Abstract
The aim of this review of hereditary and congenital ocular disease in cats is to present an overview of the most common disorders seen in this species, the pathogenesis of the problems and wherever possible, how they are treated. Several defects are common in breeds such as the Persian, Himalayan and Burmese cats and affect the anterior segment of the eye. Examples are agenesis of the eyelids, dermoids, entropion and corneal sequestrum. Other problems such as cataracts, lens luxation and retinal dysplasia, cause problems of the intraocular structures, but are less common in cats compared to dogs. Finally, various parts of the retina and in some diseases other parts of the eye, are specifically affected by hereditary diseases. Examples of these are lysosomal storage disease, Chediak-Higashi syndrome and progressive rod cone degeneration and rod cone dysplasia. Research of the latter two hereditary diseases, both described in the Abyssinian breed of cat, have made affected individuals important animal models for research into comparable diseases of humans.
In comparison to the dog species, cats are affected by relatively few inherited eye diseases. However, several inherited disorders of the anterior and posterior segments of the eye have been described especially in purebred cats. The following is a description of the most common defects diagnosed in cats in which a hereditary background is suspected or has been proven.
Anterior segment
Agenesis and dermoids
Several developmental defects may affect the anterior segment of the feline eye. Complete or partial absence of all or parts of the eyelid layers, agenesis or lid coloboma, respectively, may affect one or both eyes (Fig 1). This defect is considered to be the most common congenital eyelid abnormality in the feline species. Specifically in the snow leopard, multiple ocular colobomas have been recorded as hereditary defects (Gripenberg et al 1985). A genetic predisposition to incomplete development of the eyelids specifically at the upper lateral canthus but also infrequently in the medial canthus, is suspected in the Burmese cat (Koch 1979). Epibulbar dermoids also appear to be part of a developmental defect not only in the Burmese but also in Birman cats (Hendy Ibbs 1985). Typical findings include a circular mass of skin, more or less covered with hair, most often in the lateral angle of the eye, including bulbar conjunctiva, eyelid margin and skin. Moreover, it should be noted that it is not uncommon that external ocular abnormalities are accompanied by internal malformations, thus rendering intra-ocular examination (ie, ophthalmoscopy and biomicroscopy), always of importance in conjunction with external ocular defects.
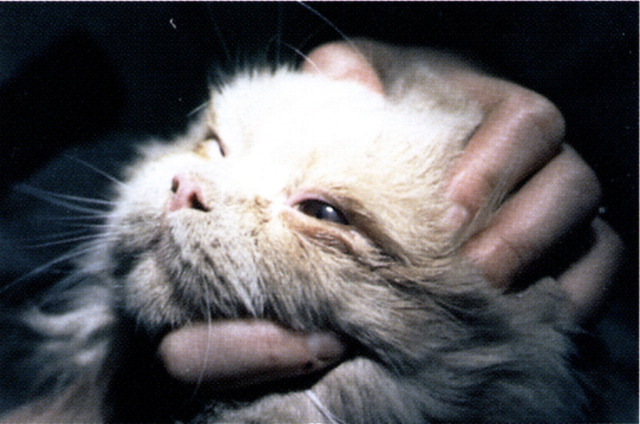
Fig 1.
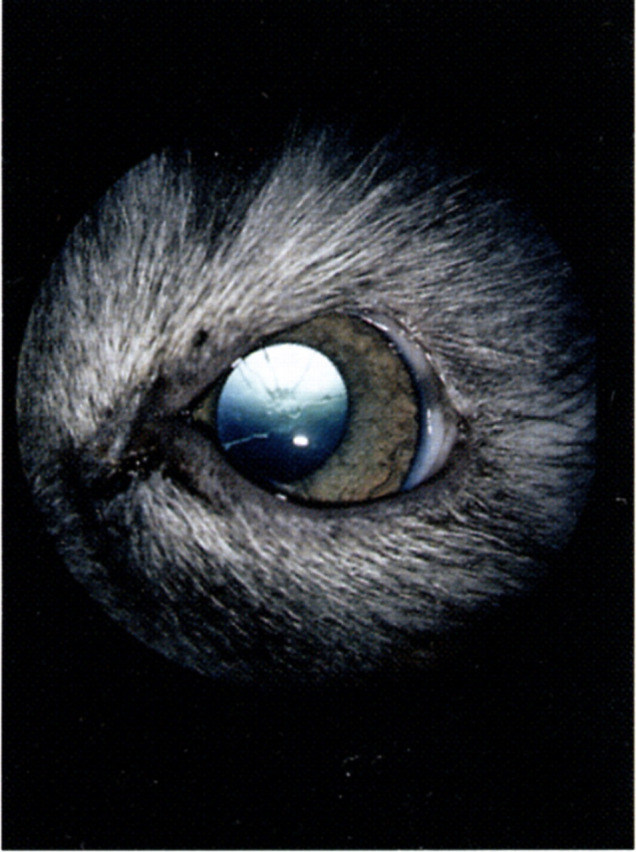
Lid coloboma of the temporal part of the left upper eyelid in a young Birman cat. There are also persistant pupillary membranes and a minor anterior capsular cataract in the affected eye.
