Abstract
Between 1990 and 1997 vegetative endocarditis was diagnosed in six neutered cats (three males, three females) aged between 3 and 9 years. Two cats were purebred (one Persian and one Tonkinese), the remaining four being domestic short haired cats. The diagnosis was made using echocardiography (five cases) or at necropsy (one case). Concurrent involvement of the aortic and mitral valve was noted in four cats, the aortic valve alone was affected in one case, and the tricuspid valve in another. A likely microbiological diagnosis was obtained in three cats: a Bartonella species in two cats based on positive blood culture and a Streptococcus species in one cat based on Gram stain of valve lesions at necropsy. In another cat, Gram-positive cocci were demonstrated histologically in tricuspid vegetations. Invariably, cats had signs of congestive heart failure (left-sided in five cats, right-sided in one), and this was the major factor contributing to mortality in four cases. Signs referable to sepsis were prominent in only two patients. Appropriate medical therapy, consisting of antimicrobials and drugs to treat congestive heart failure, resulted in survival for 5 and 11 months, respectively, in two cases. The other cats died within 2 weeks of diagnosis, including two which received aggressive treatment in hospital.
Bacterial endocarditis has been well reported in the dog and large case series have documented the range of clinical, microbiological and echocardiographic findings, and correlated these with disease outcomes (Calvert 1982, Lombard & Beurgelt 1983, Sisson & Thomas 1984, Calvert et al 1985, Elwood et al 1993). Although the diagnosis is not always straightforward, most dogs have physical findings that reflect both bacterial sepsis and left-sided cardiac dysfunction. Thus, canine patients with bacterial endocarditis typically show constitutional signs (including fever and malaise), an inflammatory leukogram and evidence of systemic embolization (such as septic arthritis, myositis, uveitis, myocarditis, etc). As well, signs of acute cardiac dysfunction often appear with the development of murmurs, arrhythmias, bounding (water hammer) or asymmetric femoral pulses, and pulmonary oedema, especially when there is involvement of the aortic valve (Calvert 1982, Calvert et al 1985, Sisson & Thomas 1984, Elwood et al 1993).
Generally the disease runs an acute to subacute course, dogs usually presenting with severe disease within 2–4 weeks of the onset of signs. A diagnosis can be confirmed by echocardiography and positive blood culture, although this is easier when the aortic valve is affected because of difficulties in differentiating myxomatous degeneration of the mitral valve leaflets (endocardiosis) from lesions of vegetative endocarditis (Lombard & Buergelt 1983).
There are few reports of vegetative endocarditis in cats (Shouse & Meier 1956, Yamaguchi et al 1983, Noakes 1988, Liu 1988, Bonagura 1989, Malik et al 1992, Goodwin & Miller 1997, Caney et al 1997, Caney 1998, De Jonghe et al 1998) and most of these concern a single affected cat. There is a suggestion that in some cats bacterial endocarditis may follow a more indolent course to that encountered in canine patients, permitting the infected valves to undergo dystrophic calcification (Noakes 1988, Malik et al 1992). The present report describes six cats seen between 1990 and 1997 in which vegetative bacterial endocarditis was diagnosed echocardiographically or at necropsy. Unfortunately the clinical data for these animals is somewhat incomplete, as several cases were diagnosed by an outpatient echocardiography service offered by The University of Sydney Veterinary Centre (UVC). Case 1 has been reported previously in a brief communication (Malik et al 1992).
Case histories
Salient features concerning six cats with vegetative bacterial endocarditis are summarised in Table 1. Echocardiography was performed in cases 2–5 using an ATL Ultramark 9 and 7 MHz and 3–5 MHz phased array transducers. A Toshiba Sonolayer V SSA-90A with a 5 MHz phased array transducer was used in Case 1. Reference ranges used for M-mode echocardiography were those reported by Sisson et al (1991).
Table 1.
Signalment, clinical and laboratory information concerning six cats with vegetative endocarditis
| Case | Age | Sex | Breed | Valve affected | Culture/Histology |
|---|---|---|---|---|---|
|
| |||||
| 1 | 3 | FN | DSH | aortic, mitral | ND |
| 2 | 9 | FN | DSH | aortic | Bartonella spp |
| 3 | 6 | MN | Persian | aortic, mitral | Bartonella spp |
| 4 | 8 | MN | DSH | aortic,?mitral | ND |
| 5 | 6 | MN | DSH | mitral, aortic | Streptococcus spp |
| 6 | 6 | FN | Tonkinese | tricuspid | Gram positive cocci |
FN—spayed female, MN—castrated male, DSH—domestic short haired cat, ND—not done, mitral—left atrioventricular valve, tricuspid—right atrioventricular valve.
Case 1
A 3-year-old spayed domestic short haired (DSH) cat was presented to a colleague for laboured breathing which developed abruptly and was accompanied by anorexia. The cat had been observed to cough over the preceding few weeks. It was afebrile (38.5°C). A loud cardiac murmur was detected, while thoracic radiographs demonstrated generalised cardiomegaly and pulmonary vascular engorgement. An episode of haemoptysis was observed during the cat's hospitalisation. Frusemide (10 mg by mouth every 24 h) was administered and resulted in an improvement in its breathing pattern.
