Abstract
Inflammatory bowel disease (IBD), while a popular diagnosis, may not occur as commonly as it is diagnosed. It is a diagnosis of exclusion, meaning that it is important to eliminate diseases that mimick it. Dietary intolerance or allergy in particular, can have the same clinical and histologic appearance as IBD. Likewise, well-differentiated alimentary lymphosarcoma can also be confused with it. Intestinal biopsies are useful, but must be taken carefully and then evaluated by someone with interest and expertise in alimentary tract pathology. Therefore, it behoves the clinician to carefully consider the diagnosis instead of starting multiple drug therapy in a cavalier fashion. Well constructed dietary therapy can often be beneficial for both dietary problems and IBD.
Idiopathic inflammation of the feline alimentary tract has been recognised since the 1970s when cats with ulcerative colitis (Ewing 1972) and histiocytic colitis (van Kruiningen & Dobbins 1979) were described. However, it was in the mid-1980s that the terms ‘lymphocytic-plasmacytic’ and ‘inflammatory bowel’ started to be heard with increasing frequency at veterinary meetings and to be read in veterinary journals (Nelson et al 1984, Willard et al 1985, Tarns 1986a, 1986b, Edwards & Russell 1987, Ghermai 1989, van Kruiningen et al 1983). Inflammatory bowel disease (IBD) became a popular discussion topic as well as a favourite diagnosis. Since then, lectures on IBD have become expected in major veterinary meetings and textbooks. However, despite this popularity and tenure of approximately 15 years, there still exists controversy over the definition, diagnosis, and treatment of feline IBD.
In this paper, IBD will be understood to refer to idiopathic inflammation of the small or large intestines, as opposed to inflammatory problems for which an underlying cause can be found. Such idiopathic inflammatory disease is typically named by describing the predominate inflammatory cell type infiltrating the mucosa. This review will focus on lymphocytic and plasmacytic infiltrates (ie, lymphocytic–plasmacytic enteritis and colitis), because they are the most common types (Lecoindre & Chevallier 1997).
Clinical presentation
There is not an obvious breed predilection for feline IBD, and middle aged (5–8 years old) to older cats (>8 years old) seem to be more commonly affected (Hart et al 1994, Dennis et al 1992, 1993, Jergens et al 1992a, Lecoindre & Chevallier 1997). However, IBD occurs in cats as young as 6 months, and as many as 23% of the affected cats have been less than 1-year-old in some studies (Ghermai 1989).
Clinical signs in affected cats vary substantially. Severity of these signs does not correlate well with the severity of the infiltrate, and correlation of signs with the site of involvement is not clear-cut. In a study of 14 cats with small bowel involvement, vomiting and weight loss were reported commonly (10/14, each) while diarrhoea was less frequent (7/14) (Dennis et al 1992). Lethargy, decreased appetite, and increased appetite each occurred less than 50% of the time. Hematochezia was the most commonly mentioned clinical sign in 14 cats with large bowel involvement (13/14), while diarrhoea was less frequent (11/14) (Dennis et al 1993). Faecal mucus, increased frequency of defecation, malodorous faeces, tenesmus, and vomiting occurred still less often. In 60 cats diagnosed with enterocolitis, weight loss was the most common sign (38/60), followed by vomiting (36/60), anorexia (24/60), diarrhoea (21/60), ravenous appetite (10/60), lethargy (10/60) and haematochezia (7/60) (Hart et al 1994). Similarly, in a study of 26 cats in which those with small bowel disease were not distinguished from those with large bowel disease, vomiting and anorexia/weight loss were the most common signs (11/26, each), while diarrhoea was infrequent (3/26) (Jergens et al 1992a). A study from Germany stated that approximately 70% of 47 affected cats were lethargic while 60% were diarrhoeic and 35% had both vomiting and diarrhoea (Ghermai 1989). A study from France found that 70% of 51 cats with various sites of involvement had small bowel diarrhoea while 55% had weight loss and 43% had vomiting (Lecoindre & Chevallier 1997).
Based on these six series, vomiting and weight loss appear to be the most common signs of small intestinal IBD while haematochezia is the primary sign of large intestinal IBD. However, there is enough variation that one cannot be dogmatic in expecting specific signs to consistently predict specific site involvement. Furthermore, some cats have simultaneous involvement of small and large intestines (Lecoindre & Chevallier 1997), meaning that almost any clinical sign could be ‘expected’. Weight loss despite an adequate or increased caloric intake is particularly consistent with small intestinal disease (and diabetes mellitus and hyperthyroidism, for that matter), but some patients with small intestinal IBD may have a poor appetite. Diarrhoea seems less common in pure small intestinal disease, but appears to be relatively common in cats with large intestinal disease. The reason that diarrhoea is not a more consistent sign in cats with IBD probably relates to the cat's ability to conserve water from the kidneys and colon. Excess water in the faeces is what produces diarrhoea, and cats are remarkable in their ability to conserve water. However, regardless of the signs involved, chronicity (ie, >2–4 weeks) is typical (Ghermai 1989, Dennis et al 1992).
