Abstract
Type-1 diabetes, resulting from immune-mediated destruction of beta cells, appears to be rare in cats. Type-2 diabetes, characterised by inadequate insulin secretion and impaired insulin action, is the most common form of diabetes in cats. Other specific forms of diabetes constitute a substantial minority of cases. The most common is pancreatic destruction from pancreatic adenocarcinoma. Less frequent causes are insulin resistance from other endocrinopathies including acromegaly. Diabetes in cats is characterised by variable loss of insulin secretory capacity and insulin resistance. Glucose toxicity, islet amyloid-deposition, and pancreatitis contribute to further loss of beta cells and failure of insulin secretion. A significant number of cats undergo remission of their diabetes, usually 1–3 months after good glycaemic control is instituted. Obesity, old age, and Burmese breed are recognised risk factors for the development of diabetes in cats.
Diabetes is one of the most frequent endocrinopathies in cats, with an incidence of between 1 in 100, and 1 in 400 (Panciera et al 1990, Rand et al 1997). Anecdotal evidence suggests that the incidence is increasing in cats, as it is in humans, as the frequency of predisposing factors such as obesity increase (Harris 1995). Management of the feline diabetic patient can be a frustrating experience for the veterinarian and expensive for the owner. Cats are very prone to stress hyperglycaemia, which may make interpretation of blood glucose concentration difficult, and lead to the inappropriate adjustment of insulin dose. Although the majority of cats are insulin-dependent with currently available therapies, a substantial proportion of cats will have remission of their diabetes if good glycaemic control can be achieved. Knowledge of the pathogenesis of feline diabetes helps us to better manage our diabetic patients, and understand why some of the problems with management occur.
Diagnostic criteria
Currently, there are no internationally accepted criteria for the diagnosis of diabetes in cats. Glucose concentrations above the renal threshold are indicative of diabetes, because they result in glycosuria and clinical signs typical of diabetes. However, using the renal threshold blood glucose concentration to define diabetes is not ideal, because it may vary between diabetic cats, as it does in humans (Johansen et al 1984). This would result in a different blood glucose concentration being used to define diabetes in each cat. The renal threshold for glucose in normal cats is 16 mmol/l (Kruth & Cowgill 1982), but subjectively the renal threshold in diabetic cats is around 12 mmol/l.
In humans, criteria are based on fasting blood glucose concentration or blood glucose concentrations 120 min after an oral glucose challenge (Expert Committee 1997).
Use of fasting blood glucose is preferred because of its simplicity, cost effectiveness, and greater reproducibility than results obtained from glucose tolerance tests (Expert Committee 1997, Sparkes et al 1996). A recent study recommended an upper limit for normal fasting blood glucose of 9.5 mmol/l or 171 mg/dl for clientowned cats (Link & Rand 1998). This is higher than reported upper values of 6.7–7 mmol/l, which were derived from research cats, and reflects the confounding effect of stress hyperglycaemia in the diagnosis of feline diabetes (O'Brien et al 1985, Sparkes et al 1996). Criteria for differentiating the intermediate state of impaired glucose tolerance from diabetes have not been defined for cats, although a cutpoint of 12.6 mmol/l was suggested based on extrapolation from human values (Rand 1997).
In humans, diabetic criteria evolved from studies which demonstrated a relationship between blood glucose concentration and microvascular complications, such as retinopathy (Dorf et al 1976, Rushford et al 1979, Expert Committee 1997). The cutpoint between impaired glucose tolerance and diabetes was defined as the blood glucose concentration at which secondary complications occurred with markedly increased frequency (Expert Committee 1997). Further studies are needed to determine diabetic criteria in cats.
Diabetes mellitus causes persistent hyperglycaemia, and it is important to recognise that transient hyperglycaemia up to 16 mmol/l may occur in healthy stressed cats (Kinnaird et al 1998). Non-diabetic sick cats have been reported to have glucose concentrations above 20 mmol/l (Leidinger et al 1989). In humans, diagnosis must be confirmed by a second test on a subsequent day (Expert Committee 1997). This is recommended in cats, especially if blood glucose is less than 20 mmol/l, other intercurrent disease is present which could cause stress hyperglycaemia, or typical clinical signs of diabetes (polyuria, polydipsia and weight loss) are absent. A second test is not usually required if blood glucose is greater than 20 mmol/l and plasma fructosamine or betahydroxybutyrate are elevated.
Classification
Diabetes mellitus is defined as a group of metabolic diseases characterised by hyperglycaemia, which result from defects in insulin secretion, insulin action, or both (Expert Committee 1997). The current classification divides diabetes mellitus into type-1, type-2, and other specific types of diabetes (Expert Committee 1997). Other specific types of diabetes include those caused by diseases of the endocrine pancreas and other endocrinopathies such as acromegaly.
Type-1 diabetes
Type-1 diabetes, in humans previously called insulin-dependent (IDDM) or juvenile onset diabetes, is caused by immune-mediated destruction of beta cells by antibody and T cells (Boitard & Avner 1991). In humans, antibodies to islet cell components are detectable in 85–90% of patients at diagnosis, and are usually present for more than 12 months prior to the onset of clinical signs (Schiffrin et al 1993). Type-1 diabetes is an uncommon cause of diabetes in cats based on histologic studies and absence of islet cell antibodies (Gooseens et al 1998, Hoenig 1999). However, lymphocytic insulitis has been reported, and islet cell antibodies were found in a diabetic kitten, suggesting that type-1 diabetes does rarely occur in cats (Nakayama et al 1990, Woods et al 1994). In dogs, type 1 appears to be the predominant form of diabetes, and 50% of diabetic dogs have islet cell antibodies (Hoenig 1995).
In humans, a genetic predisposition to type-1 diabetes is recognised, but development of the disease is triggered by environmental factors such as enterovirus (Coxsackie B) infection, and early exposure to cow's milk (Helfand et al 1995, Verge et al 1994). Casein, the protein found in cow's milk, has been shown to have sequence homology with beta-cell proteins (Cavallo et al 1996). An immune response to casein may inadvertently trigger the autoimmune destruction of beta cells (Cavallo et al 1996). In type-1 diabetes, the immune-mediated destruction eventually results in almost total loss of beta cells, and insulin therapy is required for survival (Boitard & Avner 1991).
Type-2 diabetes
Based on the typical presentation in humans, type-2 diabetes was previously called non-insulin dependent (NIDDM) or adult-onset diabetes. In 1997, the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus recommended that in humans the term type-2 diabetes be used instead of terms based on clinical description. This recommendation was made because many human patients were insulin-dependent, and some developed the disease at a young age. (Expert Committee 1997). A similar recommendation was made for cats in 1995, because of the high percentage that were insulin-dependent (Lutz & Rand 1995). Type-2 diabetes appears to be the most common form of diabetes in cats, based on islet histology, risk factors, and clinical behaviour of the disease. Most cats are older than 7 years of age by the time that signs develop, with a peak incidence between 10–13 years of age (Panciera et al 1990, Rand et al 1997). More diabetic cats are males, with a ratio of approximately 1.5:1 (Panciera et al 1990, Rand et al 1997).