The cat was referred to the UVC 7 days later. The patient was afebrile, heart sounds were prominent and a loud heart murmur and gallop rhythm were audible on auscultation. Although the systolic component was loudest, a diastolic component was also detected. The cat had a rapid respiratory rate (80 breaths/min), and breath sounds were harsh. The left femoral pulse was much weaker than the right. Thoracic radiographs confirmed generalised cardiomegaly with pulmonary venous distention. In addition, two radio-opaque nodules were visible superimposed on the cardiac silhouette. Echocardiography demonstrated highly echogenic vegetative lesions on the right coronary cusp of the aortic valve and the posterior leaflet of the mitral valve, thickening of the mitral valve apparatus, marked left atrial dilatation (30 mm diameter), increased echogenicity of the left ventricular endocardium and left ventricular dilation with normal fractional shortening.
The cat was discharged on the dose of frusemide prescribed previously. The owners were requested to return the cat for further studies including blood culture. The scheduled appointment was not kept and telephone follow-up indicated that the cat had died 10 days after discharge.
Case 2
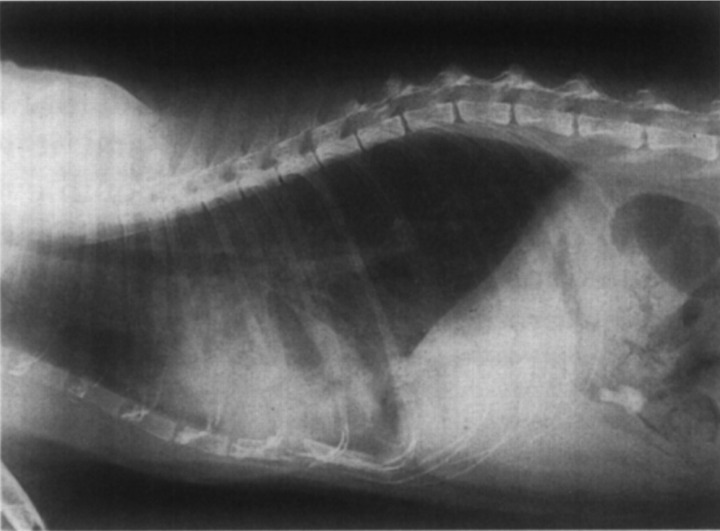
A 9 year-old spayed DSH with a long history of pruritic, seasonal, ‘miliary’ dermatitis which had been treated intermittently over several years using various drugs including megestrol and prednisolone was presented to the referring veterinarian for acute onset of distress and anorexia. It was in poor bodily condition, normothermic (38.7°C) and severely dyspnoeic. A loud heart murmur and moist breath sounds were detected during auscultation. Thoracic radiographs showed cardiomegaly and changes consistent with pulmonary oedema (Fig 1). The cat was treated using frusemide (10 mg by intramuscular injection) and referred for echocardiography the following day.
Fig 1.
Lateral thoracic radiograph of Case 2 prior to therapy. Cardiomegaly is present, and there are several patchy areas of increased pulmonary parenchymal density, consistent with pulmonary oedema.
At referral, the cat was thin (3.6 kg) and had papulocrustular dermatitis. A loud systolic heart murmur was detected and considered loudest at the right sternal edge. Femoral pulses were normal. Echocardiography demonstrated thickening of the aortic valve leaflets (Fig 2a), particularly the non-coronary cusp (Fig 2a and b). The peak velocity of blood flow through the aortic valve was increased (3.5 m/s, Fig 2c; Stepien & Bonagura 1991) and aortic insufficiency was detected using colour flow mapping. The echocardiographic diagnosis was vegetative endocarditis of the aortic valve resulting in aortic stenosis and insufficiency. Blood was collected aseptically for aerobic and anaerobic culture (Love 1979). Very small numbers of small Gram-negative coccobacilli were detected after an exhaustive search of stained smears of buffy coat preparations of peripheral blood. A pure growth of a Bartonella species was detected after 14 days by conventional blood culture (Love 1979) using brain heart infusion broth with subculture onto chocolate agar incubated in 5% carbon dioxide in air.
Fig 2.