The extent of the eyelid abnormalities determine the severity of clinical signs. Increased tearing and irritation from the eye are typical signs.
Treatment consists of cleaning and protection of the cornea by using artificial tear elements for lubrication. This may be enough in minimally affected cases, while a more aggressive approach must be initiated in more severely affected cases. Reconstructive surgery of the upper or lower eyelid is often advocated in agenesis or lid colobomas, although surgery is usually postponed until the cats are old enough for surgery and have more adult-like eyes. Epibulbar dermoids are usually treated by surgical excision. Incision should be done into normal skin tissue and closed starting at the canthus, using 6/0 silk or polyglactin.
Entropion
Entropion is an inward turning (inversion) of the eyelid which causes hairs from the surrounding skin to rub on the cornea (Fig 2). The defect may be primary or secondary. The primary type describes an anatomical entropion, with a loose or somewhat long eyelid, and is usually present at an early age. It usually involves part or all of the lower eyelid and is often bilateral. Primary entropion is not an uncommon finding in Persian cats and hereditary factors are suspected to be implicated in the condition. The secondary type of entropion is often of the spastic type, secondary to pain in the anterior segment, or cicatricial type, a result of scarring. Any breed of cat may be affected by the secondary type.
Fig 2.
Bilateral entropion in a 2-year-old Persian cat.
Investigation of entropion consists of elucidating whether the condition is secondary or primary. Topical anaesthesia is an essential part of the investigational procedure. In a secondary, spastic type of entropion, the inward turning of the eyelid is corrected when the eye is anaesthetised and/or the primary condition is mainly resolved and surgery of the eyelid not needed. In the primary type of entropion surgery is often indicated, however. A Hotz–Celsus procedure is used closing the wound with 5–6/0 silk sutures and removing the sutures after 10 days. Care should be taken not to over-correct the entropion so that there will be an ectropion (outward turning) of the eyelid instead.
Corneal sequestrum
Corneal sequestrum (cornea nigrum, black body) is a condition of unknown cause that is unique to the cat (Startup 1988). There is definitely a breed disposition in the condition, which often is bilateral in Colorpoint, Persian, Siamese, Himalayan, Birman and Burmese cats. Mixed breed cats may also be affected. Often then the condition is unilateral, which indicates that other factors such as chronic irritation or tear film disorders may be involved in the corneal disease. Recent studies have shown that many clinical cases are associated with FHV-1 infection (Basher et al 1995, Nasisse et al 1996).
The sequestrum is usually located in the central or paracentral corneal stroma as a brownish to black circular lesion (Fig 3). Often the lesion is circumscribed by loose corneal epithelium. The depth of corneal involvement is often mid or deep stroma, although occasionally the sequestrum may extend all the way to Descemet's membrane. The appearance of the lesion thus varies, most probably depending on the stage of development and the pathogenesis.