In contrast to type-1 diabetes, the cause of type-2 diabetes is much less clear. Type-2 diabetes is characterised by inadequate insulin secretion and impaired insulin action, that is, insulin resistance. The relative severity of these two defects varies between patients, both feline and human (Lutz & Rand 1996, Sieradzki 1998). The defect in beta-cell function is usually progressive, and in some cats and humans results in complete loss of insulin secretion (Lebovitz 1994, Lutz & Rand 1996, Sieradzki 1998).
Other specific types of diabetes
A substantial minority of diabetic cats have other specific types of diabetes, previously called secondary or type 3 diabetes (Lutz & Rand 1995, Goossens et al 1998, Expert Committee 1997). Diseases in this category that have been reported in cats include diseases causing insulin resistance, and those resulting from non-specific destruction of pancreatic tissue (O'Brien et al 1986, Rand 1997, Goossens et al 1998). The most frequent is pancreatic adenocarcinoma, accounting for up to 19% of feline diabetes (O'Brien et al 1985, Goossens et al 1998). Pancreatitis is a common histological finding in diabetic cats (Goossens et al 1998), but whether it is a cause or a result of diabetes is unclear. In most cases, the lesion does not appear sufficiently severe to cause diabetes by itself, but may contribute to beta-cell loss. Histologic evidence of pancreatitis is also present in many humans with type-2 diabetes (Clarke et al 1995). Pancreatitis may be triggered by the hyperlipidaemia associated with diabetes. Evidence for this comes from humans, where pancreatitis is a major complication of diseases causing marked hypertriglyceridaemia (Greenberger 1973, Thompson 1988)
Rare causes of naturally-occurring insulin resistance in cats include growth-hormone producing tumours resulting in acromegaly, and hyperadrenocorticism (Peterson et al 1990, Feldman & Nelson 1995). Iatrogenic administration of megestrol acetate or long-acting steroids is associated with the development of diabetes in some cats (Feldman & Nelson 1995). Relatively few cats develop diabetes after prolonged use of these drugs, so it is possible that affected cats had underlying impaired glucose tolerance which the drugs exacerbated.
Since type-2 diabetes is the most common form of feline diabetes, the following discussion will focus on this form of the disease.
Pathogenesis of type-2 diabetes
In humans, there is considerable debate as to whether insulin resistance or beta-cell dysfunction is the first or primary abnormality in type-2 diabetes (Olefsky 1992, Pimenta et al 1995). There is evidence that the primary abnormality may vary in different human populations (Olefsky 1992). Cats with impaired glucose tolerance have elevated insulin to glucose ratios, suggesting that insulin resistance is an early feature of the disease (O'Brien et al 1985). Paradoxically, some researchers have interpreted increased insulin concentrations to represent beta-cell dysfunction. They postulate that hypersecretion of insulin is the primary abnormality, which in turn induces insulin resistance and beta-cell exhaustion (Zimmet & O'Dea 1993).
Beta-cell dysfunction
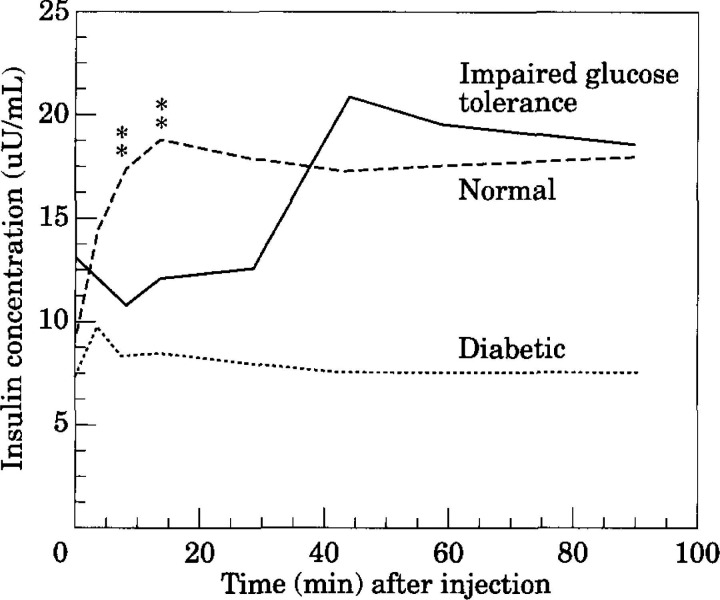
In humans, several stages of beta-cell dysfunction are recognised as beta-cell failure progresses (Atkinson & Maclaren 1994, Hoenig 1998). Some of these stages have also been identified in cats with naturally occurring diabetes (O'Brien et al 1985, Lutz & Rand 1996). Insulin secretion is abnormal in cats and humans with impaired glucose tolerance, an intermediate state between normal glucose tolerance and diabetes. The first phase of insulin secretion, which occurs within 2–15 min after an intravenous glucose bolus, is markedly reduced. The second phase of insulin secretion is delayed and exaggerated (Fig 1) (Porte 1991, Lutz & Rand 1995). In diabetic cats and humans, progression of glucose intolerance results in the loss of second phase of insulin secretion (Fig 1). In humans, overt diabetes develops when approximately 80–90% of beta-cell function is lost (Porte 1991). Once persistent hyperglycaemia occurs, insulin secretion is further suppressed by the phenomenon of glucose toxicity (Yki-Järvinen 1992, Link & Rand 1996a).
Fig 1.
In cats with impaired glucose tolerance, the insulin secretion that occurs in the first 10 min (first phase secretion) after an increase in glucose is very reduced or absent compared to normal cats. Second phase insulin secretion is often exaggerated. Diabetic cats often have minimal insulin secretion in response to glucose. Stars indicate where cats with impaired glucose tolerance are significantly different from normal cats. (Adapted from Lutz & Rand 1995). Pathogenesis of feline diabetes. In: Diabetes Mellitus, Peterson M. & Greco, D, eds. Veterinary Clinics of North America 25, 527–552).
In an experimental model of feline diabetes, in which insulin resistance induced by growth hormone and dexamethasone is the primary abnormality, stages of beta-cell failure are similar to those reported in humans (Hoenig 1998). These are characterised by initial hypersecretion, and mild to moderate alterations in the biphasic pattern of insulin release as the severity of glucose intolerance increases (Hoenig 1998). Once overt diabetes occurs, there is markedly decreased insulin secretion and a change to a monophasic pattern of secretion, which eventually becomes erratic.
Insulin resistance
Insulin sensitivity is defined as the magnitude of the change in blood glucose in response to a given amount of insulin (Olefsky 1992). In insulin resistance, more insulin has to be secreted to produce the same glucose lowering effect, compared to when insulin sensitivity is normal (Porte 1991). Insulin sensitivity is not static, and insulin resistance is not necessarily pathologic. Changing insulin sensitivity is a potent mechanism for maintaining glucose homeostasis. This is evident in cats after intravenous injection of arginine, where a four-fold increase in insulin concentration occurs with only a small decrease in glucose concentration (Link & Rand 1996b). In this scenario, rapid and profound insulin resistance is necessary for blood glucose to be maintained. However, persistently decreased insulin sensitivity (insulin resistance) is abnormal, and is a classic feature of human type-2 diabetes (Porte 1991). A recent study found that diabetic cats are approximately six times less sensitive to insulin than normal cats. This is similar to humans with type-2 diabetes (Feldhahn et al 1998).