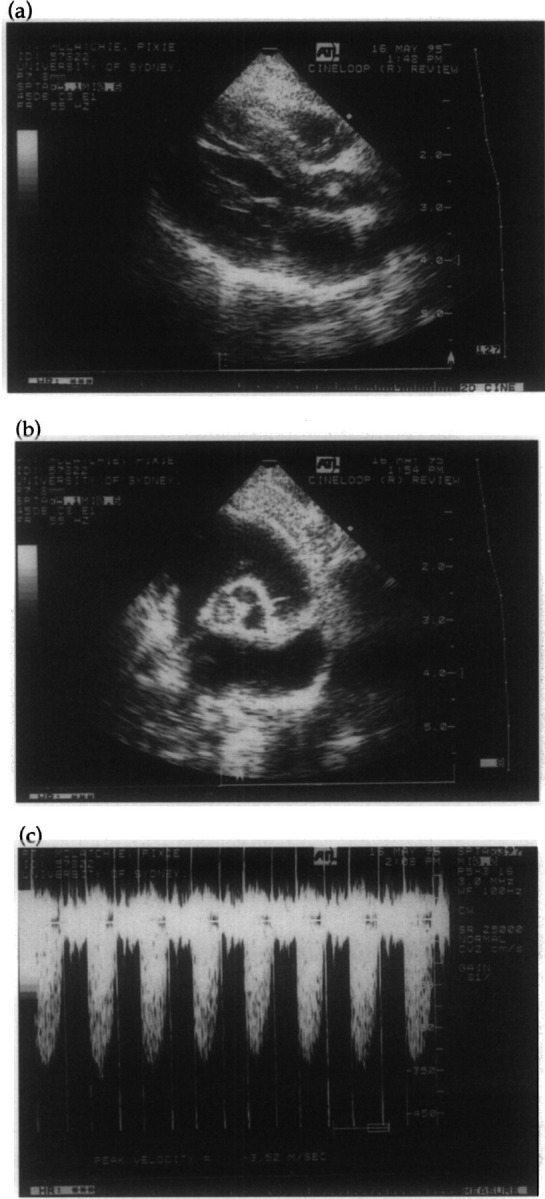
Two-dimensional (a) and (b) and spectral Doppler (c) echocardiograms of Case 2 taken the day after admission to the referring veterinarian. The right parasternal long axis view (a) demonstrates thickening of the aortic valve leaflets, especially the non-coronary cusp. The parasternal short axis view (b) demonstrates thickening of the non-coronary cusp of the aortic valve. Interrogation of the aortic valve from the left caudal parasternal window using continuous wave Doppler (c) demonstrates increased peak blood flow velocity (3.5 m/s) indicative of acquired aortic stenosis.
The cat was treated by the referring veterinarian with frusemide (2 mg by mouth every 12 h), enalopril (1.25 mg by mouth once a day), ciprofloxacin (125 mg by mouth every 12 h) and amoxycillin/clavulanic acid (100 mg by mouth every 12 h). On the day following echocardiography the cat was bright, alert and eating well. It was discharged 2 days later. One week after discharge it suddenly developed laboured breathing, became opisthotonic, collapsed and died. Permission for necropsy was denied.
Case 3
A 6-year-old castrated Persian cat (5.5 kg) was first seen by the referring veterinarian because of rapid shallow breathing. The cat improved following empirical treatment using doxycycline (dose unrecorded). Three months later the cat was represented with extreme dyspnoea. Rectal temperature (37.7°C) was normal. Thoracic radiographs showed a massive, globular cardiac silhouette (Fig 3a and b) and changes in the lung fields consistent with pulmonary oedema. Echocardiography demonstrated massive pericardial effusion, left atrial dilatation and changes erroneously attributed to hypertrophic cardiomyopathy. Laboratory examination of 150 ml of clear fluid drained from the pericardial sac suggested a transudate (protein 21 g/l, albumin 18 g/l, 2.2×199 cells/l [neutrophils, lymphocytes and macrophages]). The cat was subsequently treated using nasopharyngeal oxygen, cage rest, frusemide (10 mg every 8 h) and diltiazem (7.5 mg by mouth every 8 h). After 24 h the cat was much improved, and it was discharged on frusemide (4 mg by mouth every 8 h) and diltiazem. However, 12 days later the cat re-presented for recurrence of coughing and dyspnoea. Chest radiographs showed recurrence of the pericardial effusion and 200 ml of sanguineous fluid was drained from the pericardial sac before the cat was referred for further assessment.
Fig 3.
