyroidectomy and was associated with a high rate of post-treatment hypothyroidism. 7
The aims of this study were to evaluate the scintigraphic and histopathological features of feline thyroid carcinoma, to assist diagnosis and treatment planning, and to assess the response and long-term outcome of patients treated solely with high-dose radioiodine.
Materials and Methods
Seventeen cases of feline thyroid carcinoma presented to the University of Bristol between 1994 and 2006 were evaluated retrospectively. The criteria for inclusion in the study were a pre-mortem histopathological diagnosis of thyroid carcinoma, scintigraphic evaluation and sole treatment with high-dose radioiodine. Eight cases met the inclusion criteria.
The signalment, presenting signs, physical abnormalities, scintigraphic findings, treatment and outcome were recorded. Cases were classified on the basis of initial serum thyroxine concentration, measured at an external reference laboratory, as hypersecretory (HS; ie, hyperthyroid) or non-hypersecretory (NHS). Full clinical evaluation including assessment of serum biochemistry, haematology, urinalysis, systolic blood pressure, imaging (thoracic and abdominal) and scintigraphy was performed in all cases to determine suitability of the patient to receive radioiodine therapy and undergo an extended period of isolation post-treatment. Scintigraphic examination was conducted in all cases following the same protocol described by Harvey et al. 13
All cases received a single dose of 1100 MBq of I131 administered subcutaneously. Anti-thyroid medication was discontinued 2 weeks before treatment. Eleven weeks following administration of radioiodine, full physical examination, serum biochemistry, haematology, urinalysis and thyroxine measurement were repeated in each case.
A telephone questionnaire was conducted to obtain follow-up information from the referring veterinarians. Details regarding recurrence of hyperthyroidism, development of any other illness, serum biochemistry, haematology, total thyroxine, medication prescribed and the time and reason for death or euthanasia were recorded.
Histopathological findings were reviewed by a single board certified pathologist (MJD). Criteria used to support the diagnosis of thyroid carcinoma were: (1) extracapsular invasion, (2) vascular invasion, (3) cellular pleomorphism and (4) mitotic activity. 5,7
Results
Signalment and clinical presentation
Six cats were domestic shorthairs (DSH) and two were domestic longhairs (DLH). All of the cats were neutered; four male and four female. The age of the cats ranged from 7 to 15 years (mean 11 years). Seven cats were hyperthyroid (HS) and one was euthyroid (NHS). Total thyroxine levels (taken as highest level recorded at a reference laboratory pre-referral) ranged from 52 to >320 nmol/l in the HS group (reference range 15–40 nmol/l) and measured 33 nmol/l in the NHS case.
Initial presenting complaints and physical abnormalities were typical of hyperthyroidism in the HS cats. The NHS case was presented due to the discovery of a cervical mass by the owners. A discrete thyroid nodule or cervical masses were palpated in six cases at the time of referral.
Prior treatment of thyroid disease (Table 1)
Table 1.
Treatment and outcome of eight thyroid carcinoma cases
| Case number | Secretory activity | Medical treatment (and dose) prior to I131 treatment | Response to medical treatment | Duration of medical treatment prior to referral | Number of surgical procedures prior to referral for I131 | Surgical procedures prior to referral | Duration of thyroid disease from initial presentation to I131 treatment | Treatment at time of referral | T4 pre-treatment (nmol/l) * | T4 post-I131 treatment (nmol/l) * | Status at time of survey | Period of time elapsed post-I131treatment | Reason for death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HS | M 10 mg/day | Euthyroid | 14 weeks | 1 | Unilateral TE | 11 months | I131 | 172 | 14 | Dead | 181 days | Pancytopenia- euthanased |
| 2 | HS | M 10 mg/day caused vomiting, switched to C 10 mg/day | Euthyroid | 56 weeks | 2 | Unilateral TE+incisional biopsy | 24 months | I131 | 52 | 6.8 | Alive | 540 days | n/a |
| 3 | HS | M 10 mg/day | Not measured | 6 weeks | 1 | Incisional biopsy | 4 months | I131 | 97 | 17 | Alive | 611 days | n/a |
| 4 | HS | M 10 mg/day and then C 5 mg/day | Fluctuated euthyroid-80 nmol/l | 256 weeks | 3 | 2×unilateral TE+1×excisional biopsy | 7 years | I131 | 180 | 11.7 | Alive | 208 days | n/a |
| 5 | HS | C 5 mg/day, switched to M 10 mg/day at recurrence | Remained hyperthyroid (90 nmol/l) | 98 weeks | 2 | Unilateral TE+incisional biopsy | 40 months | I131 | >320 | 294 | Unknown | Lost to follow-up after 229 days | n/a |
| 6 | HS | M 10 mg/day | Remained hyperthyroid (>300 nmol/l) | 4 wks | 2 | Bilateral TE+incisional biopsy | 25 months | I131 | >300 | 6 | Dead | 1201 days | Died unknown cause |
| 7 | HS | C 15 mg/day | Remained hyperthyroid (80 nmol/l) | 12 weeks | 2 | Unilateral TE+incisional biopsy | 20 months | I131 | >300 | 9 | Dead | 2381 days | Head tilt, ataxia and anorexia — died |
| 8 | NHS | None | n/a | n/a | 3 | 2×unilateral TE+cervical lymph node excision | 29 months | I131 + radical surgical excison of bilateral thyroid glands and local tissue 6 months later | 33 | <4 | Dead | 428 days | Hind limb paresis and seizures — euthanased |
HS=hypersecretory, NHS=non-hypersecretory, M=methimazole, C=carbimazole, I131=radioactive iodine, TE=thyroidectomy, n/a=not applicable.
reference range 15–40 nmol/l.
