Abstract
Since the first description of feline hyperthyroidism in 1978, numerous treatment options for hyperthyroidism have been reported. Surgical removal of enlarged, autonomously functioning thyroid glands is one of the most commonly used treatment options. Affected cats must have a careful pre-operative evaluation to detect concurrent medical conditions such as renal disease or cardiomyopathy. Since more than 80% of hyperthyroid cats have neoplastic changes in both thyroid glands, bilateral thyroidectomy is necessary for treatment of the majority of hyperthyroid cats. Several different thyroidectomy techniques have been developed in an attempt to minimise potential post-operative complications associated with bilateral thyoidectomy such as hypocalcemia or recurrence of hyperthyroidism. Damage to or removal of all four parathyroid glands during bilateral thyroidectomy causes hypocalcemia, the most common post-operative complication. Recurrence of hyperthyroidism can occur months after initial thyroidectomy if residual adenomatous thyroid tissue is retained in the surgical site. The most effective surgical techniques for bilateral thyroidectomy involve preservation of at least one external parathyroid gland on the surface of the thyroid capsule. Additionally, the majority of the thyroid capsule must be removed to ensure that all neoplastic thyroid tissue is removed. The most recently described feline thyroidectomy techniques involve sequential removal of bilaterally afftected thyroid glands. Staging a bilatcral thyroidectomy allows time for ipsilateral parathyroid tissue to revascularise before the second thyroid gland is removed and the blood supply to the contralateral parathyroid glands is potentially interrupted. Thyroidectomy is a very effective treatment option for hyperthyroid cats. Surgical treatment of hyperthyroidism in cats offers permanent cure without chronic medical management. No specialised equipment other than standard surgical instrumentation and facilities are necessary. With practice, feline thyroidectomy can become a routine procedure in most veterinary hospitals.
One of the first diagnoses of feline hyperthyroidism was made in 1976 at the Angel Memorial Hospital in Boston, Massachusetts (Holzworth et al 1980). An astute veterinarian measured the serum thyroxine concentration in an older, emaciated, hyperactive cat and found the thyroxine level to be highly elevated. Since that time, the diagnosis of feline hyperthyroidism has become commonplace throughout many developed countries in the world.
The classic hyperthyroid cat is greater than 10-years-old, hyperactive, thin despite a ravenous appetite, and passes voluminous stools. However, some hyperthyroid cats may be depressed, anorexic, and vomit (Graves & Peterson 1994). Serum T4 concentration is elevated in 98% of hyperthyroid cats and serum T3 concentration is elevated in 71% (Broussard et al 1995). The thyroid glands undergo adenomatous change, similar to toxic nodular goiter in humans (Gerber et al 1994). Greater than 70% of hyperthyroid cats have bilateral thyroid enlargement (Thoday & Mooney 1992, Peterson & Becker 1984, Feldman & Nelson 1996). It is rare for the thyroid glands to undergo malignant transformation, less than 5% of hyperthyroid cats have thyroid carcinoma (Feldman & Nelson 1996). The cause of hyperthyroidism is unknown, however it seems to be a relatively recent disease. Surveys of feline necropsy studies conducted before 1979 have shown some thyroid adenomas in cats, but many of these were small masses not similar to the overall glandular enlargement that is present in hyperthyroidism (Leav et al 1976, Lucke et al 1964). Furthermore, the clinical syndrome is so obvious that it is unlikely that it would have existed for years and yet not been reported until 1980.
Since feline hyperthyroidism is a relatively new disease, treatments for the disease are also relatively new. Most of the treatments have been borrowed from effective treatments for thyroid disease in humans. Since feline hyperthyroidism is caused by hyperfunctional thyroid tissue, the use of drugs that interfere with thyroxine synthesis are effective. The most commonly used medical treatment for feline hyperthyroidism in the United States is the administration of methimazole (10–15 mg/day PO), a product that interferes with the iodination of thyroglobulin, the precursor of thyroxine stored in the thyroid follicles (Peterson et al 1988). In Europe, carbimazole (10–15 mg/day PO) which is rapidly converted to methimazole in serum has been used successfully (Mooney et al 1992). Neither methimazole nor carbimazole induce thyroid tumour regression, thyroid tumours remain palpable after treatment. However, the circulating thyroxine concentration is normalised by appropriate dosage. Many cats become nauseous and anorexic during the initial treatment with methimazole or carbimazole, however, this is usually transient. Some cats may develop long-term complications such as thrombocytopenia (Peterson et al 1988).