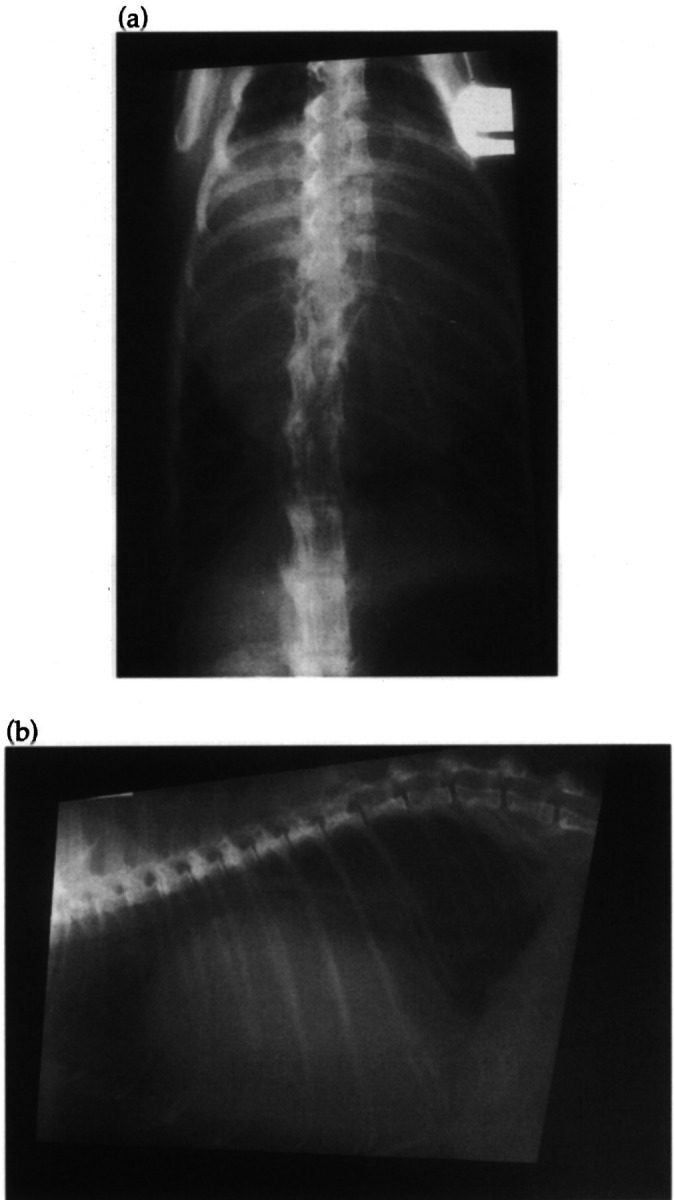
Dorsoventral (a) and lateral (b) thoracic radiographs of Case 3 at initial presentation. The cardiac silhouette is markedly enlarged and globular due to a massive pericardial effusion.
Physical finding included laboured breathing and muffled heart sounds. As preliminary echocardiographic examination demonstrated abundant pericardial fluid, the cat was sedated (15 mg ketamine and 2 mg midazolam subcutaneously), 200 ml of sanguineous pericardial fluid was removed using a 23 gauge butterfly needle and a detailed echocardiographic examination was performed. Findings included thickening of the mitral and aortic valve leaflets, particularly the right coronary cusp of the aortic valve (Fig 4a), left atrial dilatation (24 mm) and left ventricular dilation with normal fractional shortening. Continuous wave interrogation of the aortic valve demonstrated mild acquired aortic stenosis (peak velocity 1.9 m/s) and marked aortic insufficiency (Fig 4b). Blood (2 ml) was collected and prepared for aerobic and anaerobic blood culture using the lysis centrifugation method (Breitschwerdt et al 1995). The echocardiographic diagnosis was vegetative endocarditis of the mitral and aortic valves. A moderate pure growth (approximately 30 colonies per plate) of a Bartonella species was observed after 10 days growth on chocolate agar supplemented with vitamin K and haemin and cultured at 37°C in an atmosphere containing 5% carbon dioxide in air.
Fig 4.
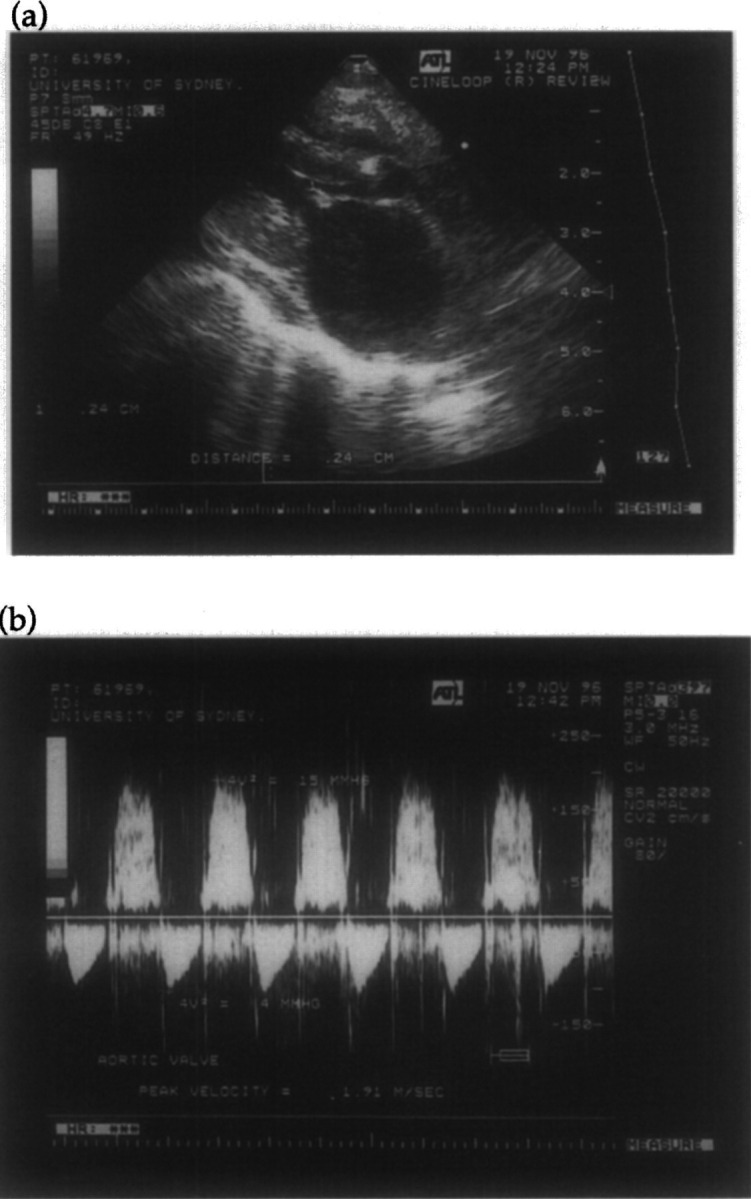
Two-dimensional (a) and spectral Doppler (b) echocardiograms of Case 3 at referral. The right parasternal long axis view (a) shows a small vegetative lesion on the anterior leaflet of the mitral valve, a larger vegetation on the right coronary cusp of the aortic valve and left atrial dilatation. Interrogation of the aortic valve from the left caudal parasternal window (b) using continuous wave Doppler demonstrates increased peak blood flow velocity (1.9 m/s), indicating mild aortic stenosis, and prominent aortic insufficiency.