In humans, insulin resistance is largely inherited, but is increased by environmental or lifestyle factors and other acquired factors (Porte 1991). These same acquired factors are likely to be important in cats.
Insulin resistance and obesity
Obesity is a recognised risk factor for the development of diabetes in cats and humans, although not all cats or humans with type-2 diabetes are overweight (Panciera 1990, McCarthy & Hitman 1993). In humans, obesity causes insulin resistance, adding to the inherited level of insulin resistance (Porte 1991). Obese cats are also probably insulin resistant because insulin concentrations are increased relative to glucose concentrations, and glucose half-life is significantly longer compared to normal-weight cats (Nelson et al 1990).
In humans, the longer the duration of obesity, the greater the risk of diabetes (Everhart et al 1992). The pattern of fat deposition is also important (Yamashita et al 1996). Deposition of fat in the abdomen (central obesity) increases the risk of diabetes significantly, compared to deposition in the thighs (peripheral obesity) (Harris 1995). There are no studies covering the effect of fat distribution on diabetes prevalence in cats.
Insulin resistance and chronic hyperglycaemia
Chronically elevated blood glucose also causes insulin resistance (Yki-Järvinen 1992). Once overt diabetes mellitus with persistent hyperglycaemia occurs, the added insulin resistance further compounds the problem of inadequate insulin secretion. This has implications for therapy, because once glucose concentrations are decreased with treatment, insulin sensitivity may improve.
Insulin resistance and the thrifty-gene theory
Insulin resistance is largely genetically determined in humans and is widespread in some indigenous populations with very high incidences of diabetes. This suggests there is an evolutionary advantage for insulin resistance (Neel 1962, Brand Miller & Colagiuri 1994). Some interesting theories have been advanced, including the thrifty-gene theory and the carnivore-connection theory (Neel 1962, Brand Miller & Colagiuri 1994). Both theories propose that resistance to the glucose lowering effects of insulin developed at a time when our ancestors were eating a high-protein, low-carbohydrate diet. In the thrifty-gene theory, selective resistance to the glucoregulatory but not the fat forming (lipogenic) effects of insulin, facilitated efficient conversion of excess energy to fat when food was plentiful (Neel 1962). This ready deposition of fat conferred survival advantages during famine. In this theory, diabetes develops when there is a constant supply of excess calories, together with physical inactivity (Neel 1962). This scenario results in obesity and added insulin resistance, leading to hyperinsulinemia, and eventually beta-cell failure and diabetes (Zimmet & O'Dea 1993).
Insulin resistance and the carnivore connection theory
In the carnivore connection theory, resistance to the glucose lowering effects of insulin evolved during the ice ages to maintain normoglycaemia on a high-protein, low-carbohydrate diet (Brand Miller & Colagiuri 1994). Even on a low-carbohydrate diet, insulin secretion is essential for its anabolic effects. Maintenance of normoglycaemia is essential for brain function and reproduction, and is achieved by resistance to the glucose lowering effects of insulin. In this theory, diabetes develops because of life-long ingestion of large amounts of highly-digestible carbohydrate. In the presence of inherited insulin resistance, a high carbohydrate diet requires high levels of insulin secretion, eventually resulting in beta-cell exhaustion.
The life-style of domestic cats, when compared to feral cats, has undergone changes similar to those postulated to contribute to diabetes in humans. These include a change to plentiful food, physical inactivity, and substantial amounts of highly digestible carbohydrate.
Genetics of type-2 diabetes
In humans with type-2 diabetes and in some cats, there is strong evidence for a genetic basis for the disease (McCarthy & Hitman 1993, Wade et al 1999). Population studies have demonstrated a higher incidence of diabetes within some families of cats and ethnic groups of people (Harris 1995, Wade et al 1999). In Australia, Burmese cats are over-represented amongst diabetic cats, and constitute approximately 20% of diabetics (Rand et al 1997). Pedigree analysis of New Zealand Burmese-cats has demonstrated a genetic basis for diabetes, with more than 10% of individuals affected in some families (Wade et al 1999). In humans, the specific genes predisposing to diabetes have not yet been identified, but there is general agreement that type-2 diabetes is a polygenic disease with significant genetic and environmental interaction (McCarthy & Hitman 1993). In New Zealand Burmese, although a genetic basis was shown, and dominant inheritance unlikely, it could not be determined whether the inheritance was autosomal recessive, multigenic, or polygenic (Wade et al 1999).
In humans, it is considered likely that the multiple genes involved in predisposition to type-2 diabetes are both primary genes controlling insulin secretion and action, as well as secondary genes, influencing factors such as propensity to obesity (McCarthy & Hitman 1993). In obesity studies in cats, there are clearly some cats which are predisposed to obesity, and others that are relatively resistant to obesity. This observation suggests that secondary genetic factors also play a role in feline diabetes.
Although there is strong evidence for a genetic basis for diabetes, other factors including obesity, age, and physical activity have a important modifying effect on the genotype, as shown in studies of twins (Harris 1995). In identical twins, diabetes only develops in 20–40% of the co-twins, if the other twin has diabetes. In non-identical twins, less than 10% of the co-twins develop diabetes (Newman et al 1987, Harris 1995).
Environmental factors and type-2 diabetes
Obesity and physical inactivity
Environmental influences interact with genetic influences, and play an important role in the development of diabetes in humans, and most likely in cats (McCarthy & Hitman 1993). Environmental or lifestyle factors shown to be important in humans and probably in cats, include obesity, physical inactivity, dietary factors, and urban rather than rural residence (McCarthy & Hitman 1993). The lifestyle of many domestic cats has changed similarly to that of humans, with inactivity and obesity increasing in urban cats. Exclusively indoor cats are usually less active than outdoor domestic cats that hunt and defend territory, and significantly less active than feral cats, which have to hunt to obtain all their nutrition. In humans and rats, exercise has been shown to increase insulin sensitivity (Arslanian et al 1990). Lack of exercise impairs insulin action, and adds to the underlying level of genetically-determined insulin resistance. This effect of physical inactivity on insulin sensitivity occurs as both a direct effect at the post-receptor level and an indirect effect by contributing to obesity (Houmard et al 1991, Regensteiner et al 1991, Harris 1995).
Diet and type-2 diabetes
In both cats and humans, obesity has been shown to be a major risk factor for diabetes (Panciera 1990, Porte 1991). Overfeeding of highly palatable, calorie-dense food in cats with reduced physical activity, likely contributes to obesity, and hence diabetes. In humans, a number of dietary factors have been identified as contributing to diabetes. Excessive consumption of refined carbohydrate, lack of dietary fibre and high levels of dietary fat have all been implicated (Yudkin 1964, Trowell 1975, Marshall et al 1991). In humans, the influence of fat and fibre is compounded by other environmental factors, such as obesity and physical inactivity, and genetic factors (Knowler et al 1981).