The duration of thyroid disease prior to referral ranged from 4 months to 7 years. The seven HS cases had all received medical treatment ranging from 4 weeks to 4 years in duration (mean 63 weeks, median 14 weeks). Four out of seven cases had been refractory to medical stabilisation. The number of surgical procedures performed prior to referral ranged from one to three.
Scintigraphy (Table 2)
Table 2.
Physical and scintigraphic features of thyroid carcinoma
| Case number | Tumour secretory activity | Last surgical procedure | Time interval between last surgical procedure and scintigraphy | Description of thyroid gland on palpation at presentation | Identification of functional thyroid tissue on scinitiscan | Location of radionuclide uptake | ||
|---|---|---|---|---|---|---|---|---|
| Cervical uptake | Thoracic inlet uptake | Intrathoracic uptake | ||||||
| 1 | HS | Unilateral TE left thyroid gland | 7 months | Large discrete cervical goitre RS | Positive | 2 discrete areas medium-sized LS+RS | None | Diffuse pulmonary miliary pattern |
| 2 | HS | Incisional biopsy left thyroid gland | 12 months | Large cervical mass LS extending larynx to mid-cervical trachea | Positive | 1 large area LS extending to thoracic inlet. Irregular margin | None | None |
| 3 | HS | Incisional biopsy | 6 weeks | 1 small ventral cervical mass | Positive | 2 small discrete areas LS+RS | None | 1 small discrete area |
| 4 | HS | Excisional biopsy (debulk) | 3 months | Irregular mass ventral cervical midline | Positive | 3 small discrete areas; 2 LS; 1 RS | None | 2 large discrete areas |