Hyperfunctional thyroid tissue can be destroyed by treatment with radioiodine, 131I (Turrel et al 1984, Guptill et al 1995). This radioactive iodine is concentrated in the thyroid gland where it causes local destruction of the thyroid tissue. The radiation damage is very localised, not even the parathyroid tissue on the surface of the thyroid gland is affected by the radiation. The administration of radioiodine is limited to institutions that are licensed to handle radiation. 131I is administered subcutaneously and the cats are housed in a radioactive holding area until the amount of radiation excreted in the urine and faeces is reduced below legal limits. This usually requires 2 weeks of hospitalisation.
Surgical removal of hyperfunctioning thyroid glands is a third option for treatment of feline hyperthyroidism. Since the adenomatous goiter that characterises the majority of hyperthyroid cats is a benign condition, surgical removal of the thyroid glands is curative. Thyroidectomy avoids prolonged medical therapy for hyperthyroidism. Furthermore, the surgical technique is relatively simple, does not required specialised instruments, and can be done in most veterinary hospitals.
Surgical anatomy
The paired thyroid glands of the cat are located just caudal to the larynx attached to the medial aspect of the sternothyroideus muscles. The normal thyroid glands are tan, flat and approximately 10 mm long, 4 mm wide and 2 mm thick. Blood is supplied by a cranial thyroid artery and drained by cranial and caudal thyroid veins. The recurrent laryngeal nerves are fine, 1 mm diameter linear structures that ascend in the neck, dorsomedial to the normal thyroid glands within the peritracheal fascia. The bilateral carotid sheaths which each contain the carotid artery, internal jugular vein, and the vagosympathetic trunk run parallel and dorsal to the recurrent laryngeal nerves. The carotid sheaths are approximately 2 cm dorsal to the thyroid glands.
Adenomatous thyroid glands may triple or quadruple in size and are nodular (Feldman & Nelson 1996). Due to the increased mass of the adenomatous gland and the quadruped stance of the cat, the thyroid glands in hyperthyroid cats migrate ventral and caudal from the normal location. Adenomatous thyroid glands are often located in the midcervical region, ventrolateral to the trachea. Some tumourous thyroids may migrate to the thoracic inlet.
There are two parathyroid glands associated with each thyroid gland (Nicholas & Swingle 1925). One parathyroid gland is usually located within the thyroid parenchyma and the other is attached to the external thyroid capsule, usually near the cranial pole of the thyroid gland. The external parathyroid gland in the cat is white, flat, oval, and approximately 3–4 mm in diameter. Fine blood vessels can usually be seen around the periphery of the gland.
Pre-operative evaluation
Since most hyperthyroid cats are greater than 10 years old, a careful pre-anaesthetic evaluation should be done to detect any concurrent or associated diseases. A complete blood count and serum chemistry panel should be done. Many hyperthyroid cats have elevations of serum alkaline phosphatase and serum alanine aminoransferase but relatively few have functional liver abnormalities (Feldman & Nelson 1996, Broussard et al 1995). Many hyperthyroid cats may have mild to moderate renal compromise that can be masked by the increased glomerular filtration rate (GFR) associated with hyperthyroidism (Graves et al 1994, DiBartola 1996). Once the hyperthyroidism is treated, the renal disease may become clinically apparent. It is prudent to treat azotemic hyperthyroid cats with anti-thyroid medical management to determine the extent of renal disease prior to thyroidectomy. If severe renal disease is unmasked by euthyroidism, then thyroidectomy is contraindicated and it may be necessary to induce partial reduction of T4 concentration and maintain modestly elevated GFR using subtherapeutic amounts of anti-thyroid medication.