Physical examination may help eliminate diseases that mimic IBD, but it is seldom helpful in diagnosing IBD. The most that physical examination can usually contribute toward a diagnosis of IBD is finding thickened intestinal loops, but that is a subjective assessment that, even when present, can be due to other diseases (eg, lymphosarcoma). Loss of muscle mass and thickened loops of intestine have occurred as often as 50% of the time in some studies (Hart et al 1994). Likewise, clinical pathology abnormalities are common, but seldom of major diagnostic usefulness other than to help eliminate other diseases. Haemoconcentration and lymphopenia occurred in 50% of cases in one study (Hart et al 1994), but were not as frequent in other studies (Dennis et al 1992, 1993, Jergens et al 1992a). Non-specific leukocytosis was common in some reports (Hart et al 1994, Ghermai 1989) but uncommon in others (Jergens et al 1992a). Increased ALT was commonly reported (22 of 36 by Hart et al 1994, seven of 14 by Dennis et al 1992, eight of 14 by Dennis et al 1992), but increased SAP was inconsistently noted (11 of 30 by Hart et al 1994). The cause of the increased ALT is unknown, but may be conjectured to be mild hepatocyte damage secondary to absorption of bacterial toxins/metabolites from the diseased intestines. Hypoalbuminemia was uncommonly mentioned, but occasionally occurred (Dennis et al 1992, Hart et al 1994, Jergens et al 1992a). Hyperglobulinemia was reported in 14/23 cats in one paper (Hart et al 1994).
Diagnosis
The first diagnostic step in a cat with suspected IBD is to eliminate diseases that mimic IBD. Complete blood count, serum biochemistry panel, urinalysis, serum free T4 concentration, FeLV/FIV test, and faecal flotations/direct smears are typically performed. Hyperthyroidism, diabetes mellitus, and renal failure are examples of such diseases which can be diagnosed by such analysis. Intestinal parasitism such as giardiasis and cryptosporidiosis should be eliminated by faecal analysis (eg, flotations utilising zinc sulfate and sugar solution, respectively; or ELISA methodology for antigen detection). Once such diseases are eliminated, the clinician must decide whether to recommend more tests or a therapeutic trial. Intestinal mucosal biopsy is often the most useful diagnostic test after the initial blood, urine, and faecal analyses. IBD, alimentary lymphosarcoma, and dietary allergy/intolerance are usually the three major differential diagnoses to consider in patients with apparent small intestinal disease. In cats with chronic large bowel disease (eg, haematochezia or diarrhoea without weight loss), IBD and food allergy/intolerance usually assume prominent places on the list of differentials, although Clostridial colitis might also be reasonably common.
Additional faecal analyses are sometimes performed. Faecal smears looking for undigested fat, and rectal cytology are often elected, but in the author's experience they are seldom useful. Faecal cultures are reasonable if the history suggests contagion. However, without such a history, faecal cultures are notoriously low-yield procedures that seem to do little except increase the client's bill.
Abdominal imaging with plain and/or upper gastrointestinal contrast radiographs is seldom helpful when evaluating a cat prior to intestinal biopsy. Obstruction occurs more commonly with lymphoma, but may also be secondary to inflammatory strictures caused by IBD (Oyer et al 1995). Occasionally the small intestines of cats with IBD become dilated to the point of suggesting obstruction, even when there is no resistance to luminal flow. Properly performed barium contrast radiographs of the intestines may detect focal lesions that are best approached surgically rather than endoscopically. However, such radiographs require so much time, effort, and expense to do them correctly, that they are seldom performed; or, if they are performed, they are often done incorrectly and may then miss lesions.
In distinction to contrast radiographs, ultrasonography is relatively quick and easy. Furthermore, ultrasonography is generally more useful because it may reveal masses, marked lymphadenopathy, or localised intestinal thickening that were not detected during abdominal palpation or by radiographs. Marked intestinal thickening (ie, up to 20 mm, Grooters et al 1994) is suggestive of lymphosarcoma, but lesser thickening may occur in cats with either IBD or lymphosarcoma (Hart et al 1994, Weichselbaum et al 1994, Ghermai 1989, Penninck et al 1994, Grooters et al 1994). Hypoechoic, transmural thickening with loss of the normal layered appearance of the intestinal wall may be particularly suggestive of lymphoma. Finding enlarged mesenteric lymph nodes is often inadequate to distinguish IBD from lymphosarcoma. Some cats with alimentary lymphosarcoma have normal-sized mesenteric lymph nodes while others have enlarged lymph nodes. Unfortunately, some cats with IBD also have enlarged mesenteric lymph nodes. While ultrasound-guided fine needle aspirates can be performed on such lesions, one must understand that cytologic preparations may diagnose lymphosarcoma but can never definitively eliminate lymphosarcoma or diagnose IBD. It is important to note that mesenteric lymph nodes are typically more reactive than most peripheral lymph nodes, and this reactivity may mask lymphosarcomatous infiltrates. The major benefits of ultrasonography are that it helps the clinician know whether there are focal lesions out of ‘reach’ of an endoscope (eg, a focal thickening of the midjejunum) which would make surgery the preferred method to biopsy the intestines, and it allows direct aspiration of selected lesions, usually without the need for anesthesia. A major disadvantage of ultrasound is that the value of the examination is extremely operator dependent. Untrained operators can easily miss lesions, or misinterpret lesions that are found.