| 5 | HS | Incisional biopsy | 3 weeks | 2 large cervical masses (1 LS+1 RS) extending from larynx to thoracic inlet | Positive | 1 large area with bilobed appearance extending from thoracic inlet to cervical region | None | |
| 6 | HS | Incisional biopsy right thyroid gland | 4 weeks | Small ventral cervical mass RS | Positive | 1 large area RS with irregular margins | None | None |
| 7 | HS | Incisional biopsy | 3 weeks | Thickened irregular tissue ventral cervical region on LS+RS | Positive | None | None | 3 discrete areas |
| 8 | NHS | Unilateral thyroidectomy left thyroid gland | 4 weeks | Not palpable | Positive | 1 small area in midline with irregular margins | None | None |
HS=hypersecretory, NHS=non-hypersecretory, TE=thyroidectomy, RS=right side, LS=left side.
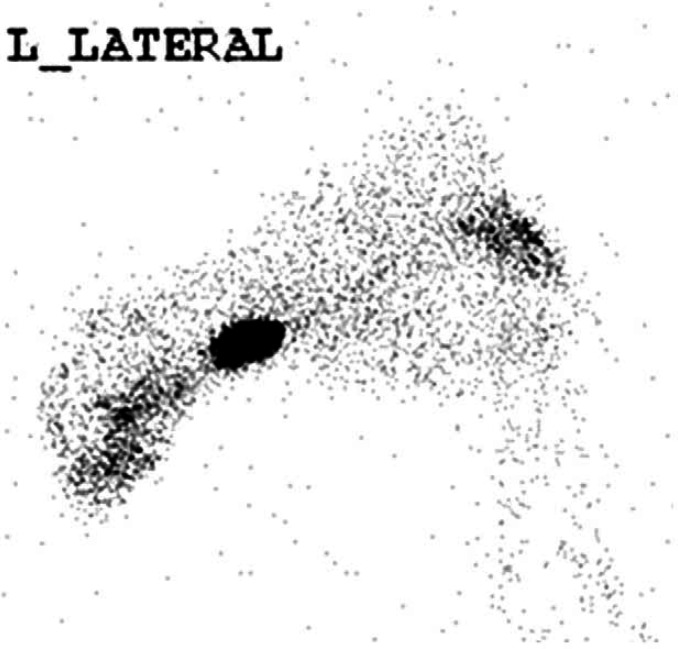
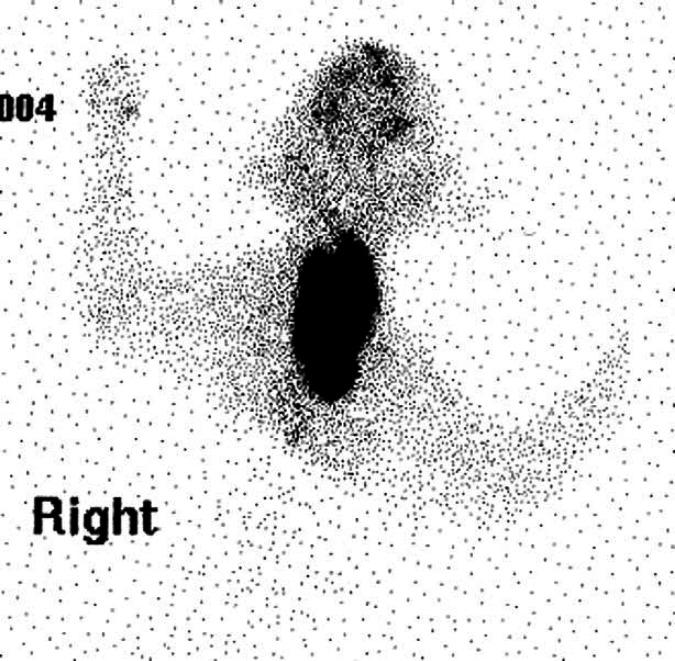
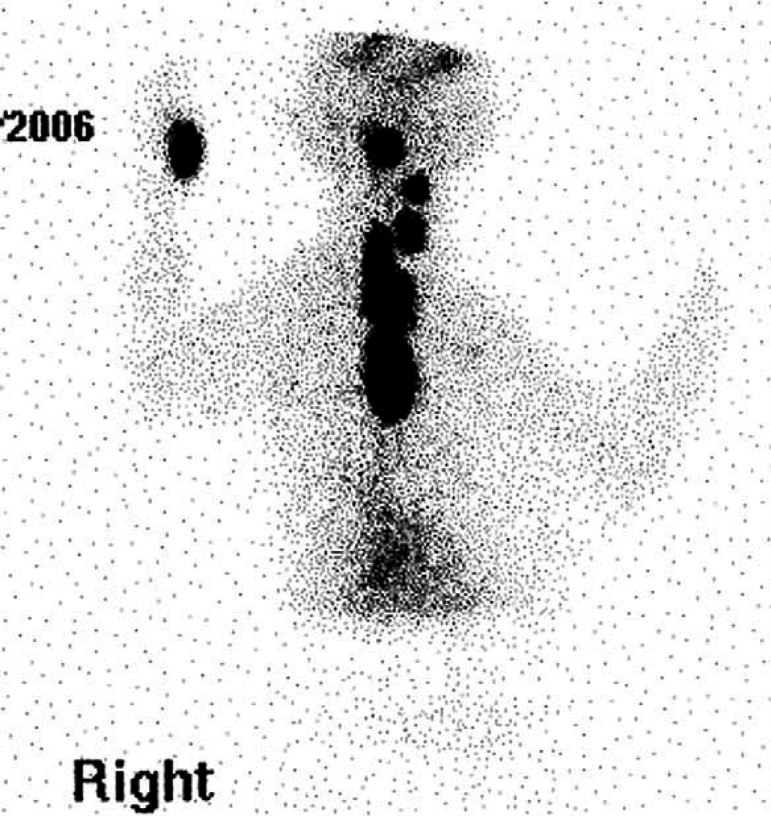
Scintigraphy identified hyperfunctional thyroid tissue in all eight cases. A single region (cervical, thoracic inlet or intrathoracic area) of increased radionuclide uptake (IRU) was identified in five cases (Figs 1, 2a and 3). Three cases had IRU in both the cervical and intrathoracic location; case 1 had two discrete cervical areas of IRU and diffuse pulmonary radionuclide uptake consistent with pulmonary metastatic disease (Figs 4 and 5a). Thoracic radiographs identified a diffuse miliary bronchointerstitial pulmonary pattern (Fig 5b). Four out of the eight cases (all HS) had intrathoracic areas of IRU.
Fig 1.
Case 6. Scintigraphic image – single cervical area of IRU.
Fig 2.
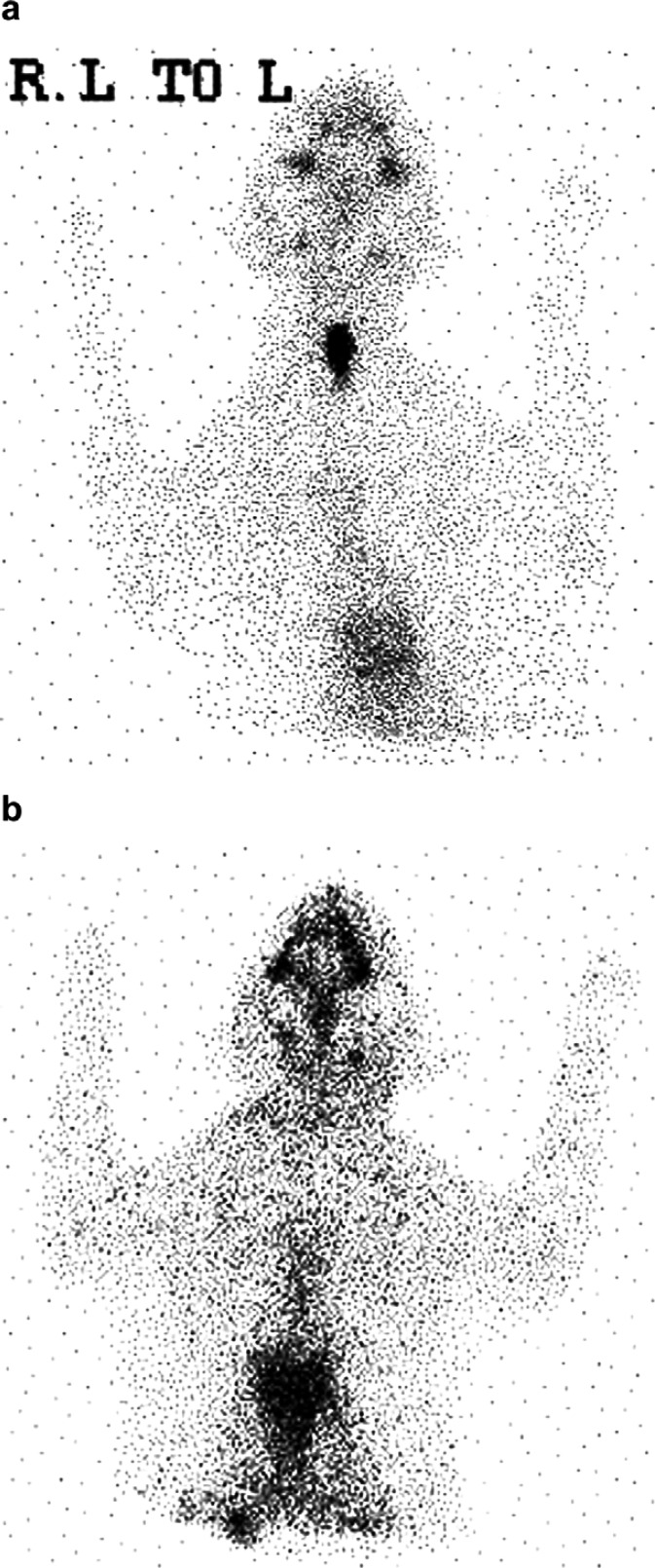