The hearts of hyperthyroid cats should be asculted carefully to detect resting tachycardia or ventricular arrhythmias. Any cardiac abnormalities should be evaluated with an ECG, thoracic radiographs, and if possible, an echocardiogram. Hyperthyroidism can cause a cardiomyopathy that is associated with ventricular arrhythmias (Peterson et al 1982). If the resting heart rate is greater that 220 beats per minute, even if no arrhythmia is present, medical therapy should be instituted to reduce the resting heart rate to 180–200 beats per minute. The slower heart rate permits better coronary artery perfusion during diastole. The heart rate can be reduced by treatment with methimazole. However, this treatment requires at least 2 weeks to produce euthyroidism. Furthermore, surgical treatment of hyperthyroidism is often selected in order to avoid methimazole therapy. An excellent alternative to methimazole for treatment of tachycardia or tachycardia-based arrhythmias is propranolol (Flanders et al 1987, Feldman & Nelson 1996). Propranolol is a beta-adrenergic blocking agent that has a rapid onset of action. Besides slowing the heart rate, propranolol reduces some of the catecholamine-related side effects of hyperthyroidism such as anxiety and hypertension (Lennquist et al 1985). Propranolol is administered at a dosage of 0.5 mg/kg orally BID to TID for 2–5 days, to effect. Another beta-adrenergic blocking agent that may be used to treat hyperthyroid cats is atenolol. Atenolol is a specific β1 adrenergic blocker that can be administered once per day at a dose of 6.25 to 12.5 mg/cat. Both atenolol and propranolol should given cautiously to cats with congestive cardiomyopathy.
General anaesthesia
Hyperthyroid cats are premedicated for general anaesthesia with acepromazine (0.1 mg/kg IM) or ketamine hydrochoride (5 mg/kg IM), or meperidine (5–10 mg/kg IM). Anticholinergic agents are generally avoided due to pre-existing tachycardia. Anesthesia may be induced by an injectable anaesthetic such as propofol (2–4 mg/kg IV to effect). Mask or chamber induction with an isoflurane/oxygen or halothane/oxygen mixture is also effective. Once anaesthesia is induced, the cats are endotracheally intubated and anaesthesia is maintained with isoflurane/oxygen or halothane/oxygen mixture administered through a rebreathing circuit. Balanced electrolyte solution should be administered during surgery (10 ml/kg/h) and during the postoperative recovery period (2–4 ml/kg/h) for blood pressure support and to help maintain renal perfusion. Blood pressure and ECG should be monitored during surgery.
Surgical approach to the thyroid glands
The thyroid glands are approached surgically through a ventral midline cervical incision that extends from the larynx to the manubrium. Deep to the skin, the oblique fibres of the thin sphincter coli muscle are incised to reveal the paired, strap-like sternohyoideus muscles. The sternohyoideus muscles are separated on midline to reveal the trachea. The normal thyroid glands will be located just caudal to the larynx on the medial aspect of the sternothyroideus muscles. Adenomatous thyroid glands can be located lateral or ventral to the trachea anywhere between the larynx and the manubrium. Exposure of the caudal aspect of the cervical region requires separation of the obliquely oriented sternocephalicus muscles on the midline. Once the adenomatous thyroid glands are identified and excised, the cervical approach is closed by apposing the sternohyoideus muscles on the midline with absorbable suture material in an interrupted pattern. The subcutaneous tissue is closed in a similar manner and the skin is closed with non-absorbable suture material.
Extracapsular thyroidectomy
The first surgical technique to be recommended for treatment of hyperthyroidism was thyroparathyroidectomy (Holzworth 1980). Since most hyperthyroid cats have bilateral thyroid tumours, this surgical procedure entailed removal of all thyroid tissue and all parathyroid tissue attached to the thyroid glands. A ventral cervical approach to the thyroid glands is made, as described above. The adenomatous thyroid glands are identified. The cranial and caudal blood supply is ligated. The thyroid glands and the associated parathyroid tissue are excised between the ligatures.
Since parathyroid hormone produced by the parathyroid glands is responsible for calcium homoeostasis in animals, removal of all four parathyroid glands is associated with a very high incidence of profound, potentially lethal hypocalcaemia. The risk of hypocalcaemia was weighed when bilateral thyroparathyroidectomy was considered for treatment of hyperthyroidism in cats, but based on studies from the early part of the 20th century, it was believed that ectopic parathyroid tissue located in the mediastinum would preserve parathyroid function. This was not the case, however. Thirty-three percent of hyperthyroid cats in one survey, (Table 1) and 62% in another study became severely hypocalcaemic after bilateral thyroparathyroidectomy and required intravenous calcium supplementation (Holzworth et al 1980, Flanders et al 1987). In the former study, attempts were made to spare parathyroid tissue by dissecting it off of the surface of the thyroid capsule (extracapsular thyroidectomy). In the latter study, no attempt was made to spare parathyroid tissue. Surgeons rapidly learned that ectopic parathyroid tissue could not be relied upon to maintain normocalcaemia after bilateral thyroparathyroidectomy.