The patient was discharged on enrofloxacin (50 mg by mouth every 24 h), amoxycillin/clavulanic acid (125 mg by mouth every 12 h), frusemide (5 mg by mouth every 24 h), digoxin (31.25 μg by mouth every 48 h) and enalopril (2.5 mg by mouth every 24 h). The cat was much improved on this regimen and the antimicrobials were discontinued after 3 months. Signs of congestive heart failure recurred 11 months after referral and the cat died a short time later. The heart was fixed in formalin and submitted by the referring veterinarian for examination. There was marked left atrial and auricular dilatation and thickening of the mitral valves although the aortic valve leaflets were grossly normal. Histologically, the thickened mitral valve leaflets were composed mostly of fibrous tissue, although in some areas there were large numbers of pigment-containing cells (probably siderophages). The aortic valve cusps had subintimal thickening. Myocardial pathology was variable ranging from areas of normal tissue to sections showing regions of interstitial fibrosis or moderately sized focal accumulations of lymphocytes, usually in a perivascular distribution. These findings suggested there was no residual infection of the valves or myocardium, although scarring and chronic inflammation were still in evidence.
Case 4
An 8-year-old castrated DSH was presented to an emergency veterinary service because of rapid and laboured breathing. The cat had been dyspnoeic for 48 h and anorexic for 24 h. The cat was afebrile (37.5°C), had a systolic heart murmur audible on auscultation of both sides of the chest and a respiratory rate of 88 breaths/min. Thoracic radiographs demonstrated changes consistent with pulmonary congestion and oedema. Frusemide (12.5 mg intravenously) and Hartmann's solution (150 ml subcutaneously) were administered. The cat improved somewhat overnight.
Thoracic radiographs taken at the regular veterinary practice the following day demonstrated generalised cardiomegaly and pulmonary oedema. Serum biochemistries were largely unremarkable, while haematology revealed a segmented neutrophilia (21.3×109/l). Echocardiography demonstrated a large echogenic mass on an aortic valve leaflet and possibly another on a mitral valve leaflet. Both the left atrium and left ventricle were enlarged, consistent with volume overload. The echocardiographic diagnosis was vegetative endocarditis. The cat was subsequently treated using frusemide (10 mg by mouth every 24 h), enrofloxacin (25 mg by mouth every 24 h) and enalopril (1.25 mg by mouth every 24 h), and improved markedly on this regimen.
Although the cat was still doing very well, echocardiography was repeated at the UVC 4 months after the cat had first been diagnosed with vegetative endocarditis. An echogenic vegetative lesion on the right coronary cusp of the aortic valve was still evident (Fig 5), as was left atrial dilatation (24 mm diameter) and left ventricular dilation with a normal shortening fraction. One month later the owners reported that the cat's breathing had become noisy during the evenings and the cat was unexpectedly found dead 10 days after that. Permission for necropsy was denied.
Fig 5.
Two-dimensional echocardiogram of Case 4 recorded at the UVC 4 months after initial presentation. A highly echogenic vegetation is visible on the right coronary cusp of the aortic valve in this right parasternal long axis view optimised for the left ventricular outflow tract. Marked left atrial dilatation is evident also.
Case 5
A 6-year-old castrated DSH cat was presented initially for a systemic illness that developed 10 days following a cat fight. Some physical findings, namely tachycardia (heart rate >200 beats/min), a pulse deficit and prominent jugular pulses, suggested cardiac involvement. Haematology showed a segmented neutrophilia (18.6×109/l), serum biochemistries were unremarkable and tests for feline leukaemia virus antigen and feline immunodeficiency virus antibody were negative. Offers of referral for echocardiography were declined by the owners who elected to try treatment with intravenous fluids and amoxycillin/clavulanic acid, which appeared to be successful.