Case 8. Scintigraphic images of an NHS functional carcinoma. (a) Pre-radioiodine treatment demonstrating a single cervical region of IRU. (b) Post-treatment scan revealed no further radioisotope uptake.
Fig 3.
Case 5. Scintigraphic image – large hyperfunctional bilobed area of IRU extending from cervical region to thoracic inlet.
Fig 4.
Case 4. Scintigraphic image – five discrete regions of IRU; three cervical and two intrathoracic.
Fig 5.
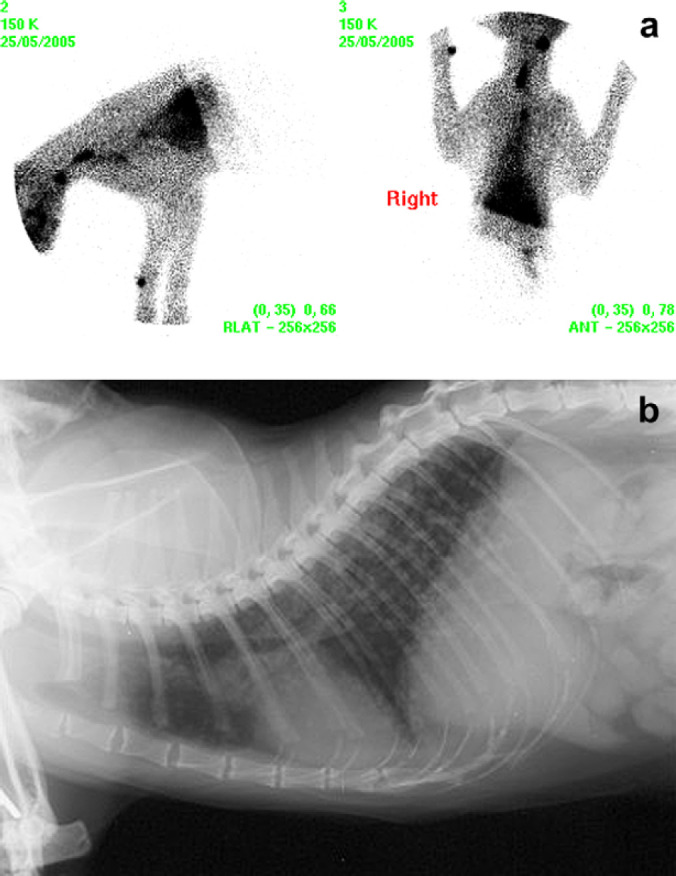
(a) Case 1 scintigraphic image – two discrete cervical regions of IRU and diffuse pulmonary uptake consistent with pulmonary metastases. (b) Case 1, thoracic radiograph (right lateral view) demonstrating diffuse bronchointerstitial pulmonary pattern.
Histopathology
In two of these eight cases (cases 1 and 4) tissue samples included a longitudinal section of an entire enlarged thyroid gland with the tumour apparently contained within the thyroid capsule. In both sections there were focal lobules of adenomatous change, suggesting that the carcinomas may have arisen from a background of benign neoplasia. Areas of atrophic normal thyroid tissue were present in sections from case 1. The remainder of the sections comprised partial fragments of the tumours with incomplete margins. Clear evidence of local invasion of surrounding adipose matrix (cases 3 and 6) or muscle (cases 2 and 8) was seen in four cases (Fig 6). In two cases (cases 1 and 4) tumour cells were noted in close apposition to the wall of blood vessels within the tumour mass or at the periphery, but there was only evidence of intra-vascular invasion in case 1. In a number of cases there was a lymphoplasmacytic inflammatory infiltration at the margin of the tumour.