In human populations with a high incidence of diabetes, both the source of carbohydrate and the way it is processed has changed relatively recently (Brand Miller & Colagiuri 1994). Introduced diets contain carbohydrate with a higher glycaemic index, that is, it produces a greater postprandial increase in glucose and insulin than traditional diets. Potatoes and some cereals common in Western diets have much higher glycaemic indices than some of the traditional sources of carbohydrate, such as native yams (Brand Miller & Colagiuri 1994). Current milling techniques for cereal result in a three-fold postprandial increase in blood glucose and subsequent insulin response, compared to the response after eating coarsely ground flour or whole grain (Heaton et al 1988, Brand Miller & Colagiuri 1994). Similarly, many commercial cat foods have higher levels of carbohydrate, especially highly digestible carbohydrate, than cats evolved to eat. The source of carbohydrate has changed, and it is processed to increase the digestibility. This increased life-long demand for increased insulin secretion, in response to increased amounts of highly digestible carbohydrate, is postulated to result in beta-cell exhaustion and type-2 diabetes (Brand Miller & Colagiuri 1994). The late age of onset of diabetes in cats and humans supports an exhaustion mechanism being involved in the disease process. Whether cats eating diets with substantial amounts of highly digestible carbohydrate have a higher requirement for insulin secretion than cats eating a carnivorous diet, is yet to be determined.
Other factors influencing insulin secretory capacity in type-2 diabetes
Amyloid
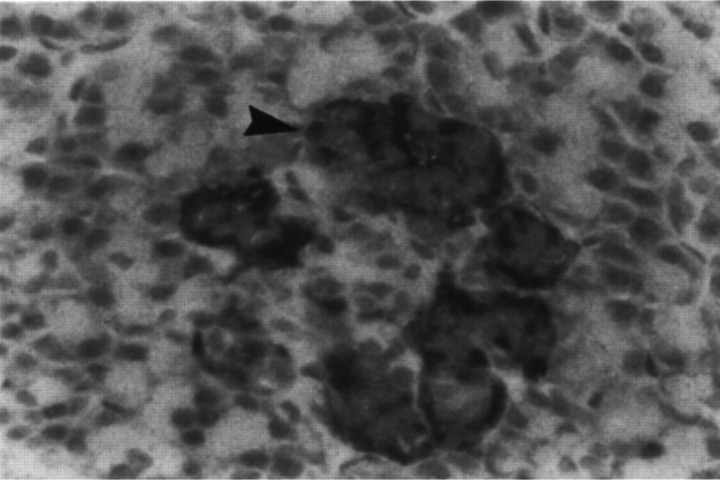
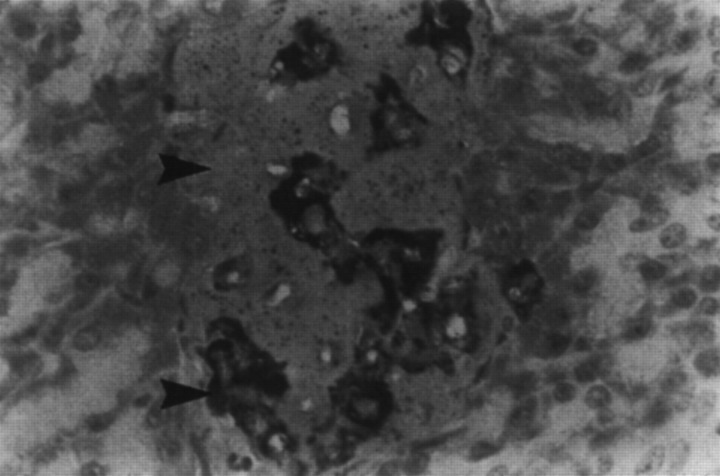
The most consistent histological finding in type-2 diabetes is amyloid deposition in the pancreatic islets (Johnson et al 1989, Clark et al 1995). Islet amyloid is derived from the hormone amylin or islet amyloid polypeptide, which is secreted with insulin from beta cells (Johnson et al 1986, 1992). Figure 2 shows a normal cat islet with an immunohistochemical label for amylin, and shows intracellular amylin and very small amounts of extracellular amyloid. Figure 3 is from a cat with diabetes, and demonstrates massive amounts of amyloid replacing islet-cells. Only a few species, including cats, humans and dogs, have an amino acid sequence in the critical portion of the amylin molecule which predisposes it to folding into beta-pleated sheets, which deposit as amyloid (Johnson et al 1992).
Fig 2.
Normal cat islet with an immunohistochemical label for amylin. Intracellular amylin (arrow, bright granular material) is present in beta cells located in the periphery of the islet. Very small amounts of amyloid (homogenous pink) are present around cells. In normal cats, amyloid does not comprise more than 20% of the islet volume. (Courtesy of Dr T Lutz, University of Zürich, Switzerland).
Fig 3.
Islet from diabetic cat, showing massive amounts of islet amyloid (arrow, homogenous pink) replacing islet cells. Reduced numbers of amylin containing beta cells are present (arrow, bright granular material). (Courtesy of Dr T Lutz, University of Zürich, Switzerland).
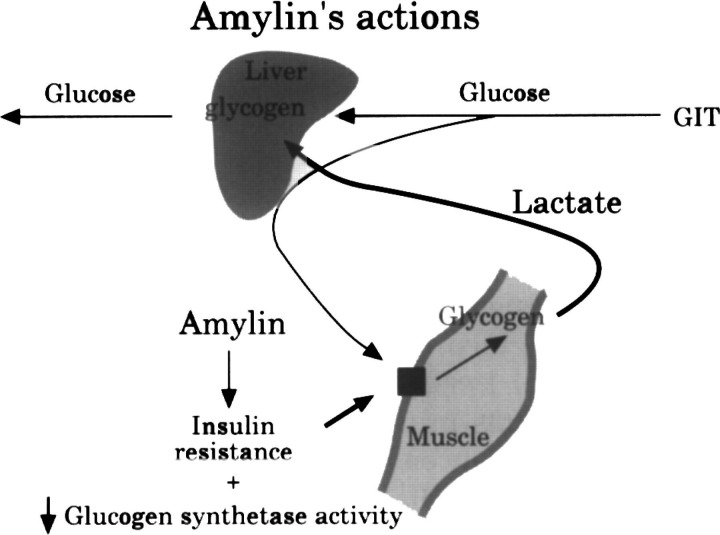
In addition to the critical amyloidogenic amino acid sequence, other factors including a high local concentration, appear to be important in the deposition of amyloid (Jordan et al 1990). Although cats and dogs both have a similar amyloidogenic sequence, in dogs, amyloid deposition only occurs associated with insulinomas, which, based on human data, probably also overproduce amylin (Jordan et al 1990). There is some evidence that cats have higher levels of circulating amylin than dogs, and therefore have higher local concentrations of amylin in the islets (Lutz & Rand 1996). The reason for higher production of amylin in cats is unknown, although it may be associated with its metabolic role. Amylin is thought to be important in maintaining blood glucose by stimulating the breakdown of muscle glycogen (Gaeta & Rink 1994). This occurs via antagonism of insulin-stimulated glucose uptake into muscle, and direct inhibition of glycogen synthetase (Fig 4) (Gaeta & Rink 1994). Breakdown of muscle glycogen releases lactate, which is used for production of glucose in the liver (gluconeogenesis) (Gaeta & Rink 1994). Cats are obligate carnivores so gluconeogenic pathways are functioning continuously to maintain blood glucose (Brand Miller & Colagiuri 1994). In other species, which obtain significant glucose from carbohydrate ingestion, gluconeogenesis only occurs between meals to maintain blood glucose (Brand Miller & Colagiuri 1994). Because there is a constant requirement for gluconeogenic substrates in cats eating a carnivorous diet, it is logical that amylin secretion is greater in cats than in species which eat more carhohydrate. In an experimental model of diabetes in cats, where insulin resistance is the primary abnormality, excessive amyloid deposition is also a feature of the diabetes, and occurs in cats with high amylin concentrations but not in cats with low concentrations (Hoenig 1998).