Table 1.
Post-operative results of published bilateral thyroidectomy techniques used in cats
| Bilateral thyroidectomy technique | Reference | Number of cats | Recurrence rate | Postoperative hypocalcaemia | |
|---|---|---|---|---|---|
|
| |||||
| Overall | Severe * | ||||
|
| |||||
| Extracapsular | Holzworth et al (1980) | 9 | 0% | NA | 33% |
| Extracapsular | Flanders et al (1987) | 11 | 9% | 82% | 62% |
| Intracapsular | Birchard et al (1984) | 53 | 8% | 15% | 8% |
| Intracapsular | Welches et al (1989) | 50 | 22% | 22% | 12% |
| Intracapsular | Swalec et al (1990) | 26 | 8% | 26% | 15% |
| Modified intracapsular | Flanders et al (1987) | 18 | 5% | 36% | 15% |
| Modified intracapsular | Welches et al (1989) | 36 | 0% | 33% | 20% |
| Modified extracapsular | Welches et al (1989) | 26 | 4% | 23% | 11% |
| Staged intracapsular | Flanders et al (1987) | 11 | 0% | 11% | 0% |
| Staged with parathyroid reimplantation | Norsworthy (1995) | 10 | 0% | 0% | 0% |
Became tetanic or required intravenous calcium therapy to prevent tetany.
Intracapsular thyroidectomy
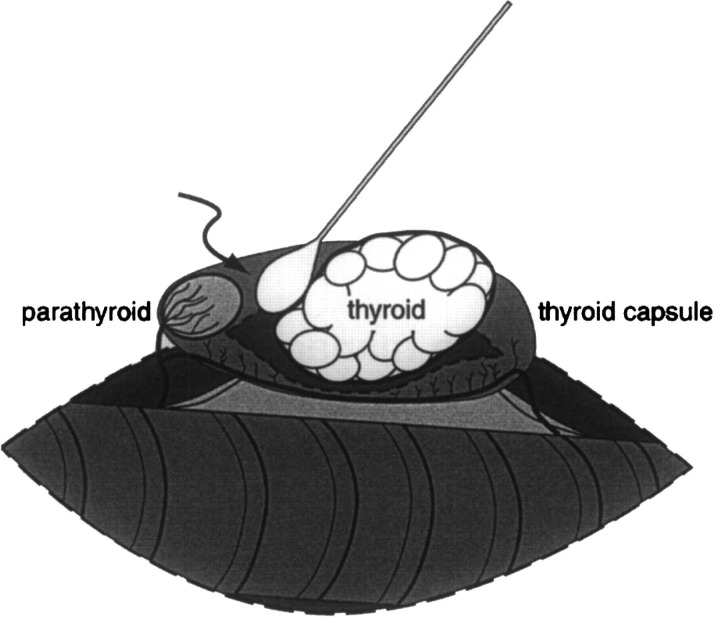
Modifications were made in the feline thyroidectomy technique in an attempt to reduce the incidence of hypocalcaemia. One of the earliest modifications was to remove the thyroid gland through an incision in the thyroid capsule distant from the external parathyroid gland (Birchard et al 1984). A longitudinal incision was made with a scalpel blade or fine iris scissors through the ventral aspect of the thyroid capsule caudal to the cranial parathyroid gland. A saline-soaked cotton tipped applicator stick was used to tease the thyroid gland away from the inside of the thyroid capsule while the external parathyroid gland and its delicate blood supply were preserved (Fig 1). The entire thyroid capsule was left in situ and the external parathyroid gland was minimally disturbed.
Fig 1.
Illustration depicting the intracapsular thyroidectomy technique. The thyroid gland adjacent to the trachea has been exposed through a ventral midline cervical approach. The ventral aspect of the thyroid capsule has been incised longitudinally and a saline-moistened cotton-tipped applicator (arrow) is used to bluntly dissect the thyroid gland out of the capsule. The vascular supply of the external parathyroid gland is preserved as it crosses the thyroid capsule. The modified intracapsular thyroidectomy technique is similar, but after the thyroid gland is removed, the thyroid capsule caudal to the parathyroid gland is excised and the blood supply caudal to the parathyroid gland is ligated.