Fig 6.
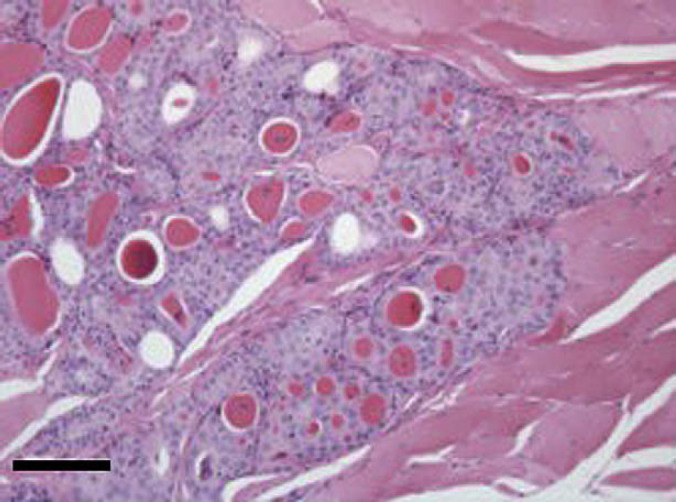
Section from thyroid carcinoma in case 2. There is invasion of muscle by lobules of well-differentiated thyroid follicular epithelial cells forming colloid-filled acinar units. Haematoxylin and eosin. Bar, 200 μm.
The microscopic appearance of the tumour cells was heterogenous. In three cases there were well-differentiated but irregular acinar units lined by cuboidal epithelium individually infiltrating the peri-thyroidal connective tissue matrix (cases 3 and 6) or forming infiltrating lobules within muscle (case 2). In the remaining five cases, the tumour appeared as compact, lobular sheets of cells (Fig 7). Four of these cases (cases 1, 5, 7 and 8) had epithelial morphology and were separated by fine connective tissue septae with low numbers of mitoses scattered throughout. In all instances of compact tumour growth, there were occasional acinar structures or accumulation of bright eosinophilic colloidal material within the tumour sheets. There was no clear microscopical distinction between HS and NHS tumours in terms of pattern of growth or cellular morphology.
Fig 7.
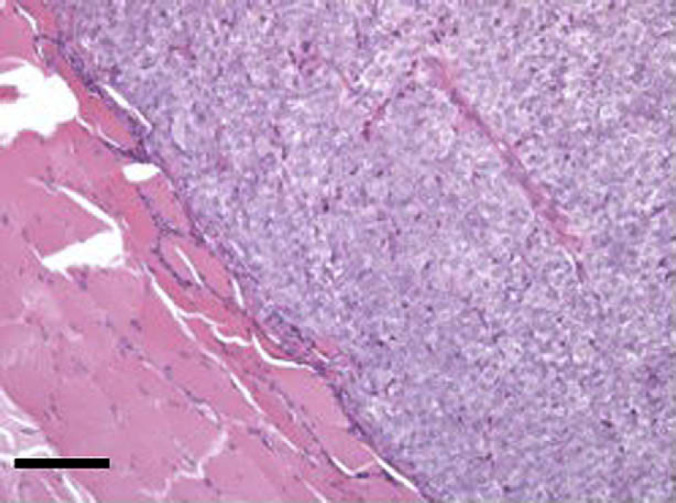
Section from thyroid carcinoma in case 8. A solid lobule of neoplastic thyroid epithelial cells infiltrates peri-thyroidal muscle. The cells comprising this lobule are uniform with packetting by fine connective tissue septae. Haematoxylin and eosin. Bar, 200 μm.
Treatment and long-term outcome (Table 1)
Six out of the seven HS cases (cases 1, 2, 3, 4, 6 and 7) were treated successfully with radioiodine, with resolution of clinical signs, weight gain and reduction in thyroxine concentrations to within or below the reference range 11 weeks post-treatment (mean 10.8 nmol/l, range 6–17 nmol/l). None of the cats developed clinical signs of hypothyroidism and there was no recurrence of hyperthyroidism in any of the successfully treated cases with follow-up periods of 6 months to 6.6 years. Case 5 failed to respond to a single dose of radioiodine and was subsequently successfully stabilised with medical treatment but lost to follow-up after 229 days; medical therapy prior to radioiodine had failed to induce euthyroidism. Cases 4 and 7 developed biochemical evidence of renal insufficiency following treatment. Renal function (judged upon serial assessment of serum urea and creatinine concentrations, and urine specific gravity) did not further decline in either case during the follow-up periods of 208 and 2381 days.
The NHS case (case 8) received radioiodine on the basis of scintigraphic findings, which revealed a small area of functional tissue (Fig 2a). Repeat scintigraphy following treatment showed no further IRU (Fig 2b). Reassessment 6 months later revealed regrowth of two thyroid masses on cervical palpation. Extensive surgical resection of both thyroid glands and deep musculature was performed. Histopathological examination revealed incomplete tumour resection however further treatment was not pursued. The cat was euthanased 428 days after initial radioiodine treatment.
Four cases were dead (three HS, one NHS) at the time of follow-up with a median survival time of 814 days (range 181–2381 days). Case 1 developed severe pancytopenia 6 months after radioiodine treatment; the cat had pulmonary metastatic disease and was feline immunodeficiency virus positive. Three HS cases were alive 208, 540 and 611 days post radioiodine treatment. The remaining case was lost to follow-up. The cause of death or reason for euthanasia was apparently unrelated to thyroid disease for all patients, however post-mortem examination was not performed in any case.