Fig 4.
Amylin is thought to be important in stimulating the breakdown of muscle glycogen This occurs via antagonism of insulin-stimulated glucose uptake into muscle, and direct inhibition of glycogen synthetase. Breakdown of glycogen releases lactate, which is used by the liver for glucose production, maintaining blood glucose between meals. (Adapted from Gaeta & Rink 1994. Amylin: a new hormone as a therapeutic target in diabetes mellitus and other metabolic diseases. Med Chem Res 3, 483).
Amyloid fibrils have been shown to be toxic to beta cells, both as they form intracellularly, and extracellularly (Lorenzo et al 1994, O'Brien et al 1985). In an in vitro study, amyloid fibrils resulted in death of the majority of islet cells within 96 h (Lorenzo et al 1994, O'Brien et al 1985). Accumulation of amyloid extracellularly replaces beta cells, reducing insulin secretory capacity (Lutz et al 1994). Cats have greater levels of islet amyloid deposition than humans, one study showed that even normal cats have up to 20% of islet volume composed of amyloid (Lutz et al 1994). Many, but not all cats and humans with diabetes, have amyloid deposition replacing islets cells (Lutz et al 1994, Clark et al 1995, Goossens et al 1998). Amyloid deposition does not appear to be an essential component of type-2 diabetes in cats or humans, but contributes to beta-cell loss and failure of insulin.
Glucose and lipid toxicity
Once persistent hyperglycaemia occurs, insulin secretion is reduced through a phenomenon termed glucose toxicity (Yki-Järvinen 1992). The mechanism for suppression of insulin secretion is unclear. There is evidence that initially, suppression of insulin secretion is functional and reversible, and is not associated with visible lesions in beta cells (Yki-Järvinen 1992, Link & Rand 1996a). With hyperglycaemia of longer than 2 weeks duration, histologic abnormalities are evident, including glycogen deposition and cell death (Dohan and Luken 1948, Imamura et al 1988, Link & Rand 1996a). The potent effect of chronic hyperglycaemia is demonstrated by the induction of permanent diabetes in dogs and cats made persistently hyperglycaemic through injection of glucose (Dohan and Luken 1948, Imamura et al 1988).
In normal cats infused with glucose to maintain glucose concentrations similar to those found in untreated diabetic cats, insulin secretion was markedly suppressed (Link & Rand 1996a). Within 3–7 days, insulin concentrations were similar to those found in insulin-dependent diabetic cats. Within 4 weeks, the infused cats developed ketotic diabetes, which was reversible with insulin therapy. The severity of the glucose toxic effect was dependent on the degree of hyperglycaemia and the duration (Link & Rand 1996a). Many mechanisms have been postulated to cause this suppression of beta-cell function, including reduced glucose transporters, a mitochondrial defect, decreased generation of ATP, and decreased cytoplasmic calcium, but the primary mechanism is unknown (Yki-Järvinen 1992).
Loss of beta cells prior to the onset of hyperglycaemia appears to predispose the remaining beta cells to permanent damage from the effects of glucose toxicity, and increases the likelihood of permanent diabetes occurring (Dohan and Luken 1948, Imamura et al 1988). It has been postulated that this increased susceptibility to glucose toxicity is associated with the hyperfunctioning state of the remaining beta cells (Kaiser et al 1991, Sako and Gill 1990).
Recent evidence suggests that increased fatty acids produce a similar effect to glucose toxicity, called lipotoxicity, resulting in suppression of insulin secretion (Unger 1995, Schermerhorn 1998). Glucose toxicity and lipotoxicity cause increased concentrations of long chain acyl-CoA's molecules in beta cells so they may act through a common pathway to inhibit beta-cell function (Prentki and Corkey 1996; Schermerhorn 1998). It is difficult to establish the primary mechanism for glucose and lipid toxicity, because chronic hyperglycaemia results in hyperlipidaemia and increased fatty acids (Schermerhorn 1998). It is possible that multiple and different mechanisms operate in glucose and lipid toxicity, with the mechanisms causing the initial reversible suppression of insulin secretion being different from those causing death of beta cells.
The clinical implications of glucose and lipid toxicity are very important. It is vital that effective therapy be instituted as soon as possible to reduce hyperglycaemia in diabetic cats, if beta-cell function is to be preserved. This is important because data in humans indicates that patients with residual beta-cell function have better glycaemic control when treated with insulin, than patients with no significant endogenous insulin secretion (Service and Nelson 1980). Secondly, it is important because a substantial number of cats will undergo remission of their diabetes, if the effects of glucose toxicity are minimised (Rand 1997).
Diabetic remission or transient diabetes
Diabetic remission occurs in 20–40% of cats if treated adequately (Nelson et al 1992, Martin & Rand, unpublished data). Remission occurs most commonly after 1–3 months of insulin therapy, and in some cats, glucose tolerance is normal in remission (Kirk 1993, Martin & Rand, unpublished data). Based on experience, remission is more likely if glycaemic control is adequate, so beta cells can recover from glucose toxicity. There is anecdotal evidence that if oral hypoglycaemicdrugs are used as the primary mode of therapy, and hyperglycaemia is poorly controlled, the rate of diabetic remission is low. Prolonged use of oral hyperglycaemic-agents in cats which have poor glycaemic control potentially contributes to greater loss of beta cells. Similarly, cats inadequately treated with insulin to control hyperglycaemia are unlikely to undergo remission. This is seen most frequently when lente insulin is used once daily instead of twice. Data obtained from our laboratory suggests that if a nadir blood glucose of 5–9 mmol/l is obtained after insulin administration, a substantial number of cats will undergo diabetic remission. Remission lasts from weeks to years, and appears more likely if steroids or progesterone drugs were used prior to the onset of diabetes (Martin & Rand, unpublished data). If diabetes reoccurs, early and diligent treatment in many cats will result in another remission occurring. North Americans of African decent have diabetic remissions with very similar characteristics to those of diabetic cats (Banerji and Lebovitz 1990). These occur in patients that present with severe, symptomatic hyperglycaemia, and remission is not dependent on weight loss or resolution of stress, but appears to be dependent on intensive treatment to control hyperglycaemia. Remission may last months or years, and glucose tolerance may be normal in remission. Reoccurrence of diabetes occurs independently of weight gain and acute stressful events. In contrast, in most humans with type-2 diabetes, remission is uncommon if initial blood glucose is very high at presentation (Banerji and Lebovitz 1990).