Intracapsular dissection of the thyroid gland reduced the incidence of post-operative hypocalcaemia (Table 1) after bilateral thyroidectomy (15–26% hypocalcaemia). Transient hypocalcaemia occurred in some cats due to trauma to the delicate parathyroid blood supply during surgical manipulation. Additionally, 8–22% of hyperthyroid cats undergoing intracapsular thyroidectomy had a recurrence of hyperthyroidism within 1 year of initial surgery (Birchard et al 1984, Welches et al 1989, Swalec & Birchard 1990). Small fragments of adenomatous thyroid tissue that remained attached to the thyroid capsule after dissection had continued to grow and had reached sufficient size within 1 year to produce hyperthyroidism.
In order to maintain the low rate of hypocalcaemia and reduce the incidence of hyperthyroidism recurrence, the intracapsular thyroidectomy technique was modified (Flanders et al 1987, Welches et al 1989). After removing the thyroid gland from the incised thyroid capsule, the thyroid capsule was resected, leaving a small rim of capsule around the external parathyroid gland. Resecting the majority of the thyroid capsule greatly reduced the chance that a small piece of adenomatous thyroid tissue would remain in the cat's neck. This modification resulted in a 0% recurrence rate in one study (Welches et al 1989).
Modified extracapsular thyroidectomy
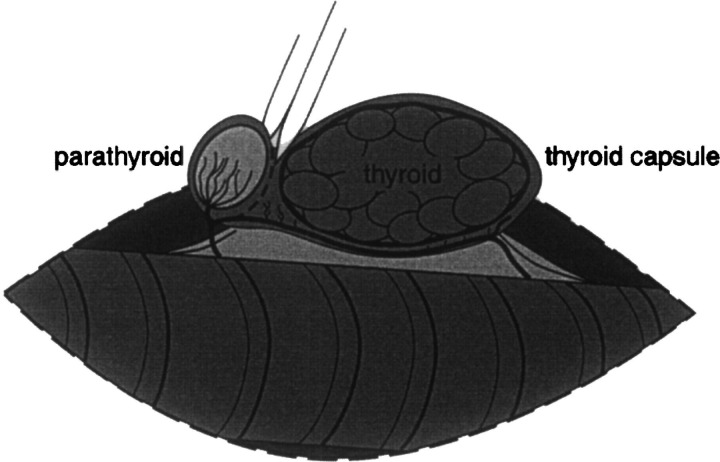
Another modification of the thyroparathyroidectomy involves incision of the thyroid capsule approximately 300° around the external parathyroid gland. (Flanders 1986, Welches et al 1989). The incision line can be pre-marked using bipolar cautery. After partially encircling the parathyroid gland, the capsular incision follows the fine parathyroid vessels that arise from the thyroid artery (Fig 2). The result is a paddle-shaped incision that extends from the thyroid artery and encircles the parathyroid gland. The handle of the paddle encompasses the parathyroid vasculature. The external parathyroid gland and the surrounding thyroid capsule are gently dissected away from the remainder of the capsule and thyroid gland. The caudal blood supply of the thyroid gland is ligated and transected. Then the thyroid gland and approximately 90% of the thyroid capsule are removed from the cat, leaving the external parathyroid gland and a small sleeve of thyroid capsule surrounding the parathyroid gland in situ.
Fig 2.
Illustration depicting the modified extracapsular thyroidectomy technique. The thyroid gland adjacent to the trachea has been exposed through a ventral midline cervical approach. Bipolar cautery has been used to demarcate the thyroid capsule 300° around the external parathyroid gland. A pair of iris scissors is used to incise the capsule around the parathyroid gland. A paddle-shaped portion of thyroid capsule that contains the external parathyroid gland and the parathyroid blood supply is preserved and left in situ. The remainder of the thyroid capsule is removed when the thyroid gland is excised. The blood supply caudal to the parathyroid gland is ligated and transected.
The modified extracapsular thyroidectomy technique has resulted in an incidence of postoperative hypocalcaemia (23%) and hyperthyroid recurrence (4%) similar to the modified intracapsular dissection technique (Table 1) when used in cats with bilateral thyroid adenomas (Welches et al 1989). Selection of modified intracapsular dissection or extracapsular dissection for bilateral thyroidectomy is a matter of surgeon's preference.