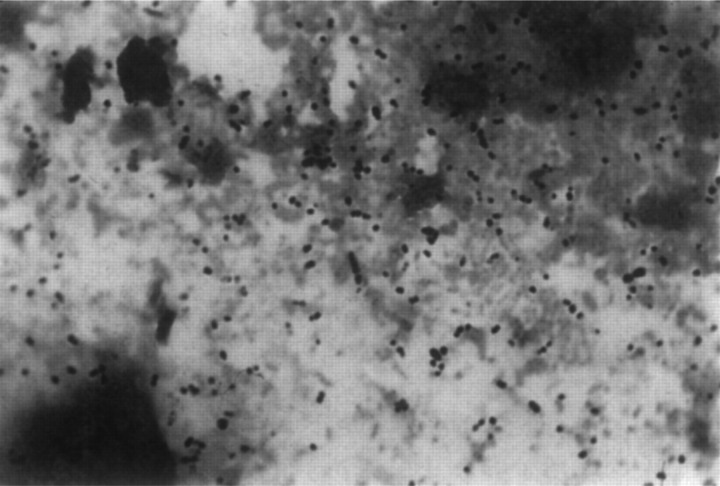
Six months later the cat was presented for collapse following a 5-day period of inappetence progressing to anorexia. The oral mucosae were pale and the cat was referred for further investigation. On admission to the UVC, the cat was extremely depressed and had borderline hypothermia (37.5°C). A systolic heart murmur, loudest over the left ventricular apex, was detected and the oral mucous membranes were ash white. Haematology showed a regenerative anaemia (haematocrit 0.12 l/l, haemoglobin 30 g/l, uncorrected reticulocyte count 11%, eight nucleated red blood cells per 100 leukocytes), and a marked inflammatory leukogram (segmented neutrophil count 84×109/l, band neutrophils 0.91×109/l, monocytes 1.82×109/l), thrombocytopenia and absolute eosinopenia. Intracellular cocci were seen within some (approximately one in 800) neutrophils (Fig 6). The plasma was moderately icteric and several serum biochemical abnormalities were detected including mild azotaemia (urea 18 mmol/l with a normal creatinine concentration) and a modest elevation in ALT activity (237 U/l, reference range <60).
Fig 6.
Diff Quik stained peripheral blood smear collected from Case 5 on the day of referral. Two intracellular cocci can be seen within the central cytoplasm of the lowermost neutrophil in the centre of the microscopic field. Original magnification ×754.
Echocardiography showed severe vegetative endocarditis affecting the aortic valve, mitral valve and the mural surface of the left ventricular endocardium (Fig 7). Despite blood transfusions, intravenous fluids, parenteral antibiotics (amoxycillin/clavulanic acid and enrofloxacin), frusemide, benazopril, digoxin and nutritional support, red blood cell destruction continued and the cat developed intractable pulmonary oedema. It was put down 7 days after admission. Necropsy confirmed bacterial endocarditis affecting principally the mitral valve and adjacent portions of ventricular endocardium. A smaller vegetative lesion was adherent to the aortic valve via a flimsy fibrin tag (Fig 8). Squash preparations of an affected mitral valve leaflet demonstrated large numbers of Gram-positive cocci (Fig 9), some in chains.
Fig 7.
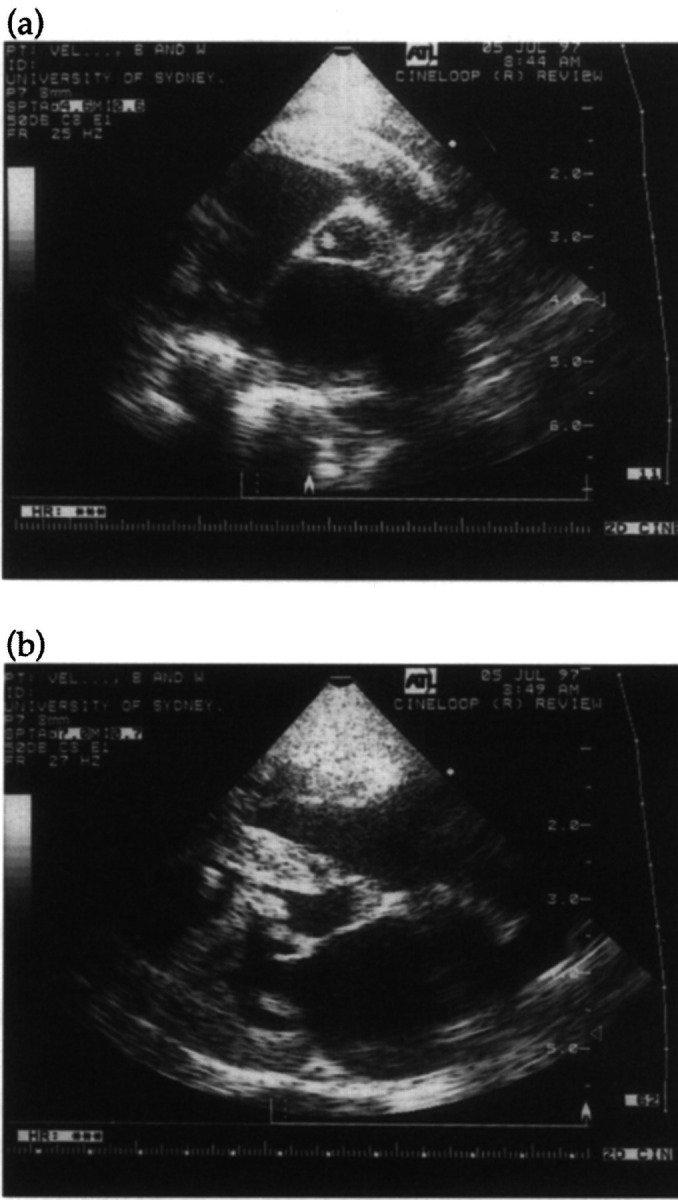
Two-dimensional echocardiograms of Case 5 taken on the day following referral. This right parasternal short axis view (a) is slightly skewed but demonstrates a small vegetation on the non-coronary cusp of the aortic valve and marked left atrial dilatation. The right parasternal long axis view (b) demonstrates thickening of both leaflets of the mitral valve, and vegetations attached to the left ventricular endocardial surface. In real time, these endocardial vegetations moved ‘like a curtain’ within the left ventricular lumen.
Fig 8.
Photographs of the heart of Case 5 obtained at necropsy. Note the small vegetation (corresponding to the one evident in Figure 7(a) attached to an aortic valve leaflet.