Stress hyperglycaemia
Stress hyperglycaemia is more pronounced in cats than in dogs or humans. The mechanism is poorly understood, and appears to result from both psychological stress and illness (Leidinger et al 1989, Opitz 1990, Kinnaird et al 1998). Stress hyperglycaemia can complicate diagnosis of diabetes, but is more of a problem in monitoring glycaemic control in diabetic patients for adjustment of insulin dose. A recent study of healthy cats stressed by a spray bath, found that stress hyperglycaemia occurred by the end of the 5 min bath, and persisted for 1–2 h (Kinnaird et al 1998). Blood glucose did not exceed 16 mmol/l (288 mg/dl), and was correlated with struggling and increased plasma lactate concentrations. Hyperglycaemia in response to an acute ‘flight or fight’ stressor probably results from increased hepatic glucose production from muscle lactate, rather than from decreased peripheral utilisation (insulin resistance) (Feldhahn et al 1998, Kinnaird et al 1998). In the bath study, stress hyperglycaemia was not associated with more passive indicators of stress, such as vocalisation. Clinicians should be aware that stress hyperglycaemia is likely to affect blood glucose estimations in cats if struggling occurs prior to the sample being taken. Blood glucose may be increased by as much as 10 mmol/l above baseline, resulting in values up to 16 mmol/l in normal cats. In sick cats, hyperglycaemia as high as 34 mmol/l has been reported (Leidinger et al 1989).
Summary
Understanding the features of feline diabetes is important for good patient management, and may help to decrease the incidence of diabetes. Obesity is a recognised risk factor for diabetes, and is more likely if highly palatable, caloriedense foods are fed to physically inactive cats. Many cats have type-2 diabetes, although the majority are insulin-dependent with currently available therapies. Loss of insulin secretion results from the primary disease process in type-2 diabetes, and is compounded by amyloid deposition replacing islet cells, pancreatitis, and glucose toxicity. Glucose toxicity is a potent suppressor of insulin secretion and if prolonged, leads to loss of beta cells and insulin-dependent diabetes. Many diabetic cats will go into diabetic remission within 1–3 months, if good glycaemic control is instituted. Other specific types of diabetes account for a substantial minority of diabetes in cats, of which pancreatic adenocarcinoma is the most common. Part 2 of this article will discuss current recommendations for management of feline diabetes.
References
- Arslanian S, Nixon PA, Becker D, Drash AL. (1990) Impact of physical fitness and glycaemic control on in vivo insulin action in adolescents with IDDM. Diabetes Care 13, 9–15. [DOI] [PubMed] [Google Scholar]
- Atkinson MA, Maclaren NK. (1994) The pathogenesis of insulin dependent diabetes. New England Journal of Medicine 331, 1428–1436. [DOI] [PubMed] [Google Scholar]
- Banerji M, Lebovitz H. (1990) Remission in non-insulin-dependent diabetes mellitus: Clinical characteristics of remission and relapse in black patients. Medicine 69, 176–185. [DOI] [PubMed] [Google Scholar]
- Bergman RN, Finegood DT, Ader M. (1985) Assessment of insulin sensitivity in vivo. Endocrine Reviews 6, 45–86. [DOI] [PubMed] [Google Scholar]
- Boitard C, Avner P. (1991) The immunology of diabetes mellitus. Nature 351, 519.2046761 [Google Scholar]
- Brand Miller JC, Colagiuri S. (1994) The carnivore connection: Dietary carbohydrate in the evolution of NIDDM. Diabetologia 37, 1280–1286. [DOI] [PubMed] [Google Scholar]
- Cavallo MG, Fava D, Monetini L, et al. (1996) Cell-mediated immune response to beta case in in recent-onset insulin-dependent diabetes: Implications for disease pathogenesis. Lancet 348, 926–928. [DOI] [PubMed] [Google Scholar]
- Clark A, De-Koning EJ, Hattersley AT, et al. (1995) Pancreatic pathology in non-insulin dependent diabetes (NIDDM). Diabetes Research and Clinical Practice S39, 47. [DOI] [PubMed] [Google Scholar]
- Dohan FC, Lukens FDW. (1948) Experimental diabetes produced by the administration of glucose. Endocrinology 42, 244–262. [DOI] [PubMed] [Google Scholar]
- Dorf A, Ballintine EJ, Bennett PH, Miller M. (1976) Relationships to glucose level, duration of diabetes, age at diagnosis of diabetes, and age at examination in a population with a high prevalence of diabetes mellitus. Diabetes 25, 554–560. [DOI] [PubMed] [Google Scholar]
- Everhart JE, Pettitt DJ, Bennett PH, Knowler WC. (1992) Duration of obesity increases the incidence of NIDDM. Diabetes 41, 235–240. [DOI] [PubMed] [Google Scholar]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (1997) Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 20, 1183–1197. [DOI] [PubMed] [Google Scholar]
- Feldhahn JR, Rand JS, Martin GM. (1998) Insulin sensitivity in normal and diabetic cats, and normal cats under stress. Journal of Veterinary Internal Medicine 12, 238. [Google Scholar]
- Feldman EC, Nelson RW. (1995) Canine Diabetes Mellitus. In: Canine and Feline Endocrinology and Reproduction, 2nd ed. Feldman EC, Nelson RW, eds. Philadelphia: Saunders, pp. 256, 340. [Google Scholar]
- Gaeta LSL, Rink TJ. (1994) Amylin: A new hormone as a therapeutic target in diabetes mellitus and other metabolic diseases. Medical and Chemical Research 3, 483. [Google Scholar]
- Goossens J, Nelson R, Feldman EC, Griffey SM. (1998) Response to insulin treatment and survival in 104 cats with diabetes mellitus (1985–1995). Journal of Veterinary Internal Medicine 12, 1–6. [DOI] [PubMed] [Google Scholar]
- Greenberger NJ. (1973) Pancreatitis and hyperlipemia. New England Journal of Medicine 289, 586–587. [DOI] [PubMed] [Google Scholar]
- Harris MI. (1995) Epidemiologic studies on the pathogenesis of non-insulin-dependent diabetes mellitus (NIDDM). Clinical and Investigative Medicine 18, 231–239. [PubMed] [Google Scholar]
- Heaton KW, Marcus SN, Emmett PM, Bolton CH. (1988) Particle size of wheat, maize, and oat test meals: Effects on plasma glucose and insulin responses and on the rate of starch digestion in vitro. American Journal of Clinical Nutrition 47, 675–682. [DOI] [PubMed] [Google Scholar]
- Helfand R, Gary H, Freeman C, Anderson LJ. (1995) Serologic evidence of an association between enteroviruses and the onset of type 1 diabetes mellitus. Journal of Infectious Diseases 172, 1206–1211. [DOI] [PubMed] [Google Scholar]
- Hoenig M. (1995) Pathophysiology of canine diabetes. In: Diabetes Mellitus. Peterson M, Creco D, eds. Veterinary Clinics of North America 25, 253–256. [DOI] [PubMed] [Google Scholar]
- Hoenig M. (1998) Stages of beta cell failure: The cat as a model for diabetes mellitus. Proceedings. American College of Veterinary Internal Medicine Forum 16, 575–576. [Google Scholar]
- Hoenig M. (1999) Beta cell and inulin antibodies in untreated diabetic cats. Proceedings. American College of Veterinary Internal Medicine Forum, in press.