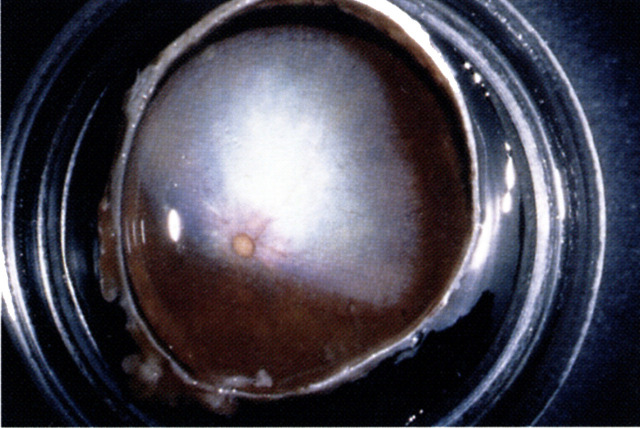
Fig 3.
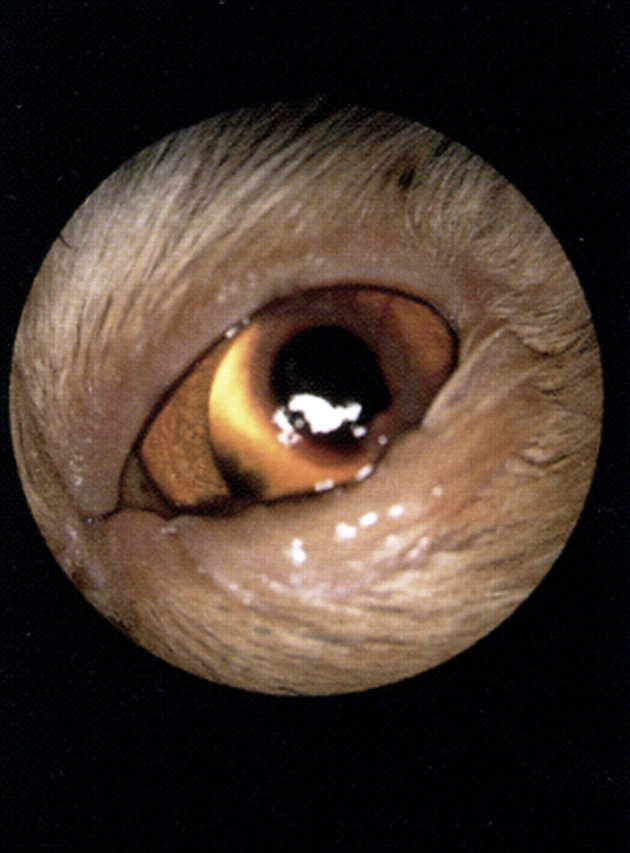
A large corneal sequestrum with surrounding vascularisation in a middle-aged Persian cat. There is also secondary entropion in this eye.
Obvious discomfort causes clinical signs such as increased tearing and a dark yellow to brownish ocular discharge, blepharospasm and sometimes secondary entropion. The dark material found in the lesion has not been identified but is believed to be derived from the ocular tear film. Histopathologically a collagen degeneration is found together with non-specific inflammatory cells.
Treatment varies depending on the extent of the lesion and the amount of ocular discomfort caused by the problem. Either the lesion can be allowed to slough naturally, which often occurs through vascular ingrowth around and under the lesion, or surgical excision should be performed. If the sequestrum is not producing any major discomfort, treatment with tear substitutes is indicated until sloughing and corneal healing occurs, which usually takes between 2–6 months. If there is major irritation and secondary ocular and periocular problems, keratectomy is indicated with or without a partial conjunctival flap. The latter is indicated if the sequestrum is deep in the stroma. In some cases penetrating keratoplasty is used where involvement of Descemet's membrane is suspected.
Lysosomal storage disorders
Cats are affected by a number of specific enzyme deficiency syndromes that lead to the accumulation of abnormal products within the cell lysosomes. Diffuse corneal clouding is a specific sign of these recessively inherited disorders, which include mucopolysaccharidosis VII, GM1 and 2, gangliosidosis and mannosidosis. Also there is accumulation of abnormal products in the retinal pigment epithelium which causes focal or more generalised retinopathies and blindness in most types of disorders (see below). Except for the ocular signs, neurological signs are common and facial dysmorphia may be part of the clinical picture (Haskins & Patterson 1987). The disorders are progressively devastating.