Fig 9.
Burke's Gram stain of a squash preparation from an aortic valve vegetation collected from Case 5 at necropsy. Note the enormous number of Gram-positive cocci, some of which form chains. Original magnification ×754.
Case 6
A 6-year-old spayed Tonkinese cat was presented with multisystem disease. The cat had been missing for 7 days, and was very unwell when it returned home. The cat was ataxic with signs of right-sided vestibular disease, and it had an abscess on its left thoracic limb. It was afebrile (38.2°C) but severely dehydrated. Blood was collected for laboratory analyses and the cat treated with intravenous fluids and amoxycillin (dose unrecorded). Apart from mild hypokalaemia, serum biochemistry was non-contributory. Haematology demonstrated non-regenerative anaemia (haematocrit 0.17 l/l, haemoglobin 62 g/l, reticulocyte count 0.34%), neutrophilia (22.7×109/l) with a left shift (band neutrophils 1.32×109/l), monocytosis (2.9×109/l) and absolute eosinopenia, suggesting both inflammation and stress. Antimicrobial therapy was changed to chloramphenicol (250 mg by mouth every 12 h), but despite intravenous fluid therapy and nutritional support the cat did not improve and it was referred to the UVC after 2 days.
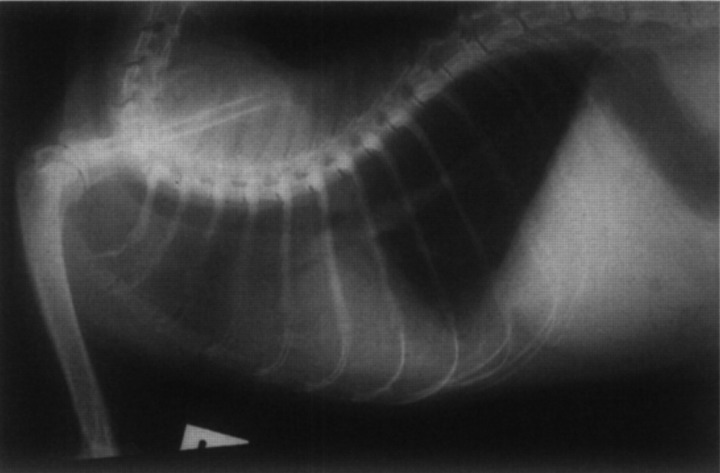
Physical findings included emaciation (2.8 kg), hypothermia (36.6×C), bilateral nasal discharge, dyspnoea and polypnoea (60 breaths/min), pale oral mucosa, bilaterally dilated pupils, head tilt, nystagmus and a tendency to roll to the right. A detailed neurological examination demonstrated the cat had right vestibular disease and a left menace deficit. It was not possible to discern whether the cat had central or peripheral vestibular involvement. Chest radiographs demonstrated a severe alveolar pattern in both portions of the left cranial lung lobe, most consistent with pneumonia (Fig 10). Needle aspirates from consolidated lung showed suppurative inflammation. Our assessment was that the cat had bacterial pneumonia and a middle ear infection or central nervous system disease. The cat continued to deteriorate despite aggressive treatment using antibiotics (intravenous benzyl penicillin, amoxycillin/clavulanic acid intramuscularly, clindamycin orally), intravenous fluids and nutritional support and it died 4 days after referral. At necropsy the most significant gross finding was the presence of numerous nodular vegetations on the tricuspid valve leaflets. Ascites, hydrothorax, hydropericardium and congestion of the liver and splanchnic veins were evident also, reflecting backward right-sided congestive heart failure presumably secondary to tricuspid regurgitation. Histologically, there was severe ulcerative endocarditis of the tricuspid valve leaflets associated with many Gram-positive cocci. The brain was grossly and microscopically normal. The middle ear was not inspected.