Endoscopy and mucosal biopsy are usually the preferred method of obtaining a definitive diagnosis of IBD. Obvious lesions [eg, gastric ulceration and/or erosion (Jergens et al 1992b)] rarely occur in cats with IBD, and even when they do exist, they are not pathognomonic. If the mucosa has a ‘cobblestone’ appearance, looks relatively flat (ie, does not have the typical texture) with ‘mudcracks’, is covered with exudate, or has spontaneous bleeding (be sure that the bleeding is occurring ahead of the endoscope and is not due to iatrogenic trauma from the tip of the instrument) then mucosal infiltration and/or villus atrophy are suspected. Newer videoscopes magnify the duodenal mucosa, helping the endoscopist visualise such changes; however, gross examination of the intestinal mucosa is not the basis of diagnosis. Histopathology of the duodenum and colon are typically required for definitive diagnosis. Inflammatory bowel disease is typically a generalised disease; hence, there is seldom a need to distinguish diseased from non-affected portions of the duodenum or colon. Even though it has been suggested that normal-appearing intestinal mucosa seldom yields tissue samples with histologic abnormalities (Roth et al 1990), others report that normal-appearing gut mucosa often yields mucosal samples that are substantially diseased (Dennis et al 1992). The general consensus among veterinary gastroenterologists is that intestinal biopsy is indicated almost every time endoscopy or abdominal surgery is performed (Jergens et al 1994).
Even though alimentary tract biopsy is the most definitive test for IBD, there are numerous potential mistakes associated with it. First, when performing endoscopic biopsies, multiple tissue samples should be obtained at each site, no matter how normal the intestinal mucosa appears. One should keep biopsying a particular site (eg, duodenum) until at least five to eight excellent tissue samples (ie, tissue samples that comprise the entire mucosa from the submucosa to the tips of the villus and are not replete with crush artifact) of each site are obtained (Hitt 1995). Second, as many different areas of the alimentary tract (eg, duodenum, ileum, colon, jejunum) should be biopsied as possible to prevent missing unsuspected inflammatory lesions. For example, one investigator has suggested that feline colonic inflammation has probably been under diagnosed, possibly for this reason (Lecoindre & Chevallier 1997). Ileal tissue samples obtained by passing the endoscopic forceps into the ileo-colic valve and blindly biopsying mucosa occasionally are diagnostic when duodenal and colonic samples are not helpful, and are therefore recommended. Gastric biopsies are routinely performed, probably because the stomach is so easy to examine and biopsy. Unfortunately, gastric biopsies are seldom as helpful as intestinal samples in diagnosing the cause of vomiting in cats (Lecoindre & Chevallier 1997). If biopsies are obtained via laparotomy, then the stomach, duodenum, jejunum, ileum, and mesenteric lymph node should be biopsied (although not addressed in this paper, hepatic and pancreatic biopsies are usually appropriate and should also be obtained if a laparotomy is performed). Full thickness colonic biopsies should be avoided; endoscopy is the preferred method of sampling the colon.
Unfortunately, it is particularly easy to obtain poor, non-diagnostic tissue samples of the small intestine (Wilcock 1992). This problem occurs not only with endoscopic biopsies, but also with full-thickness biopsies obtained at laparotomy. The most common problems seen with endoscopically-obtained tissue samples are that they are too superficial (ie, are primarily composed of villi) (Fig 1) or have too much crush artifact. However, the author has examined a number of full-thickness intestinal samples that have so little mucosa or so much crush artifact as to make them almost useless. This problem with full thickness biopsies is often due to the surgeon taking a very small sample in an effort to avoid causing a stricture or to ‘minimise’ the chance of dehiscence. There is rarely any risk of stricture unless one removes ≥20% of the circumference of the intestine, and the size of the sample should have little or no bearing on the likelihood of dehiscence.
Fig 1.
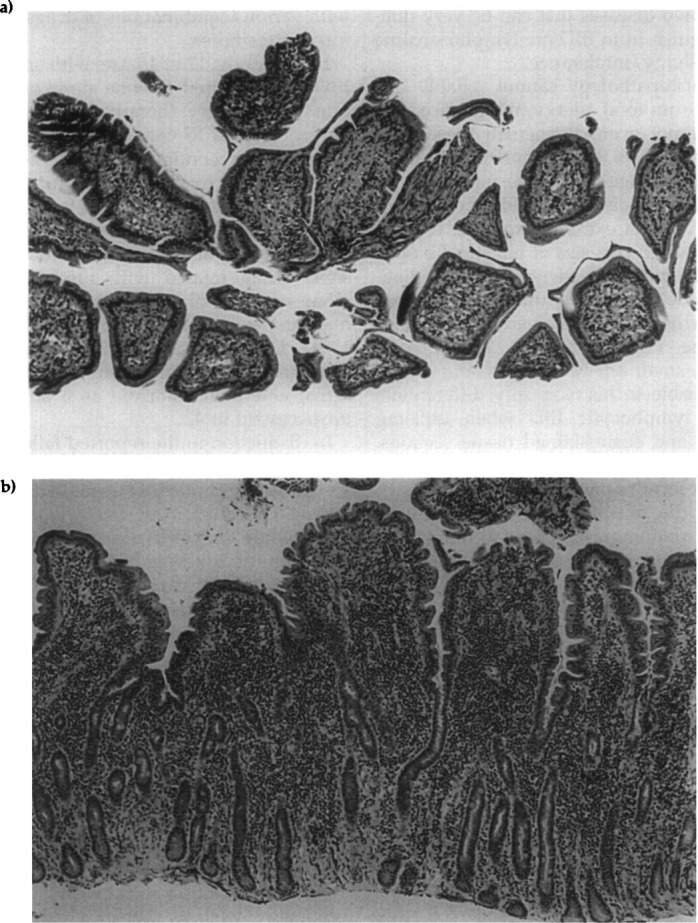
(a) A photomicrograph of an endoscopic biopsy of duodenal mucosa. This sample principally contains villi; therefore, it is inadequate for evaluating the duodenal mucosa. Compare this section of tissue to that in Fig 1b. (b) A photomicrograph of an endoscopic biopsy of duodenal mucosa. In contrast to Fig 1a, this tissue sample represents the full thickness of the intestinal mucosa, all aspects of the mucosa being available for examination. Notice also that the orientation of the tissue section allows the histopathologist to evaluate for evidence of villus blunting or collapse. The smooth lower border shows that this piece of tissue was ‘peeled’ off the submucosa. This particular section of tissue has a marked infiltrate of lymphocytes, and it is difficult to determine whether this represents marked lymphocytic inflammatory bowel disease or well-differentiated lymphosarcoma. Hematoxylin and eosin stain, photographed at an original magnification of × 100.