Cataract
Cataract is an opacity of the lens and/or its capsule and is infrequently seen in the cat species compared to how often it is diagnosed in dogs. Congenital cataract has been reported in the Persian (Peiffer & Gelatt 1975) and British short-haired breeds (Irby 1983) and presumed to have an autosomal recessive inheritance. Also Rubin reported three cases in Himalayan cats, in which the condition was bilateral and present as early as at 12 weeks (Rubin 1986). Possible cases of primary hereditary cataract in related British blue-cream cats was also recently reported (Barnett & Crispin 1998).
Primary, most probably developmental cataracts have been observed in a Birman family of related cats in Sweden (Narfström, unpublished observation). Lenticular opacities appeared in 2–5 year-old cats and the changes were bilateral and progressive. Initial findings were either posterior subcapsular and/or cortical circular or triangular cataracts, or complete cataracts. An intriguing finding was that resorbing cataracts were found in two of six cases observed (Fig 4a–c).
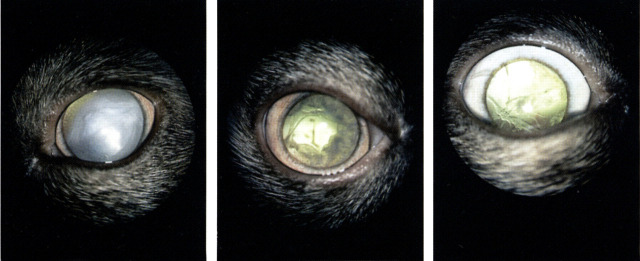
Fig 4.
(a) Complete cataract in a 3-year-old Birman cat. (b) Same eye about 1-year later: There is resorbtion of the cataracteous lens material. (c) Six months later than at the time of (b). Only thin cataracteous strands are remaining within the lens capsule. There were no clinical signs of uveitis in this cat.
Apart from the above mentioned hereditary or suspected hereditary types of cataracts, most feline cataracts are, in fact, secondary to other ocular diseases such as uveitis, ocular neoplasia and following trauma, as well as systemic and metabolic disorders, such as diabetes. Metabolic diabetic cataracts are uncommon in cats, however, and if they occur, appear to progress more slowly in cats compared to dogs. In blue smoke Persian cats the Chediak-Higashi syndrome has been reported as an autosomal recessive condition (Collier et al 1979). In this syndrome hypopigmentation of the iris and an abnormally thin iris and cataract formation is found. Cataracts secondary to inherited progressive retinal atrophy (PRA) are not seen in cats, however (Narfström 1983).
Clinical signs of cataract depend on the extension of lenticular opacifications. Minor changes cause no visual problems while complete cataracts cause blindness in the affected eye.
It is mainly the primary cataracts that are treated and this is accomplished by surgery. Phacoemulsification is usually performed of the contents of the lens (leaving the lens capsule into which an artificial lens can be inserted), a procedure that today is as successful in cats as in dogs.
Lens luxation
A breed predisposition for primary lens luxation for the Siamese cat has been described by Olivero et al (1991). Lens luxation is commonly seen in somewhat older animals, usually between the age of 7–9 years. The condition might follow a certain degree of zonular degeneration in cats. Glaucoma, secondary to lens luxation, occurs in cats as well as in dogs. Treatment, therefore, of lens luxation in cats, is surgical by performing lens extraction as soon as possible, before secondary uveitis and secondary glaucoma is manifestated.
Posterior segment
Retinal dysplasia
Retinal dysplasia (RD) is an abnormal development of the neural retina and involves central focal or multifocal, or more or less widespread parts of the retina. In less severe cases it results in bilateral retinal folds, but in more severe cases in areas of retinal degeneration and scarring. Cases of suspected hereditary multifocal type of RD have been found by the author in related Somali cats in Sweden. Other causes of retinal dysplasia in cats are perinatal infection with feline panleukopenia and leukaemia virus (FeLV) (Percy et al 1975, Albert et al 1977).
Lysosomal storage disease
Inherited enzyme deficiencies or lysosomal storage diseases affect the retina as well as other body systems, such as the cornea as described earlier. The recessively inherited diseases include alpha-mannosidosis, manifested as a dull, grey, granular appearance of the area centralis region (Blakemore 1986) and GM1.gangliosidosis, associated with focal fundus lesions in the form of multiple small spots, which represent accumulation of glycolipid within the ganglion cells (Murray et al 1977). Another lysosomal storage disease is mucopolysaccharidosis type VI, where there is a generalised retinal atrophy in most affected cats (Haskins et al 1979). This is also the case in gyrate atrophy, which represents a systemic ornithine deficiency and also results in blindness.