Staged thyroidectomy
A third technique for surgical treatment of cats with bilateral thyroid adenomas is to excise one thyroid gland and then wait 3–4 weeks before excising the second thyroid gland (Flanders et al 1987). Either the modified intracapsular or the modified extracapsular thyroidectomy techniques can be used during a staged thyroidectomy. Waiting 3–4 weeks between thyroidectomies allows time for the blood supply of the parathyroid gland left in situ to recover from surgical trauma. Removal of one adenomatous thyroid gland often resolves the clinical signs of hyperthyroidism in bilaterally affected cats. However, if the interval between thyroidectomies is greater than 6 months, the remaining thyroid gland will become large enough to produce a recurrence of hyperthyroidism.
The major drawback of staged thyroidectomy is that two anaesthetic episodes are needed, however the time required for each surgery is very brief and the patients can be discharged from the hospital the day after each surgery since the potential for post-operative hypocalcaemia is very low.
Staged thyroidectomy with parathyroid re-implantation
It has long been known that the parathyroid glands are capable of surviving heterotopic transplantation into muscles distant from the thyroid gland. The parathyroid gland induces neovascularisation and is completely functional within 2 weeks of autotransplantation to distant sites in cats, dogs, and humans (Padgett et al 1998, Wells et al 1974, Rothmund & Wagner 1983).
The most recently described technique for surgical treatment of hyperthyroid cats involves excision of the external parathyroid gland from an adenomatous thyroid gland and implantation of the parathyroid gland into a 1 cm incision made in the sternohyoideus muscle of the neck (Norsworthy 1995). If there is any question concerning the identification of the implanted tissue, a small piece can be submitted for histologic confirmation. The muscle incision is closed with fine non-absorbable suture material. The adenomatous thyroid gland is excised and the cervical incision is closed. The cat is discharged 1 day after surgery and scheduled for the second surgery in 4 weeks. This interval is sufficient to allow the transplanted parathyroid gland to neovascularise. During the second surgery, the external parathyroid gland from the remaining thyroid gland is excised and implanted in the sternohyoideus muscle and the thyroid gland is removed. The cat is discharged from the hospital the day after surgery.
Post-operative management
Hypocalcaemia is the most common postoperative complication after bilateral thyroidectomy (Flanders et al 1987, Welches et al 1989, Birchard et al 1984). Hypocalcaemia is due to hypoparathyroidism caused by intraoperative damage to the parathyroid blood supply or inadvertent parathyroidectomy. One study demonstrated that 77% of hyperthyroid cats were coincidently hyperparathyroid (Barber & Elliott 1996). The authors suggested that reversal of the hyperthyroidism in hyperparathyroid cats may predispose to hypocalcaemia in the post-operative period.
One functional parathyroid gland is sufficient to maintain calcium homeostasis in the cat. If a bilateral thyroidectomy was done, serum calcium should be measured 18–24 h after surgery. If a cat is unilaterally hyperthyroid, or if a staged thyroidectomy is done, then post-operative serum calcium monitoring is not essential. Measuring total serum calcium, without correcting for albumin binding, is sufficient to assess the calcium status of a cat. The association between serum calcium and albumin is not as strong in the cat as it is in the dog, so a valid formula correcting serum calcium for albumin binding cannot be constructed (Flanders et al 1989). Measurement of ionised calcium is readily available and represents a more accurate assessment of calcium status.
Clinical signs of hypocalcaemia associated with iatrogenic hypoparathyroidism usually occur within 1–3 days of surgery. If serum calcium is within the normal range 24 h after bilateral thyroidectomy, it is unlikely that the cat will become hypocalcaemic. The cat should be observed for an additional day and the serum calcium measured approximately 48 h after surgery. If the serum calcium is still within the normal range 48 h after surgery, it is highly unlikely that the cat will become hypocalcaemic and can be discharged to the owner. If the serum calcium concentration falls below 7.0 mg/dl calcium supplementation should be instituted. Cats usually do not show signs of tetany until the serum calcium concentration falls below 6.5 mg/dl.