Even when good quality tissue samples are obtained, there are limitations to what can be determined histopathologically. While various types of inflammatory cells may infiltrate the intestinal mucosa (eg, eosinophils, neutrophils), the types most commonly associated with IBD are lymphocytes and plasma cells (hence the name, ‘lymphocytic-plasmacytic’ enteritis or colitis) (Wilcock 1992). These two cell types are always present in normal feline intestinal mucosa; therefore, the pathologist must decide whether there are excessive or normal numbers of these cells. Unfortunately, there are no generally agreed upon criteria for making this determination (Wilcock 1992). Consequently, there is an apparent lack of uniformity in how different pathologists may describe the same section of intestine.
In some early reports (Nelson et al 1984, Willard et al 1985), it was simply stated that there were excessive numbers of lymphocytes and/or plasma cells. One group of investigators has gone so far as to count the number of lymphocytes/standardised area of mucosa (Yamasaki et al 1996). While such an approach is less subjective than some others, it is so tedious that it is seldom employed, especially by commercial pathology laboratories. Starting in the early 1990s, more involved grading schemes began to be implemented (Dennis et al 1992, 1993). Initially, these criteria were modified from those published for people (Whitehead et al 1975) and dogs (Roth et al 1990). These methods usually evaluate epithelial and architectural changes as well as numbers of mucosal lymphocytes and plasma cells (Jergens et al 1992b, Weiss et al 1996, Dennis et al 1993, Hart et al 1994). However, there is still a lack of uniformity between the criteria used by different clinicians and pathologists. For example, epithelial cell necrosis is used as a criteria in one report (Weiss et al 1996) but not in another (Dennis et al 1992). Fibrosis is likewise inconsistently considered in these two papers.
Unfortunately, not only is the histologic interpretation of intestinal tissue subjective, but the pathologist evaluating the intestinal tissue typically does not work directly with the patient and seldom knows how it responded or failed to respond to various therapies. Therefore, pathologists (including those who see hundreds of such samples) are typically unaware of the ultimate consequences of their assessment of the biopsy sample. This dichotomy can make it difficult to ascertain whether the changes seen in a tissue section are clinically significant.
Diseases that mimic IBD
Even if the pathologist can confidently state that intestinal inflammation exists, IBD has not necessarily been diagnosed. This inflammation must be idiopathic before it can be attributed to IBD. Inflammatory bowel disease is a diagnosis of exclusion; all other causes of intestinal inflammation must be eliminated before IBD can be correctly diagnosed. Bacteria, food antigens, parasites, and neoplasia in particular must be excluded. Laboratory tests (eg, CBC, serum biochemistry profile, FeLV/FIV, etc) as well as therapeutic trials (eg, elimination diets, fibre supplemented diets, antibiotics for Clostridial colitis) must often be performed to accomplish such a diagnosis. Two diseases that can be very difficult to distinguish from IBD are lymphosarcoma and dietary allergy/intolerance.
Intestinal histopathology cannot reliably distinguish IBD from food allergy/intolerance, and sometimes cannot even distinguish these from lymphosarcoma. Cats that ultimately respond best to dietary change may have inflammatory infiltrates that are distinguishable from those found in IBD. As an extreme example, there is one report in which two sets of intestinal biopsy samples taken months apart were both diagnosed as demonstrating lymphosarcoma, but the cat's diarrhoea was resolved with dietary therapy alone (Wasmer et al 1995). Well-differentiated, small cell lymphosarcoma can be almost impossible to histologically differentiate from severe lymphocytic IBD when utilising haematoxylin and eosin-stained tissue sections. Immunohistochemical staining of lymphocytes in such tissue sections can allow the pathologist to determine the phenotype of the infiltrating lymphocytes: monomorphic populations of lymphocytes are suggestive of lymphosarcoma. Therefore, if the pathologist cannot distinguish IBD from alimentary lymphosarcoma, and immunohistochemical staining is not available, the only way to distinguish these two diseases may be to treat for IBD (or food allergy) and observe the cat's long-term (ie, at least 6–12 months) response to therapy.
Therapy
The word ‘glucocorticoid’ typically appears in the same sentence as ‘IBD’, so seemingly established are drugs like prednisolone in the treatment of feline IBD. One reason for this association includes the generally accepted fact that many cats diagnosed with IBD ‘respond’ to such therapy. Fortunately, cats have relatively few adverse complications from long-term glucocorticoid therapy, and these complications (eg, diabetes mellitus, iatrogenic hyperadrenocorticism, urinary tract infection) seemingly occur infrequently. More fortunately, infectious diseases that could be made worse by administration of glucocorticoids [eg, histoplasmosis, toxoplasmosis (Peterson et al 1991), salmonellosis] rarely cause chronic alimentary tract disease in cats. Unfortunately, it is hard to objectively compare results of therapy for IBD between different studies because most papers report varying numbers of cats having been treated with various combinations of drugs and/or diets for various times.