Chediak–Higashi syndome
As described above, Chediak–Higashi syndome (CHS) is an autosomal recessive disease of cats and other species (Collier et al 1979). Affected kittens have hypopigmentation not only of iris but also of retinal pigment epithelium (RPE) and changes in the tapetal cells. The tapetal cells do not seem to reach maturity and ophthalmoscopically there is a lack of visible tapetum in affected cats. Ultrastructurally there is a loss of RPE cells and of tapetal cells with time. A membrane defect has been reported in CHS, involving an increase in unsaturated fatty acid content. This could lead to increased peroxidation of both phagosome-derived and intracellular CHS-RPE membranes, making them resistant to lysosomal degradation.
Progressive retinal atrophy (PRA)
PRA was for many years reported in only a few breeds of cats and in few individuals in each report. Retinal degeneration with a suspected hereditary basis was described in Siamese (Barnett 1965, Carlisle 1981), Persian (Rubin & Lipton 1973) and in mixed-breed domestic cats in the US (West-Hyde & Buyukmihci 1982). The only breed so far in which there is hereditary PRA, proven through genetic studies, is the Abyssinian. It has also been shown that in the Abyssinian cat there are two specific types of retinal diseases and each one has a different mode of inheritance for the defect. Both are bilateral and more or less symmetrical in the two eyes and progressively lead to blindness.
The first type described in the Abyssinian cat is a progressive rod cone degenerative disease with an autosomal recessive inheritance (Narfström 1981, 1983). Classical early signs of PRA are found ophthalmoscopically in affected cats at the age of 1.5–2 years; tapetal colour changes and slight changes in tapetal reflectivity and some vascular attenuation mainly in the midperipheral and peripheral tapetal fundus. With progression of the disease there are more widespread changes indicating neuro-retinal thinning, showing as hyper-reflectivity and severe vascular attenuation. The late stage is seen 3–4 years after the initial findings when there is a complete bilateral retinal atrophy (Figs 5a–c).
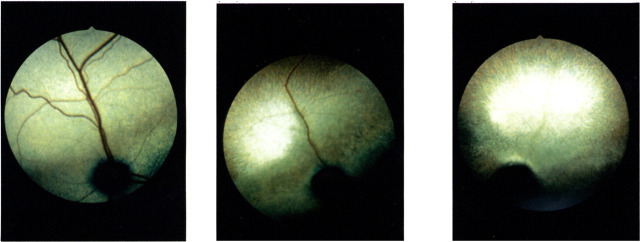
Fig 5.
(a) Six-months-old Abyssinian cat homozygous for hereditary rod cone degeneration. Note the slight greyish discoloration above and on either side of the disc. Otherwise the fundus is normal appearing (stage 1, Narfström 1985). (b) Same eye 2 years later. The discolored streak is still obvious above the disc and there is now severe vascular attenuation and the midperipheral and peripheral parts of the fundus are hyperreflective (stage 3). (c) Completely atrophic fundus seen at the age of 5 years (stage 4).
Functionally it has been shown by using electroretinography (ERG) and morphologically, by using light- and electron microscopy, that the retina mainly develops normally in affected cats up to the age when the retina is adult-like, at 5 months in cats (Narfström 1985, 1986). After that age there is a drop out primarily of rod photoreceptors and much later also of cones (Narfström & Nilsson 1989). The neuro-retinal cell layers are successively lost starting in the midperiphery and periphery (Fig 6). The central retina is spared until late in the disease process.
Fig 6.
In preparation for histologic studies the eyecup of a 3-year-old Abyssinian cat with hereditary rod cone degeneration is seen. Note the hyperreflective tapetal fundus and the marked vascular attenuation.