Discussion
Hyperthyroidism associated with benign thyroid adenoma is the most frequently diagnosed endocrinopathy in feline geriatric patients. Thyroid carcinoma is, however, rare. 2 The first aim of this study was to characterise scintigraphic and histopathological features of malignancy to assist in making a pre-mortem diagnosis of thyroid carcinoma. Our results, however, revealed that in most cases there were no typical scintigraphic features to reliably distinguish carcinoma from adenoma. Histopathology revealed typical criteria of malignancy in all cases with mitotic activity described most frequently (7/8 cases), followed by infiltration of local tissues (4/8 cases). The second aim was to assess the response to high-dose radioiodine as a sole treatment for thyroid carcinoma and long-term outcome. Three quarters of the cases were successfully treated and therapy was well tolerated, without the development of clinical hypothyroidism in any case.
The clinical presentation of HS carcinoma was typical of hyperthyroidism. The sole NHS case presented following discovery of a cervical mass, with no other significant clinical signs. Only one other case of NHS carcinoma has been mentioned previously, therefore, there is sparse information available regarding the behaviour, functionality and response to treatment for this form of carcinoma. 7 A further four cases were treated at the UOB during the study period but did not meet all of the inclusion criteria. All of these cases were presented for investigation of cervical masses, two underwent scintigraphic evaluation and both were found to have functional tissue.
Case details including thyroid gland morphology and response to prior medical and surgical treatment were evaluated to try to identify distinguishing clinical features of malignancy. Descriptions of each thyroid mass were highly variable and ranged from an extensive chain of tissue running from the larynx to thoracic inlet, to a single large mass surrounding the lateral and ventral aspect of the larynx. A large thyroid mass is not, however, indicative of malignancy, as many benign thyroid adenomas become large when cystic. 14 Seven out of eight cases were reported by the referring veterinarian to have an atypical appearance of the thyroid gland at surgery (increased vascularity or invasion of local structures, an unusual shape, size or extent of the mass); in 6/8 cases surgical biopsies were performed due to these atypical features instead of planned thyroidectomy. Hyperthyroidism had frequently been difficult to control in the HS group, with 5/7 cats having undergone multiple thyroid surgeries and 4/7 being refractory to medical stabilisation. Any case with an unusual appearance to a cervical mass on palpation or at surgery, and cases of hyperthyroidism requiring repeat surgical procedures or showing an inadequate response to medical therapy should, therefore, raise suspicion of malignancy. However, as not all cases exhibited such features, histopathology should be performed routinely following any thyroid surgery.
The histopathological features of this series of tumours were similar to those described in the literature. 5,14,15 Three of the eight tumours had follicular morphology and five were compact carcinomas. In each case the tumour displayed at least one of the pre-defined criteria for malignancy; seven tumours displayed mitotic activity, four were infiltrative of peri-thyroidal tissue and one had evidence of intra-vascular metastasis and mitotic activity. In keeping with previous reports there was little pleomorphism amongst the neoplastic population. Of note was the observation of mixed adenomatous and carcinoma tissue in two cases, which could be interpreted to suggest that the carcinoma arose from a background of benign neoplasia.
Scintigraphy enables localisation of functional thyroid tissue. However, malignancy cannot necessarily be inferred from the scan. Functional tissue may be present in cats with benign or malignant disease and in both HS and NHS carcinoma. Turrel et al 5 reported a metastatic rate of up to 71% in feline thyroid carcinoma based on necropsy evaluation, with regional lymph nodes and lungs most commonly affected. The scintigraphic identification of metastatic lesions is, however, complicated by ectopic thyroid tissue. As ectopic tissue may be present from the base of the tongue caudally to within the thoracic cavity, 16 it is, therefore, impossible to know whether the focal IRU seen in cases 3, 4 and 7 represents metastases or multiple primary tumours in ectopic sites, as may occur with adenomatous hyperplasia. Several authors have previously described such lesions as metastases. 5,8 There was no histological evidence of vascular metastasis in tumour tissue in cases 3, 4 or 7. Convincing evidence of metastatic disease was identified only in case 1, where there was neoplastic vascular invasion on histopathogical examination and diffuse pulmonary radionuclide uptake consistent with pulmonary metastasis (Fig 5a and b).
Additional scintigraphic features that have been suggested to help distinguish carcinoma from benign disease, include multiple and extensive areas of IRU, a heterogenous pattern of uptake with irregular, spiculated margins, linear multifocal patterns (suggestive of tumour extension along fascial planes) and uptake within the cranial mediastinum. 5,8,17,18 However, all the scintigraphic features from cats in this study (except the diffuse pulmonary uptake shown in Fig 5a) have also been described by the authors in cats with benign disease, suggesting that there are no definitive scintigraphic features that can reliably distinguish carcinomas from adenomatous hyperplasia and that a pre-mortem diagnosis of malignancy relies principally upon histopathological evaluation. 19 Despite this limitation scintigraphy remains an important technique in assessing patients diagnosed with thyroid carcinoma (both HS and NHS), to enable localisation of thyroid tissue and determination of tumour functionality, which is paramount to assessing whether the patient is suitable to receive radioiodine.