In one study, 12 of 13 cats with small intestinal IBD received prednisolone alone or in combination with dietary therapy (Dennis et al 1992). Seven of these 13 cats improved (none became normal), but continued steroid administration was necessary to sustain this improvement. In a study of 60 cats (in which it is not clear which had large and which had small bowel disease), 39 cats had total or partial resolution of clinical signs when treated with prednisolone, either alone or in combination with other drugs (ie, metronidazole, cimetidine, tylosin) (Hart et al 1994). Dietary trials in 52 of these cats were unsuccessful at adequately resolving clinical signs, and metronidazole as a sole agent was unsuccessful in 42.
In distinction to the reported failure of dietary therapy in cats with small intestinal IBD, six of six cats with colonic IBD treated with a high fibre diet had resolution or improvement of clinical signs, while four of five cats treated with both a high fibre or controlled diet plus various drugs also responded (two of these five were later controlled by diet alone) (Dennis et al 1993). This is similar to a report of six cats diagnosed with lymphocytic-plasmacytic colitis in which five cats had resolution of clinical signs after being fed a hypoallergenic diet (Nelson et al 1984). Only one of these cats required azulfidine for resolution of signs. In another study, two cats diagnosed with lymphoplasmacytic colitis and treated with dietary change alone had resolution of clinical signs (Hart et al 1994).
Cats with large bowel IBD without small intestinal involvement are typically not losing weight or condition rapidly and often respond reasonably well to dietary change. Therefore, a therapeutic trial with a high fibre and/or elimination diet is reasonable, even before biopsying the intestines. The biggest problem with such therapeutic trials is that many cats will not comply with the veterinarian's dietary recommendations, and already frustrated owners can become even more frustrated if their pet refuses to eat the more expensive or inconvenient diet recommended by the veterinarian. Yet such trials are often desirable. Patients with alimentary tract manifestations of dietary allergy/intolerance are more likely to respond to dietary change than to glucocorticoid therapy similar to what occurs with cutaneous manifestations of dietary allergy (Rosser 1993a). Unfortunately, it can be difficult to find a diet that will alleviate clinical signs in patients that are apparently intolerant or allergic to a large number of dietary antigens. Failure of one hypoallergenic or elimination diet does not mean all such diets will fail. Remember that it may take weeks or months for allergic dogs to show clinical response to a hypoallergenic diet (Rosser 1993b), and some cats might respond to an appropriate hypoallergenic diet similarly.
If there are signs of small bowel disease (eg, weight loss), either with or without large bowel involvement, then it is reasonable to biopsy the intestines before starting a therapeutic trial, especially if there is substantial loss of weight or condition. Once IBD has been diagnosed, pharmacologic management is appropriate because many cats with small intestinal IBD respond well to prednisolone therapy (Table 1). In fact, many clinicians administer glucocorticoids as a therapeutic trial in place of biopsy. While such pharmacologic therapy without histopathologic evidence of IBD is less desirable, there is no denying that many cats' signs are alleviated in this manner. However, this approach has the risk of delaying appropriate therapy in cats whose disease will progress during the trial.
Table 1.
Selected drugs that have been used or recommended for the treatment of feline IBD
| Drug | Dose |
|---|---|
|
| |
| Prednisolone | 2.2 mg/kg (can go as high as 4.4 mg/kg) once daily, or can split the dose into 1.1 mg/kg twice daily |
| Dexamethasone | 0.22 mg/kg up to once daily |
| Metronidazole | 10–15 mg/kg twice daily |
| Chlorambucil | 1 mg twice weekly in cats ≤7 lbs |
| 2 mg twice weekly in cats >7 lbs | |
| Azathioprine | 0.3 mg/kg every other day |
| Azulfidine | 10–20 mg/kg, 1–3 times daily for 7–10 days |
If prednisolone therapy appears to effect resolution of clinical signs (which usually happens within 1–2 weeks), then I typically continue the current therapy without change for another 2–4 weeks. There are no data showing the optimal time to use the initial dose of prednisolone before lowering it. However, if the steroid dose is reduced too quickly, relapse of clinical signs will be difficult to interpret. While the clinician's first assumption upon seeing such a relapse is typically that the cat needs a higher dose of prednisolone for a longer time, it might be that the initial, transient clinical improvement was due to something besides the steroids. Therefore, the initial dose of prednisolone should be used long enough so that the clinician is convinced that the clinical improvement was due to the steroid and was not a fortuitous event unrelated to the therapy. The steroid dose is then decreased every 2–3 weeks until the lowest effective dose is determined.
If a cat with IBD does not respond to prednisolone, one may change to another type of steroid or add other drugs. The author has found rare cats that responded to parenteral administration of dexamethasone but not oral administration of prednisolone. Nonetheless, I prefer orally administered prednisolone initially because of its short half life compared to dexamethasone and triamcinolone, and because prednisolone seems to cause fewer side effects. If prednisolone is being used and the cat's signs become worse, then it is easy to stop administering the steroid and quickly return the cat to the condition it was in prior to steroid therapy. If a cat responds to oral prednisolone therapy but resists swallowing pills, then parenteral administration of a long lasting steroid (eg, methylprednisolone acetate, triamcinolone acetonide) may be useful.