The pathogenesis of this rod cone degenerative disease is so far not elucidated and further studies are in progress in order to find the gene defect. Early diagnosis of affected cats can be made by careful ERG studies at the age of 8 months (Narfström et al 1988), well before retinal changes are diagnosed by ophthalmoscopy. The frequency of the disease was calculated to be unusually high, up to 45% of affected animals in Sweden, when first found (Narfström 1983). Sixteen years later, only sporadic cases of the disease are observed in northern Europe and mostly still in Scandinavia.
In the second type of retinal disorder in the Abyssinian the defect has been shown to have an autosomal dominant mode of inheritance (Barnett & Curtis 1985). Affected cats are congenitally visually impaired and some have nystagmus (involuntary fast horizontal movements of the eyes). The first observable clinical sign is mydriasis or dilated, unresponsive pupils, already at the age of 4 weeks. Most affected cats then show further severe symptoms of visual impairment or blindness at 12–16 months.
Morphologically, rods and cones are affected equally in the dominantly inherited type of Abyssinian PRA. As early as 14 days of age, disorganised rod and cone outer segments as well as diminutive inner segments can be detected (Leon & Curtis 1990). The photoreceptor cell layer is progressively lost from the central to the peripheral retina. This early-onset retinopathy of Abyssinian cats has been classified as a rod cone dysplasia (Curtis et al 1987), in contrast to the earlier described recessively inherited rod cone degeneration.
This dominantly inherited disease of Abyssinians is unlikely to be encountered in the clinical practice since the disease was discovered and further investigated under laboratory conditions in England. Both the dominant and the recessive types of photoreceptor disorders of the Abyssinian cat have, since their discoveries, become important animal models for a similar group of diseases in humans, namely retinitis pigmentosa (RP).
References
- Albert DM, Lahaw M, Colby ED, Shadduck JA, Sang DN. (1977) Retinal neoplasia and dysplasia. I. Induction by feline leukemia virus. Investigative Ophthalmology Visual Science 16, 325–337. [PubMed] [Google Scholar]
- Barnett KC. (1965) Retinal atrophy. Veterinary Record 77, 1543–1560. [PubMed] [Google Scholar]
- Barnett KC, Crispin SM. (1998) Feline Ophthalmology. An atlas and text. London: WB Saunders Co Ltd, p. 114. [Google Scholar]
- Barnett KC, Curtis R. (1985) Autosomal dominant progressive retinal atrophy in Abyssinian cats. Journal of Heredity 76, 168–170. [DOI] [PubMed] [Google Scholar]
- Basher A, Roberts S, Collins J, Getzy D, Carman J. (1995) Immunoperoxidase analysis for feline herpesvirus in surgically excised corneal sequestra from cats. Proc Am Coll Vet Ophthalmol 26, 3. [Google Scholar]
- Blakemore WF. (1986) A case of mannosidosis in the cat: Clinical and histopathological findings. Journal of Small Animal Practice 27, 447–455. [Google Scholar]
- Carlisle JL. (1981) Feline retinal atrophy. Veterinary Record 108, 311. [DOI] [PubMed] [Google Scholar]
- Collier LD, Bryan GM, Prieur DJ. (1979) Ocular manifestations of the Che'diak-Higashi Syndrome in four species of animals. Journal of the American Veterinary Medical Association 175, 587–595. [PubMed] [Google Scholar]
- Curtis R, Barnett KC, Leon A. (1987) An early-onset retinal dystrophy with dominant inheritance in the Abyssinian cat. Clinical and pathological findings. Invest Ophthalmol Vis Sci 28, 131–139. [PubMed] [Google Scholar]
- Gripenberg U, Blomqvist L, Pamillo P, et al. (1985) Multiple ocular coloboma in Snow Leopards (Panthera uncia): Clinical report, pedigree analysis, chromosome investigations and serum protein studies. Hereditas 103, 221–229. [DOI] [PubMed] [Google Scholar]
- Haskins ME, Patterson DF. (1987) Inherited metabolic diseases. In Holzworth J. (ed.) Diseases of the cat. Philadelphia: WB Saunders, pp. 808–819. [Google Scholar]
- Haskins ME, Jezyk PF, Patterson DF. (1979) Mucopolysaccharide storage disease in three families of cats with arylsulfatase B deficiency: Leukocyte studies and carrier identification. Paedriatic Research 13, 1203–1210. [DOI] [PubMed] [Google Scholar]
- Hendy Ibbs PN. (1985) Familial feline epibulbar dermoids. Veterinary Record 116, 13–14. [DOI] [PubMed] [Google Scholar]
- Irby NI. (1983) Hereditary cataracts in the British Shorthair Cat. In American College of Veterinary Ophthalmology: Genetic workshop.