- Houmard JA, Egan PC, Neufer PD, et al. (1991) Elevated skeletal muscle glucose transporter levels in exercise-trained middle-aged men. American Journal of Physiology 261, 437–443. [DOI] [PubMed] [Google Scholar]
- Imamura T, Koffler M, Helderman JF, et al. (1988) Severe diabetes induces in subtotally depancreatized dogs by sustained hyperglycemia. Diabetes 37, 600–609. [DOI] [PubMed] [Google Scholar]
- Johansen K, Aaby Svendsen P, Lorup B. (1984) Variations in renal threshold for glucose in type I (insulin-dependent) diabetes mellitus. Diabetologia 26, 180–182. [DOI] [PubMed] [Google Scholar]
- Johnson KH, Hayden DW, O'Brien TD, Westermark P. (1986) Spontaneous diabetes mellitus—islet amyloid complex in adult cats. American Journal of Pathology 125, 416. [PMC free article] [PubMed] [Google Scholar]
- Johnson KH, O'Brien TD, Betsholtz C, Westermark P. (1989) Islet amyloid, islet-amyloid polypeptide and diabetes mellitus. New England Journal of Medicine 321, 513. [DOI] [PubMed] [Google Scholar]
- Johnson KH, O'Brien TD, Betsholtz C, Westermark P. (1992) Biology of disease: Islet amyloid polypeptide: Mechanisms of amyloidogenesis in the pancreatic islets and potential roles in diabetes mellitus. Laboratory Investigation 66, 522. [PubMed] [Google Scholar]
- Jordan K, Murtaugh MP, O'Brien TD, et al. (1990) Canine IAPP cDNA sequence provides important clues regarding diabetogenesis and amyloidogenesis in type 2 diabetes. Biochemical and Biophysical Research Communications 169, 502. [DOI] [PubMed] [Google Scholar]
- Kaiser N, Corcos AP, Sarel I, Cerasi E. (1991) Monolayer culture of adult rat pancreatic islets on exracellular matrix: Modulation of β-cell function by chronic exposure to high glucose. Endocrinology 129, 2067–2076. [DOI] [PubMed] [Google Scholar]
- Kinnaird ER, Rand JS, Baglioni A, Blackshaw J. (1998) Stress hyperglycemia in cats (abstr). In: Journal of Veterinary Internal Medicine 12, 213. [DOI] [PubMed] [Google Scholar]
- Kirk CA, Feldman EC, Nelson RW. (1993) Diagnosis of naturally acquired type-I and type-II diabetes mellitus in cats. American Journal of Veterinary Research 54, 463. [PubMed] [Google Scholar]
- Knowler WC, Pettitt DJ, Savage PJ, Bennett PH. (1981) Diabetes incidence in Pima Indians: Contributions of obesity and parental diabetes. American Journal of Epidemiology 113, 144–156. [DOI] [PubMed] [Google Scholar]
- Kruth SA, Cowgill LD. (1982) Renal glucose transport in the cat (abstr). In: Proceedings. American College of Veterinary Internal Medicine Forum 1, 78. [Google Scholar]
- Lebovitz HF. (1994) Oral antidiabetic agents. In: Joslin's diabetes mellitus I. Kahn CR, Weir GC, eds. Philadelphia: Lea & Febiger, 508–523. [Google Scholar]
- Leidinger K, Nolte I, Eigenbrodt E. (1989) Klinische und labordiagnostische Untersuchungen sum Phanomen der Hyperglykamie der Katze. Kleintierpraxix 34, 457. [Google Scholar]
- Link KRJ, Rand JS. (1996a) Glucose toxicity in cats. Journal of Veterinary Internal Medicine 10, 185. [Google Scholar]
- Link KRJ, Rand JS. (1996b) Arginine and phentolamine response tests in cats. Journal of Veterinary Internal Medicine 10, 185. [Google Scholar]
- Link KRJ, Rand JS. (1998) Reference values for glucose tolerance and glucose status in cats. JAVMA 213 (4), 492–496. [PubMed] [Google Scholar]
- Lorenzo A, Razzaboni B, Weir GC, Yankner BA. (1994) Pancreatic islet cell toxicity of amylin associated with Type 2 diabetes mellitus. Nature 368, 756. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Rand JS. (1995) Pathogenesis of feline diabetes. In: Diabetes Mellitus. Peterson M, Greco D, eds. Veterinary Clinics of North America 25, 527–552. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Rand JS. (1996) Plasma amylin and insulin concentrations in normo- and hyperglycemic cats. Canadian Veterinary Journal 37, 27–34. [PMC free article] [PubMed] [Google Scholar]
- Lutz TA, Ainscow J, Rand JS. (1994) Frequency of pancreatic amyloid deposition in cats from south-eastern Queensland. Australian Veterinary Journal 71, 254–255. [DOI] [PubMed] [Google Scholar]
- Marshall JA, Hamman RF, Baxter J. (1991) High-fat, low-carbohydrate diet and the etiology of non-insulin-dependent diabetes mellitus: The San Luis Valley Diabetes Study. American Journal of Epidemiology 134, 590–603. [DOI] [PubMed] [Google Scholar]
- McCarthy M, Hitman GA. (1993) The genetic aspects of non-insulin-dependent diabetes mellitus. In: Causes of Diabetes. Genetic and Environmental Factors 1993. Leslie RDG, ed. Chichester, Wiley Publishers, pp. 157–183. [Google Scholar]
- Nagasawa J, Muraoka I, Sato Y. (1995) Long-lasting effect of training on insulin responsiveness in the rat. International Journal Sports Medicine 16 (2), 91–93. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Uchida K, Ono K, Goto N. (1990) Pathological observation of six cases of feline diabetes mellitus. Japanese Journal of Veterinary Science 52, 819. [DOI] [PubMed] [Google Scholar]
- Neel JV. (1962) Diabetes mellitus: A ‘thrifty’ genotype rendered detrimental by ‘progress'? American Journal of Human Genetics 14, 353–362. [PMC free article] [PubMed] [Google Scholar]
- Nelson RW, Himsel CA, Feldman EC, Bottoms GD. (1990) Glucose tolerance and insulin response in normal-weight and obese cats. American Journal of Veterinary Research 51, 1357. [PubMed] [Google Scholar]
- Nelson RW, Feldman EC, Devries SE. (1992) Use of ultralente insulin in cats with diabetes mellitus. Journal of the American Veterinary Medicine Association 200, 1828. [PubMed] [Google Scholar]
- Newman B, Selby JV, King MC, et al. (1987) Concordance for type 2 (non-insulin dependent) diabetes mellitus in male twins. Diabetologia 30, 763–768. [DOI] [PubMed] [Google Scholar]
- O'Brien TD, Butler PC, Dreutter DK, et al. (1995) A model of intracellular amyloidogenesis. American Journal of Pathology 147 (3), 609–616. [PMC free article] [PubMed] [Google Scholar]