The author generally prefers to add metronidazole to the regime of cats that do not respond adequately to prednisolone, or to use it initially in cats that appear to have more severe disease. Metronidazole not only has activity against anaerobic bacteria and protozoa, but also appears to affect cell-mediated immunity (Grove et al 1977) and have anti-inflammatory properties (Tanga et al 1975). A relatively low dose (see Table 1) is typically used, minimising the chances of neurotoxicity. Although there are no published data showing efficacy of this combination, anecdotal evidence suggests that it is useful. In distinction to the data reported by Hart et al 1994, the author and others (Jergens 1994) find that metronidazole is sometimes effective as sole therapy for cats with IBD. The reason for such dissimilar experiences is unknown. If metronidazole plus prednisolone is successful, first the steroid dose is gradually tapered until it is being given every other day. Then, the metronidazole dose is gradually decreased until the lowest effective dose is found, or the drug can be discontinued altogether.
In more seriously debilitated cats (ie, those that are so ill that you believe you only have one chance to treat them before they become terminal), cats not responding to prednisolone plus metronidazole, and cats with very intense inflammatory cell infiltrates plus severe clinical signs, cytotoxic agents (eg, azathioprine (Jergens 1994), chlorambucil) should be considered (Table 1). These two drugs have greater potential for causing adverse reactions so they should be reserved for cats having a histologic diagnosis, and they should be used in combination with other anti-inflammatory drugs. Both drugs must often be administered for 3–5 weeks to effect clinical improvement. In general, chlorambucil appears to cause fewer adverse signs in cats than azathioprine does, and is about as effective. Azulfidine may be used in cats with large bowel IBD that do not respond to dietary therapy. However, one must use a lower dose than is recommended in the dog (see Table 1) (Jergens 1994) to avoid causing salicylate toxicity.
A relatively new drug currently being used in cats with ‘resistant’ IBD is budesonide (Stewart & Bolineck 1997). This is an orally administered steroid that is essentially eliminated by first pass metabolism in the liver so that there are almost no systemic side effects. While this drug has been very useful in people with Crohn's disease, its value for feline IBD remains to be established.
After a cat has been diagnosed as having IBD and is being treated, one should still consider the possible causes of the inflammation, even though the clinician has been unable to definitively attribute the inflammation to any particular entity. The fact that the inflammation is idiopathic does not mean that it does not have a cause; it just means that the cause is unknown. One may simplistically consider that the intestines are primarily exposed to two potential causes of inflammation: food and infectious agents (especially bacteria and parasites). Therefore, it seems reasonable to assume that one or more of these is responsible for initiating and/or perpetuating IBD. Possibly the intestine is initially damaged by one thing (eg, virus) and this damage increases intestinal permeability allowing other antigens (eg, dietary or bacterial) to perpetuate the inflammation. It is interesting that normal cats often have large numbers of bacteria in their upper small intestinal lumen (Johnston et al 1993, Sparkes et al 1998). An exaggerated or abnormal response to these bacteria might be one of the mechanisms by which IBD is perpetuated. Therefore, therapy for IBD can reasonably include both symptomatic anti-inflammatory drugs as well as hypoallergenic diets and antibacterial therapy.
The author routinely recommends a combination of prednisolone, metronidazole, and a hypoallergenic diet for cats with mild to moderate IBD (adding chlorambucil in selected cases, as described above). While cats with lymphocytic-plasmacytic enteritis seldom respond to dietary therapy alone, there are still three reasons to recommend it. First, the cat could have a dietary allergy that requires weeks (instead of days) to respond, and if such is the case it might be possible to eventually stop all drug therapy. Second, a highly digestible diet may help compromised small intestines to function better, even if dietary allergy is not present. Finally, if dietary antigens are perpetuating intestinal disease by entering the mucosa secondary to increased permeability, providing a highly digestible, hypoallergenic diet may help diminish the inflammation occurring secondary to these antigens diffusing into the lamina propria.
Failure to respond to therapy
When a patient is diagnosed with IBD but does not respond to apparently appropriate therapy, then there are three main possibilities: (a) the diagnosis is wrong, (b) the diagnosis is correct but the treatment is wrong, and (c) the diagnosis and treatment are correct but the client is not following instructions. Many clinicians attribute therapeutic failure to insufficient therapy, but in the author's experience that is probably the least common of the three causes. Most patients with IBD respond at least partially to prednisolone, metronidazole, cytotoxic drugs and/or azulfidine. Failure to respond to these drugs (assuming they are properly dosed and administered) should raise concerns that another disease may be present.
In the author's experience, cats diagnosed as having large bowel lymphocytic-plasmacytic colitis that do not respond to appropriate drug therapy often prove to have a diet-responsive disease. However, finding a diet that will resolve clinical signs can be surprisingly difficult in some patients. In some extremely difficult cases that fail two or more carefully chosen diets, I will begin randomly trying various commercial diets. Cats with small bowel signs (eg, weight loss) diagnosed as having lymphocytic-plasmacytic enteritis but who fail to respond to pharmacologic therapy or only respond for a few weeks to months before relapsing sometimes have a well-differentiated alimentary lymphosarcoma.