Radioactive iodine is considered to be a highly effective treatment for feline hyperthyroidism associated with benign thyroid pathology, with reported success rates of up to 94%, however, efficacy in thyroid carcinoma is less well established. 20 The advantages of radioiodine therapy include the ability to treat all functional thyroid tissue regardless of site, (ie, ectopic or metastatic), lack of requirement for general anaesthesia and potential surgical complications. While successful treatment of seven cats with thyroid carcinoma using intravenous high-dose radioiodine has been described, radioiodine was used in conjunction with complete thyroidectomy (including removal of intrathoracic tissue in one case), and it was concluded that surgical removal of the majority of the hyperfunctional thyroid tissue played an important role in the success of the treatment. Although all of the cats in the current series had undergone surgical procedures at some point prior to referral, it is unlikely that the procedures played a significant role in reducing the volume of thyroid tissue prior to radioiodine treatment, as 6/8 cases had only undergone biopsy, significant residual hyperfunctional tissue was identified scintigraphically in all cases and half of the cases had intrathoracic thyroid tissue. An empirical dose of 1100 Mbq I131 administered subcutaneously was used, based on previously published reports. 5,7 Higher doses of radioiodine are required than for benign disease as malignant tumour cells concentrate and retain I131 less efficiently than thyroid adenomas and the tumour size is usually much larger. 12
Radioiodine treatment was successful in 6/8 cases, with complete resolution of hyperthyroidism and extended survival times ranging from 181–2381 days (median survival time 1201 days – cases 1, 6 and 7). Three cases were still alive after 208, 540 and 611 days at the time of follow-up. This is comparable to a reported median survival time of 2 years in cases of ‘benign’ hyperthyroidism treated with I131 and the two previous reports of carcinoma treatment; a median survival time of 20.5 months was reported following high-dose radioiodine treatment in conjunction with thyroidectomy and survival times of 312–656 days were described for three cases receiving high-dose radioiodine. 5,7,20
High-dose radioiodine was well tolerated in this group of cats, no acute adverse signs were observed (eg, local inflammation, sialoadenitis). Clinical hypothyroidism was also not observed in contrast to that reported by Guptill et al, where 4/7 cats required thyroxine supplementation 2–18 months following surgery and radioiodine. 7 Case 1 had evidence of a large tumour burden with diffuse metastatic pulmonary disease, and in contrast to the only other similar reported case, treatment was successful in resolving hyperthyroidism. 5 Pancytopenia developed 6 months following treatment. Transient bone marrow suppression is a well-recognised potential adverse effect of high-dose systemic radioiodine therapy in humans (cumulative doses >0.67 Gbq/kg) and has previously been reported in dogs occurring up to 4 months after radioiodine treatment. 11,21,22 While the aetiology of pancytopenia was not determined in this case, concurrent FIV infection may also have been the underlying cause.
Treatment with radioiodine was unsuccessful in two cases. Case 5 had large bilateral HS thyroid tumours (Fig 3) and remained hyperthyroid after radioiodine therapy, but subsequently became responsive to medical treatment. Case 8 (NHS, Fig 2a) had tumour recurrence 6 months after radioiodine therapy. Post-treatment scintigraphy failed to identify any residual areas of radioisotope uptake (Fig 2b), suggesting initially that treatment had been effective. I131 distribution is limited to functional cells and treatment failure may be explained by tumour heterogeneity, meaning that areas of the tumour were not exposed to adequate levels of radiation to ensure uniform cell death. 10 As functional tissue was not identified at the time of recurrence surgical resection was attempted, but was unsuccessful. Although radioiodine treatment was not curative in either of these cases, a partial response was seen in both. In such cases multimodal therapy may be necessary to provide adequate control of thyrotoxicosis and induce tumour remission. For example, in case 5 residual tumour could have been treated either with a second dose of radioiodine, surgical resection or external beam radiotherapy. Chemotherapy has not been evaluated in feline thyroid carcinoma.
In conclusion, whilst most thyroid disease in cats is benign, carcinomas do rarely occur. Although suspicion of malignancy may arise due to clinical factors such as an inadequate response to conventional treatment or an atypical physical presentation of the thyroid gland on palpation or at surgery, these features may not always be present and the diagnosis can only reliably be made histopathologically. For this reason histopathology should be performed routinely following any thyroid surgery. Scintigraphy should be performed in all cases of thyroid carcinoma, to evaluate tumour functionality and, hence, suitability to receive radioiodine, regardless of secretory activity. Sole treatment with high-dose radioiodine is well tolerated and a safe effective treatment for functional tumours. The prognosis for cats affected by thyroid carcinoma and treated with high-dose radioiodine is good, with extended survival times commonly achieved.
Acknowledgements
Angie Hibbert and Andrea Harvey hold positions funded by the Feline Advisory Bureau (FAB). The authors would like to thank all the clinicians at The University of Bristol involved in the management of these cases, the staff in the Langford Veterinary Diagnostic laboratories for laboratory analyses, Gemma Corcoran for collating the case files, and the referring veterinary surgeons for referring the cases and providing follow-up information.