- O'Brien TD, Hayden DW, Johnson EH, Stevens JB. (1985) High dose intravenous glucose tolerance test and serum insulin and glucagon levels in diabetic and non-diabetic cats: Relationships to insular amyloidosis. Veterinary Pathology 22, 250. [DOI] [PubMed] [Google Scholar]
- O'Brien TD, Hayden DW, Johnson KH, Fletcher TF. (1986) Immunohistochemical morphometry of pancreatic endocrine cells in diabetic, normoglycaemic glucose-intolerant and normal cats. Journal of Comparative Pathology 96, 357. [DOI] [PubMed] [Google Scholar]
- Olefsky JM. (1992) Insulin resistance and the pathogenesis of non-insulin dependent diabetes mellitus: Cellular and molecular mechanisms. New Concepts in the Pathogenesis of NIDDM. Advances in Experimental Medicine and Biology 334, 129–150. [DOI] [PubMed] [Google Scholar]
- Opitz M. (1990) Zur¤Strehyperglykamie bei Katzen (Stress Hyperglycemia in Cats). Berliner und Munchener Tierarzt Wochen 103, 151–158. [PubMed] [Google Scholar]
- Panciera DL, Thomas CB, Eicker SW, Atkins CE. (1990) Epizootiologic patterns of diabetes mellitus in cats: 333 cases (1980–1986). Journal of the American Veterinary Medicine Association 197, 1504. [PubMed] [Google Scholar]
- Peterson ME, Taylor RS, Greco DS, et al. (1990) Acromegaly in 14 cats. Journal of Veterinary Internal Medicine 4, 192. [DOI] [PubMed] [Google Scholar]
- Pimenta W, Korytkowski M, Mitrakou A, et al. (1995) Pancreatic beta-cell dysfunction as the primary genetic lesion in NIDDM. Evidence from studies in normal glucose-tolerant individuals with a first-degree NIDDM relative. Journal of the American Medical Association 273, 1855–1861. [PubMed] [Google Scholar]
- Porte D. (1991) β-cells in type II diabetes mellitus. Diabetes 40, 166–180. [DOI] [PubMed] [Google Scholar]
- Prentki M, Corkey BE. (1996) Are the beta-cell signalling molecules malonyl-CoA and cytosolic long- chain acyl-CoA implicated in multiple tissue defects of obesity and NIDDM? Diabetes 45, 273–283. [DOI] [PubMed] [Google Scholar]
- Rand JS. (1997) Understanding feline diabetes. Australian Veterinary Practitioner 27, 17. [Google Scholar]
- Rand JS, Bobbermein LM, Hendrikz JK. (1997) Over-representation of Burmese in cats with diabetes mellitus in Queensland. Australian Veterinary Journal, in press. [DOI] [PubMed] [Google Scholar]
- Regensteiner JG, Mayer EJ, Shetterly SM, et al. (1991) Relationship between habitual physical activity and insulin levels among nondiabetic men and women. San Luis Valley Diabetes Study. Diabetes Care 11, 1066–1074. [DOI] [PubMed] [Google Scholar]
- Rushford NB, Miller M, Bennett PH. (1979) Fasting and two-hour post-load glucose levels for the diagnosis of diabetes. The relationship between glucose levels and complications of diabetes in the Pima Indians. Diabetologia 16, 373–379. [DOI] [PubMed] [Google Scholar]
- Sako Y, Grill VE. (1990) Coupling of beta-cell desensitization by hyperglycaemia to excessive stimulation and circulating insulin in glucose infused rats. Diabetes 39, 1580–1583. [DOI] [PubMed] [Google Scholar]
- Schermerhorn T. (1998) Beta cell dysfunction in diabetes mellitus: recent developments. Proceedings of the 16th American College of Veterinary Internal Medicine Forum, pp 498–500.
- Schiffrin A, Colle E, Ciampi A, et al. (1993) Different rates of conversion to IDDM in siblings of type 1 diabetic children: The Montreal family study. Diabetes Research and Clinical Practice 21, 75–84. [DOI] [PubMed] [Google Scholar]
- Service FJ, Nelson RL. (1980) Characteristics of glycemic stability. Diabetes Care 3, 58. [DOI] [PubMed] [Google Scholar]
- Sieradzki J. (1998) Combined treatment with insulin and oral agents in type 2 diabetes mellitus. Dialogue, diabetes literature review service 2nd quarter, 15–18.
- Sparkes AH, Adams DT, Cripps PJ, et al. (1996) Inter- and intraindividual variability of the response to intravenous glucose tolerance testing in cats. American Journal of Veterinary Research 57, 1294–1298. [PubMed] [Google Scholar]
- Thompson GR. (1988) Clinical consequences of hyperlipidaemia. Journal of Inherited Metabolic Disease 11 (S1), 18–28. [DOI] [PubMed] [Google Scholar]
- Trowell H. (1975) Dietary-fibre hypothesis of the aetiology of diabetes mellitus. Diabetes 24, 762–765. [DOI] [PubMed] [Google Scholar]
- Unger RH, Grundy S. (1995) Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: Implications for the management of diabetes. Diabetologia 28, 119–121. [DOI] [PubMed] [Google Scholar]
- Verge CF, Howard NJ, Irwig L, et al. (1994) Environmental factors in childhood IDDM. A population-based, case-control study. Diabetes Care 17, 1381–1389. [DOI] [PubMed] [Google Scholar]
- Wade, Rand J. (1999) Evidence of a genetic basis for diabetes mellitus in Burmese cats (Abstr). Proceedings. American College of Veterinary Internal Medicine Forum, in press.
- Walker M, Alberti KGMM. (1993) Syndrome X. In: Causes of Diabetes. Genetic and Environmental Factors 1993. Leslie RDG, ed. Chichester, Wiley Publishers, pp 305–318. [Google Scholar]
- Woods JP, Panciera DL, Snyder PS, et al. (1994) Diabetes mellitus in a kitten. Journal of the American Animal Hospital Association 30, 177. [Google Scholar]
- Yamashita S, Nakamura T, Shimomura I, et al. (1996) Insulin resistance and body fat distribution. Diabetes Care 19, 287–291. [DOI] [PubMed] [Google Scholar]
- Yki-Jarvinen H. (1992) Glucose toxicity. Endocrine Reviews 13, 415. [DOI] [PubMed] [Google Scholar]
- Yudkin J. (1964) Dietary fat and sugar in relation to ischaemic heart disease and diabetes. Lancet ii, 4–5. [DOI] [PubMed] [Google Scholar]
- Zimmet P, O'Dea K. (1993) Thrifty Genotypes. In: Causes of Diabetes. Genetic and Environmental Factors 1993. Leslie RDG, ed. Chichester, Wiley Publishers, pp 269–290. [Google Scholar]