Extra-alimentary tract involvement
Cats with IBD may have problems involving other organ systems. It is important to recognise these potential associations so that the clinician does not forget to consider that the intestinal tract may be involved. Severe malabsorption may cause failure to absorb vitamin K, resulting in a coagulopathy (Edwards & Russell 1987). A more frequent problem in cats with IBD is accompanying hepatic disease (Weiss et al 1996). Severe intestinal inflammation may increase intestinal mucosal permeability, allowing various antigens (including bacteria) to enter the portal blood and be taken out of circulation by the liver. Bacteria appear to translocate from the intestines and cause bacterial cholangitis/cholangiohepatitis in some patients. Therefore, cats with suppurative cholangitis/cholangiohepatitis may be evaluated for IBD, and cats that are being treated for IBD and do not respond may be evaluated for hepatic disease. More importantly, if the primary signs of IBD in a cat were vomiting and anorexia, then recurrence of the IBD may cause signs indistinguishable from suppurative cholangiohepatitis. If the clinician assumes that the signs are due to IBD, more aggressive steroid therapy may result (instead of antibiotic therapy) and the patient may accordingly suffer.
A connection between IBD and pancreatitis is currently less certain than the one between IBD and hepatic disease, but seems enticingly likely. ‘Tri-itis’ is a colloquialism that is often heard in feline meetings, and refers to the apparent triology of IBD, hepatitis, and pancreatitis. Assuming that there is a relationship between the IBD and pancreatitis, questions that need to be answered include mechanisms of disease as well as significance. Regardless of whether pancreatitis can be an effect of IBD or not, the clinical significance of pancreatitis found in cats with IBD is currently unknown. Currently, the author is not routinely biopsying the pancreas in cats with suspected IBD, unless for some reason the biopsies are being obtained by laparotomy. However, pancreatic biopsy is considered in cats not responding to apparently appropriate therapy for IBD, in cats with marked abnormalities of the pancreas on ultrasonography, and in cats with biochemical abnormalities that seemingly suggest pancreatitis (eg, inappropriate hypocalcemia).
Summary
Inflammatory bowel disease is an oft diagnosed disease of cats that is still poorly understood. It is critical to remember that IBD is a diagnosis of exclusion. Therefore, when dealing with a cat that has chronic vomiting or diarrhoea or weight loss, it is best to concentrate on trying to find a definitive cause for the signs instead of quickly focusing on IBD. It must be remembered that food allergy/intolerance and alimentary lymphosarcoma in particular can closely mimic IBD, and that histopathology is not guaranteed to distinguish these entities. Finally, if intestinal biopsy is to be performed, it is critical that multiple, good-quality tissue samples be obtained and evaluated by someone who has more than a passing knowledge of intestinal pathology.
References
- Dennis JS, Kruger JM, Mullaney TP. (1992) Lymphocytic/plasmacytic gastroenteritis in cats: 14 cases (1985–1990). Journal of the American Veterinary Medicine Association 200, 1712–1718. [PubMed] [Google Scholar]
- Dennis JS, Kruger JM, Mullaney TP. (1993) Lymphocytic/plasmacytic colitis in cats: 14 cases (1985–1990). Journal of the American Veterinary Medicine Association 202, 313–317. [PubMed] [Google Scholar]
- Edwards DF, Russell RG. (1987) Probable Vitamin K—deficient bleeding in two cats with malabsorption syndrome secondary to lymphocytic-plasmacytic enteritis. Journal of Veterinary Internal Medicine 1, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing GO. (1972) Feline ulcerative colitis: A case report. Journal of the American Animal Hospital Association 8, 64–65. [Google Scholar]
- Ghermai AK. (1989) Chronisch-entzundliche darmerkrankungen der katze. Tierarztl Prax 17, 195–199. [PubMed] [Google Scholar]
- Grooters AM, Biller DS, Ward H, Miyabayashi T, Couto CG. (1994) Ultrasonographic appearance of feline alimentary lymphoma. Veterinary Radiology Ultrasound 35, 468–472. [Google Scholar]
- Grove DI, Mahmoud AAF, Warren KS. (1977) Suppression of cell-mediated immunity by metronidazole. Internation Archives of Allergy and Applied Immunology 54, 422–427. [DOI] [PubMed] [Google Scholar]
- Hart JR, Shaker E, Patnaik AK, Garvey MS. (1994) Lymphocytic-plasmacytic enterocolitis in cats: 60 cases (1988–1990). Journal of the American Animal Hospital Association 30, 505–514. [Google Scholar]
- Hitt ME. (1995) Biopsy of the Gastrointestinal Tract. In Current Veterinary Therapy XII edited by Bonagura JD, Philadelphia: W.B. Saunders Co., pp. 675–678. [Google Scholar]
- Jergens AE. (1994) Inflammatory Bowel Disease. In Consultations in Feline Internal Medicine edited by August JR, Philadelphia: W.B. Saunders Co., pp. 75–81. [Google Scholar]
- Jergens AE, Moore FM, Haynes JS, Miles KG. (1992a) Idiopathic inflammatory bowel disease in dogs and cats: 84 cases (1987–1990). Journal of the American Veterinary Medicine Association 201, 1603–1608. [PubMed] [Google Scholar]