- Koch SA. (1979) Congenital ophthalmic abnormalities in the Burmese cat. Journal of the American Veterinary Medical Association 174, 90–91. [PubMed] [Google Scholar]
- Leon A, Curtis R. (1990) Autosomal dominant rod-cone dysplasia in the Rdy cat. 1. Light and electron microscopic findings. Experimental Eye Research 51, 361. [DOI] [PubMed] [Google Scholar]
- Murray JA, Blakemore WF, Barnett KC. (1977) Ocular lesions in cats with GM1-gangliosidosis with visceral involvement. Journal of Small Animal Practice 18, 1–10. [DOI] [PubMed] [Google Scholar]
- Narfström K. (1981) Progressive retinal atrophy in the Abyssinian cat. Svensk Veterinärtidning 33, 147–150. [Google Scholar]
- Narfström K. (1983) Hereditary progressive retinal atrophy in the Abyssinian cat. Journal of Heredity 74, 273–276. [DOI] [PubMed] [Google Scholar]
- Narfström K. (1985) Progressive retinal atrophy in the Abyssinian cat: Clinical characteristics. Investigative Ophthalmology and Visual Science 26, 193–200. [PubMed] [Google Scholar]
- Narfström K, Nilsson SE. (1986) Progressive retinal atrophy in the Abyssinian cat: Electron microscopy. Investigative Ophthalmology Visual Science 27, 1569–1576. [PubMed] [Google Scholar]
- Narfström K, Nilsson SE. (1989) Morphological findings during development and maturation in hereditary rod-cone degeneration in Abyssinian cats. Experimental Eye Research 49, 611–628. [DOI] [PubMed] [Google Scholar]
- Narfström K, Nilsson SE, Andersson BE. (1985) Progressive retinal atrophy in the Abyssinian cat: Studies of the DC-recorded electroretinogram and the Standing Potential of the eye. British Journal of Opththalmology 69, 618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narfström K, Wile'n M, Andersson BE. (1988) Hereditary retinal degeneration in the Abyssinian cat: Developmental studies using clinical electroretinography. Documenta Ophthalmologica 69, 111–118. [DOI] [PubMed] [Google Scholar]
- Nasisse MP, Luo H, Wang YJ, Glover TL, Weigler BJ. (1996) The role of feline herpesvirus-1 (FEV-1) in the pathogenesis of corneal sequestrum and eosinophilic keratitis. Proc Am Coll Vet Ophthalmol 27, 80. [Google Scholar]
- Olivero DK, Riis RC, Dutton AG, Murphy CJ, Nasisse MP, Davidsson MG. (1991) Feline lens displacement. A retrospective analysis of 345 cases. Progress in Veterinary and Comparative Ophthalmology 1, 239–244. [Google Scholar]
- Peiffer RL, Gelatt KN. (1975) Congenital cataracts in a Persian kitten. Veterinary Medicine (Small Animal Clinician) 70, 1334–1335. [PubMed] [Google Scholar]
- Percy DH, Scott FW, Albert DM. (1975) Retinal dysplasia due to feline panleukopenia virus infection. Journal of the American Veterinary Medical Association 167, 935–937. [PubMed] [Google Scholar]
- Rubin LF. (1986) Hereditary cataract in Himalayan cats. Feline Practice 16, 14–15. [Google Scholar]
- Rubin LF, Lipton DE. (1973) Retinal degeneration in kittens. Journal of the American Veterinary Association 162, 467–469. [PubMed] [Google Scholar]
- Startup FG. (1988) Corneal necrosis and sequestration in the cat: A review and record of 100 cases. Journal of Small Animal Practice 29, 476–486. [Google Scholar]
- West-Hyde L, Buyukmihci N. (1982) Photoreceptor degeneration in a family of cats. Journal of the American Veterinary Association 181, 243–247. [PubMed] [Google Scholar]