Cats showing signs of tetany should receive 1–2 ml of 10% calcium gluconate IV over a period of 1 min. The cat can then be maintained on an intravenous infusion of calcium gluconate (8 ml of 10% calcium gluconate in 120 ml normal saline administered at 15 ml/h). Calcium gluconate can safely be given subcutaneously to cats if diluted in a 1:1 ratio with saline. Hypoparathyroid cats should receive 6–8 ml of diluted calcium gluconate solution (3–4 ml 10% calcium gluconate in 3–4 ml 0.9% saline) TID to QID. The dosage is adjusted to maintain normal serum calcium concentration.
Post-operative hypocalcaemia is usually transient after bilateral thyroidectomy. Thrombosis of parathyroid blood vessels usually resolves in 2–9 days. Hypoparathyroidism due to complete devascularisation of the parathyroid glands resolves in 14 days. If hypocalcaemia persists for longer than 2 weeks it is likely that complete parathyroidectomy has occurred. Either the parathyroid glands were removed with the thyroid glands or the parathyroid glands were irreversibly damaged during surgery. Vitamin D therapy can be used to maintain chronically hypocalcaemic cats. Cats can adapt to a chronic hypoparathyroid state without vitamin D, but they maintain a lower than normal serum calcium concentration and are prone to tetany if dietary calcium intake is not maintained (Flanders et al 1991).
Conclusions
Surgical treatment of hyperthyroid cats is a relatively simple surgical procedure that can result in a complete cure in the majority of affected cats. Recurrence of hyperthyroidism after surgery is rare. The modified intracapsular, modified extracapsular, and staged thyroidectomy techniques described in this paper have all been used successfully and a single technique has not been shown to be clearly superior to the other techniques. As with many surgical techniques, the incidence of complications decreases markedly with experience. Many surgeons find that the staged thyroidectomy techniques are more suitable as a learning tool, to be used during the first few bilateral thyroidectomies until some confidence is gained in thyroid gland extirpation with concurrent parathyroid gland preservation. If feline thyroidectomy is done infrequently in a particular practice, the staged techniques may be the superior choice.
References
- Barber PJ, Elliott J. (1996) Study of calcium homeostasis in feline hyperthyroidism. Journal of Small Animal Practice 37, 575–582. [DOI] [PubMed] [Google Scholar]
- Birchard SJ, Peterson ME, Jacobson A. (1984) Surgical treatment of feline hyperthyroidism: Results of 85 cases. Journal of the American Animal Hospital Association 20, 705–709. [Google Scholar]
- Broussard JD, Peterson ME, Fox PR. (1995) Changes in clinical and laboratory findings in cats with hyperthyroidism from 1983 to 1993. Journal of the American Veterinary Medicine Association 206, 302–305. [PubMed] [Google Scholar]
- DiBartola SP, Broome MR, Stein BS, Nixon M. (1996) Effect of treatment of hyperthyroidism on renal function in cats. Journal of the American Veterinary Medicine Association 208, 875–878. [PubMed] [Google Scholar]
- Feldman EC, Nelson RW. (1996) Canine and Feline Endocrinology and Reproduction (2nd ed.), Philadelphia: WB Saunders Co, pp. 118–166. [Google Scholar]
- Flanders JA. (1986) Surgical treatment of hyperthyroid cats. Modern Veterinary Practice 67, 711–715. [Google Scholar]
- Flanders JA, Harvey HJ, Erb HN. (1987) Feline thyroidectomy: A comparison of postoperative hypocalcemia associated with three different surgical techniques. Vet Surgery 16, 362–366. [DOI] [PubMed] [Google Scholar]
- Flanders JA, Scarlett JM, Blue JT, et al. (1989) Adjustment of total serum calcium concentration for binding to albumin and protein in cats: 291 cases (1986–1987). Journal of the American Veterinary Medicine Association 194, 1609–1611. [PubMed] [Google Scholar]
- Flanders JA, Neth S, Erb JN, Kallfelz FA. (1991) Functional analysis of ectopic parathyroid activity in cats. American Journal of Veterinary Research 52, 1336–1340. [PubMed] [Google Scholar]