References
- 1.Feldman E.C., Nelson R.W. Feline hyperthyroidism (thyrotoxicosis), Canine and Feline Endocrinology and Reproduction, 3rd edn, 2004, Elsevier Science: USA, 152–218. [Google Scholar]
- 2.Naan E.C., Kirpensteijn J., Kooistra H.S., Peters M.E. Results of thyroidectomy in 101 cats with hyperthyroidism, Vet Surg 35 (3), 2006, 287–293. [DOI] [PubMed] [Google Scholar]
- 3.Holzworth J., Theran P., Carpenter J.L., Harpster N.K., Todoroff R.J. Hyperthyroidism in the cat: Ten cases, J Am Vet Med Assoc 176 (4), 1980, 345–353. [PubMed] [Google Scholar]
- 4.Hoenig M., Goldschmidt M.H., Ferguson D.C., Koch K., Eymontt M.J. Toxic nodular goitre in the cat, J Small Anim Pract 23, 1982, 1–12. [DOI] [PubMed] [Google Scholar]
- 5.Turrel J.M., Feldman E.C., Nelson R.W., Cain G.R. Thyroid carcinoma causing hyperthyroidism in cats: 14 cases (1981–1986), J Am Vet Med Assoc 193, 1988, 359–364. [PubMed] [Google Scholar]
- 6.Swalec K.M., Birchard S.J. Recurrence of hyperthyroidism after thyroidectomy in cats, J Am Anim Hosp Assoc 26, 1990, 433–437. [Google Scholar]
- 7.Guptill L., Scott-Moncrieff C.R., Janovitz E.B., Blevins W.E., Yohn S.E., DeNicola D.B. Response to high-dose radioactive iodine administration in cats with thyroid carcinoma that had previously undergone surgery, J Am Vet Med Assoc 207 (8), 1995, 1055–1058. [PubMed] [Google Scholar]
- 8.Peterson M.E., Becker D.V. Radionuclide thyroid imaging in 135 cats with hyperthyroidism, J Vet Radio 25 (1), 1984, 23–27. [Google Scholar]
- 9.Schlumberger M.J., Torlantano M. Papillary and follicular thyroid carcinoma, Bailliere's Clin Endocrinol Metabol 14 (4), 2000, 601–613. [DOI] [PubMed] [Google Scholar]
- 10.Worth A.J., Zuber R.M., Hocking M. Radioiodine (I131) therapy for the treatment of canine thyroid carcinoma, Aust Vet J 83 (4), 2005, 208–214. [DOI] [PubMed] [Google Scholar]
- 11.Turrel J.M., McEntee M.C., Burke B.P., Page R.L. Sodium iodide I131 treatment of dogs with non-resectable thyroid tumours: 39 cases (1990–2003), J Am Vet Med Assoc 229, 2006, 542–548. [DOI] [PubMed] [Google Scholar]
- 12.Peterson M.E. Radioiodine treatment of hyperthyroidism, Clin Tech Small Anim Pract 21, 2006, 34–39. [DOI] [PubMed] [Google Scholar]
- 13.Harvey A.M., Hibbert A., Barrett E.L., Day M.J., Quiggan A.V., Brannan R.M., et al. Scintigraphic findings in 120 hyperthyroid cats, J Feline Med Surg 11, 2009, 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucke V.M. A histological study of thyroid abnormalities in the domestic cat, J Small Anim Pract 5, 1964, 351–358. [Google Scholar]
- 15.Leav I., Schiller A.L., Rijnberk A., Legg M.A., Der Kinderen P. Adenomas and carcinomas of canine and feline thyroid, Am J Pathol 188, 1976, 1160–1164. [PMC free article] [PubMed] [Google Scholar]
- 16.Jones T.C., Hunt R.D. Veterinary Pathology, 5th edn, 1983, Lea and Febiger: Philadelphia, 1597–1613. [Google Scholar]
- 17.Broome M.R. Thyroid scintigraphy in hyperthyroidism, Clinical Tech Small Ani Pract 21, 2006, 10–16. [DOI] [PubMed] [Google Scholar]
- 18.Daniel G.B., Brawnier W.R. Thyroid scintigraphy. Daniel G.B., Berry C.R. Textbook of Veterinary Nuclear Medicine. 2nd edn, 2006, American College of Veterinary Radiology: Harrisburg, PA, 181–199. [Google Scholar]
- 19.Harvey AM, Quiggan AV, Brannan RM, Barrett EL, Caney SMA. A retrospective study of scintigraphic findings in 80 hyperthyroid cats. In: BSAVA Congress 2005 Scientific Proceedings 2005: 536.
- 20.Peterson M.E., Becker D.V. Radioiodine treatment of 524 cats with hyperthyroidism, J Am Vet Med Assoc 207 (11), 1995, 1422–1428. [PubMed] [Google Scholar]
- 21.Adams W.H., Walker M.A., Daniel G.B., Peterson M.G., Legendre A.M. Treatment of differentiated thyroid carcinoma in 7 dogs utilizing I131, Vet Radiol Ultra 36 (5), 1995, 417–424. [Google Scholar]
- 22.Robbins R.J., Schlumberger M.J. The evolving role of I131 for the treatment of differentiated thyroid carcinoma, J Nucl Med 46 (suppl), 2005, 28–37. [PubMed] [Google Scholar]