- Jergens AE, Moore FM, March P, Miles KG. (1992b) Idiopathic inflammatory bowel disease associated with gastroduodenal ulceration-erosion: A report of nine cases in the dog and cat. Journal of the American Animal Hospital Association 28, 21–26. [Google Scholar]
- Johnston K, Lamport A, Batt RM. (1993) An unexpected bacterial flora in the proximal small intestine of normal cats. Veterinary Record 132, 362–363. [DOI] [PubMed] [Google Scholar]
- Lecoindre P, Chevallier M. (1997) Contribution to the study of feline inflammatory bowel disease: 51 cases (1991–1994). Revue of Medical Veterinary 148, 893–902. [Google Scholar]
- Nelson RW, Dimperio ME, Long GG. (1984) Lymphocytic-plasmacytic colitis in the cat. Journal of the American Veterinary Medicine Association 184, 1133–1135. [PubMed] [Google Scholar]
- Oyer DL, Grooters AM, Aronson E, Reed AL. (1995) What is your diagnosis? Journal of the American Veterinary Medicine Association 207, 1557–1558. [PubMed] [Google Scholar]
- Penninck DG, Moore AS, Tidwell AS, Matz ME, Freden GO. (1994) Ultrasonography of alimentary lymphosarcoma in the cat. Vet Radiol Ultrasound 35, 299–304. [Google Scholar]
- Peterson JL, Willard MD, Lees GE, Lappin MR, Dieringer T, Floyd E. (1991) Toxoplasmosis in two cats with inflammatory intestinal disease. Journal of the American Veterinary Medicine Association 199, 473–476. [PubMed] [Google Scholar]
- Rosser EJ. (1993a) Advances in Veterinary Dermatology II edited by Ihrke PJ. Food allergy in the cat: a prospective study of 13 cats. New York: Pergamon Press, 33. [Google Scholar]
- Rosser EJ. (1993b) Diagnosis of food allergy in dogs. Journal of the American Veterinary Medical Association 203, 259–262. [PubMed] [Google Scholar]
- Roth L, Leib MS, Davenport DJ, Monroe WE. (1990) Comparisons between endoscopic and histologic evaluation of the gastrointestinal tract in dogs and cats: 75 cases (1984–1987). Journal of the American Veterinary Medicine Association 196, 635–638. [PubMed] [Google Scholar]
- Roth L, Walton AM, Leib MS, Burrows CF. (1990) A grading system for lymphocytic plasmacytic colitis in dogs. Journal of Veterinary Diagnostic Investigation 2, 257–262. [DOI] [PubMed] [Google Scholar]
- Sparkes AH, Papasouliotis K, Sunvold G, et al. (1998) Bacterial flora in the duodenum of healthy cats, and effect of dietary supplementation with fructo-oligosaccharides. American Journal of Veterinary Research 59, 431–435. [PubMed] [Google Scholar]
- Stewart A, Bolineck J. (1997) The use of a novel formulation of budesonide as an improved treatment over prednisone for inflammatory bowel disease. Proceedings of the American College of Veterinary Internal Medicine 15, 662. (Abstract). [Google Scholar]
- Tams TR. (1986a) Chronic feline inflammatory bowel disorders. Part II. Feline eosinophilic enteritis and lymphosarcoma. Compend Cont Educ 8, 464–470. [Google Scholar]
- Tams TR. (1986b) Chronic feline inflammatory bowel disorders Part I. Idiopathic inflammatory bowel disease. Compend Cont Educ 8, 371–376. [Google Scholar]
- Tanga MR, Antani JA, Kabade SS. (1975) Clinical evaluation of metronidazole as an anti-inflammatory agent. International Surgery 60, 75–76. [PubMed] [Google Scholar]
- van Kruiningen HJ, Dobbins WO. (1979) Feline histiocytic colitis. Veterinary Pathology 16, 215–222. [DOI] [PubMed] [Google Scholar]
- van Kruiningen HJ, Ryan MJ, Shindel NM. (1983) The classification of feline colitis. Journal of Competitive Pathology 93, 275–294. [DOI] [PubMed] [Google Scholar]
- Wasmer ML, Willard MD, Helman RG, Edwards JF. (1995) Food intolerance mimicking alimentary lymphosarcoma. Journal of the American Animal Hospital Association 31, 463–466. [DOI] [PubMed] [Google Scholar]
- Weichselbaum RC, Feeney DA, Hayden DW. (1994) Comparison of upper gastrointestinal radiographic findings to histopathologic observations: A retrospective study of 41 dogs and cats with suspected small bowel infiltrative disease (1985 to 1990). Vet Radiol Ultra 35, 418–426. [Google Scholar]
- Weiss DJ, Gagne JM, Armstrong PJ. (1996) Relationship between inflammatory hepatic disease and inflammatory bowel disease, pancreatitis and nephritis in cats. Journal of the American Veterinary Medical Association 209, 1114–1116. [PubMed] [Google Scholar]
- Whitehead R, Roca M, Meikle DD, Skinner J, Truelove SC. (1975) The histological classification of duodenitis in fibreoptic biopsy specimens. Digestion 13, 129–136. [DOI] [PubMed] [Google Scholar]
- Wilcock B. (1992) Endoscopic biopsy interpretation in canine or feline enterocolitis. Seminars in Veterinary Medicine and Surgery 7, 162–171. [PubMed] [Google Scholar]
- Willard MD, Dalley JB, Trapp AJ. (1985) Lymphocytic-plasmacytic enteritis in a cat. Journal of the American Veterinary Medicine Association 186, 181–182. [PubMed] [Google Scholar]
- Yamasaki K, Suematsu H, Takahashi T. (1996) Comparison of gastric and duodenal lesions in dogs and cats with and without lymphocytic plasmacytic enteritis. Journal of the American Veterinary Medicine Association 209, 95–97. [PubMed] [Google Scholar]