- Gerber H, Peter H, Ferguson DC, Peterson ME. (1994) Etiopathology of feline toxic nodular goiter. Vet Clin N Am (Small Anim Pract) 24 (3), 541–565. [DOI] [PubMed] [Google Scholar]
- Graves TK, Olivier NB, Nachreiner RF, Kruger JM, Walshaw R, Stickel RL. (1994) Changes in renal function associated with treatment of hyperthyroidism in cats. American Journal of Veterinary Research 55 (12), 1745–1749. [PubMed] [Google Scholar]
- Graves TK, Peterson ME. (1994) Diagnostic tests for feline hyperthyroidism. Vet Clin North Am (Small Anim Pract) 24 (3), 567–576. [DOI] [PubMed] [Google Scholar]
- Guptill L, Scott-Moncrieff CR, Janovitz EB, et al. (1995) Response to high-dose radioactive iodine administration in cats with thyroid carcinoma that ha previously undergone surgery. Journal of the American Veterinary Medicine Association 207, 1055–1058. [PubMed] [Google Scholar]
- Holzworth J, Theran P, Carpenter JL. (1980) Hyperthyroidism in the cat: Ten cases. Journal of the American Veterinary Medicine Association 176, 345–353. [PubMed] [Google Scholar]
- Leav I, Schiller AL, Rijnberk A, et al. (1976) Adenomas and carcinomas of the canine and feline thyroid. American Journal of Pathology 83, 61–93. [PMC free article] [PubMed] [Google Scholar]
- Lennquist S, Jortso E, Anderberg B, Smeds S. (1985) Beta-blockers compared with antithyroid drugs as preoperative treatment in hyperthyroidism: Drug tolerance, complications, and postoperative thyroid function. Surgery 98, 1141–1147. [PubMed] [Google Scholar]
- Lucke VM. (1964) An histological study of thyroid abnormalities in the domestic cat. Journal of Small Animal Practice 5, 351–358. [Google Scholar]
- Mooney CT, Thoday KL, Doxey DL. (1992) Carbimazole therapy of feline hyperthyroidism. Journal of Small Animal Practice 33, 228–235. [Google Scholar]
- Nicholas JS, Swingle WW. (1925) An experimental and morphological study of the parathyroid glands of the cat. American Journal of Anatomy 34, 469–509. [Google Scholar]
- Norsworthy GD. (1995) Feline thyroidectomy: A simplified technique that preserves parathyroid function. Veterinary Medicine 90, 1055–1063. [Google Scholar]
- Padgett SL, Tobias KM, Leathers CW, Wardrop KJ. (1998) Efficacy of parathyroid gland autotransplantation in maintaining serum calcium concentrations after bilateral thyroparathyroidectomy in cats. Journal of the American Animal Hospital Association 34, 219–224. [DOI] [PubMed] [Google Scholar]
- Peterson ME, Keene B, Ferguson DC, Pipers FS. (1982) Electrocardiographic findings in 45 cats with hyperthyroidism. Journal of the American Veterinary Medicine Association 180, 934–937. [PubMed] [Google Scholar]
- Peterson ME, Becker DV. (1984) Radionuclide thyroid imaging in 135 cats with hyperthyroidism. Veterinary Radiology 25, 23–27. [Google Scholar]
- Peterson ME, Kintzer PP, Hurvitz AI. (1988) Methimazole treatment of 262 cats with hyperthyroidism. J Vet Intern Med 2, 150–157. [DOI] [PubMed] [Google Scholar]
- Rothmund M, Wagner PK. (1983) Total parathyroidectomy and autotransplantation of parathyroid tissue for renal hyperparathyroidism. Ann Surg 197, 7–16. [PMC free article] [PubMed] [Google Scholar]
- Swalec KM, Birchard SJ. (1990) Recurrence of hyperthyroidism after thyroidectomy in cats. Journal of the American Animal Hospital Association 26, 433–437. [Google Scholar]
- Thoday IL, Mooney CT. (1992) Historical, clinical and laboratory features of 126 hyperthyroid cats. Vet Rec 131, 257–261. [DOI] [PubMed] [Google Scholar]
- Turrel JM, Feldman EC, Hayes M, et al. (1984) Radioactive iodine therapy in cats with hyperthyroidism. Journal of the American Vetererinary Medicine Association 184, 554–558. [PubMed] [Google Scholar]
- Welches CD, Scavelli TD, Matthiesen DT, Peterson ME. (1989) Occurrence of problems after three techniques of bilateral thyroidectomy in cats. Vet Surgery 15, 392–396. [DOI] [PubMed] [Google Scholar]
- Wells SA, Burdick JF, Hattler BG, et al. (1974) The allografted parathyroid gland: Evaluation of function in the immunosuppressed host. Ann Surg 180, 805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]