Fig 10.
Lateral thoracic radiograph of Case 6 on the day of referral. Note the severe alveolar pattern evident within both portions of the left cranial lung lobe.
Discussion
The echocardiographic findings in the first five cases were virtually pathognomonic for vegetative bacterial endocarditis, as no other disease process affecting the valvular tissues is likely to give rise to such asymmetric echogenic lesions (Lombard & Buergelt 1983, Sission & Thomas 1984). However, to make a definitive diagnosis of bacterial endocarditis, positive blood culture or histological evidence of valve infection at necropsy is required, for other conditions such as congenital valvular anomalies, excessive moderator bands or calcified thrombi could conceivably give rise to lesions with a similar echocardiographic appearance. The first four cats were presented principally for signs of congestive heart failure referable to volume overload of the left side of the heart secondary to aortic and, or, mitral insufficiency, and had been systemically well until immediately prior to presentation. In none of these four patients was fever, an inflammatory leukogram, or other clinical signs of systemic bacterial infection prominent from the history or physical findings. The only evidence of systemic embolization was the asymmetrical femoral arterial pulses encountered in Case 1. A Bartonella species was isolated in pure growth from Cases 2 and 3, and we suspect the chronic, indolent clinical course of bacterial endocarditis in these two cats and possibly also in Cases 1 and 4 was compatible with such a bacterial aetiology. Bartonella henselae has been reported to have a tropism for cardiac valves and Bartonella endocarditis is increasingly well documented in the human literature as a cause of ‘culture negative’ bacterial endocarditis (Raoult et al 1996). Because of the high prevalence of Bartonella bacteraemia in ‘normal’ cats (Chomel et al 1995, Breitschwerdt & Kordick 1995, Breitschwerdt 1995), the only unequivocal way to confirm a diagnosis of Bartonella endocarditis is by demonstrating characteristic organisms in valvular lesions using special stains, culture or the polymerase chain reaction (Breitschwerdt 1995, Breitschwerdt et al 1995). Thus, in a veterinary setting, definitive diagnosis of Bartonella endocarditis is only possible at necropsy.
It is noteworthy that cardiac vegetations had mineralised by the time cats were presented for veterinary attention in Case 1 (Malik et al 1992) and another case reported previously (Noakes 1988). As dystrophic calcification of chronic inflammatory lesions probably takes a considerable time to develop, this feature suggests that a chronic disease process with an organism of relatively low pathogenicity such as a Bartonella species (Breitschwerdt 1995, Kordick & Breitschwerdt 1997), was responsible for the infection in these cats. In contrast, cats with vegetative endocarditis caused by more pathogenic species, such as E coli, Streptococcus spp or S aureus, which produce virulence factors that can produce disease in various parts of the body, are unlikely to live sufficiently long to develop calcified valve leaflets in the absence of appropriate antimicrobial therapy (Shouse & Meier 1956, Yamaguchi et al 1983, Goodwin & Miller 1997, De Jonghe et al 1988). We have observed calcification of endocarditis lesions in canine patients where antimicrobial therapy had sterilised the lesions, but not prevented irreparable valvular damage that eventually resulted in intractable congestive heart failure (Malik R, Church DB and Hunt GB, unpublished observations).
Cases 5 and 6 presented with more acute signs than the other patients, comparable to that seen typically in canine and human patients with acute or subacute bacterial endocarditis with pyogenic organisms. Both cases had recent histories of cat fight abscesses and Gram-positive cocci were documented in each patient. Case 6 developed subacute disease, while the history of Case 5 was more compatible with an acute on chronic disease process.
Case 5 presented with signs of severe systemic disease, with accelerated red blood cell destruction, prehepatic jaundice, marked neutrophilia with left shift and a bacteraemia. These changes were likely a consequence of sepsis, and it is conceivable that a streptococcal haemolysin, low grade disseminated intravascular coagulation and, or, turbulence within the left ventricle associated with the extensive vegetative lesions contributed to the severe on-going erythrocyte destruction observed in this cat. The presence of a cardiac murmur drew our attention to the heart as being involved in the disease process.
Case 6 was presented for signs of multisystem disease, but in contrast to the first five patients, physical findings suggestive of bacterial endocarditis were absent. Although the cat was treated on the basis of having a disseminated bacterial infection affecting the upper and lower respiratory tract, subcutaneous tissues, middle ear and, or, central nervous system, we did not suspect bacterial endocarditis because the murmur of tricuspid regurgitation was never detected. The difficulty in appreciating tricuspid insufficiency murmurs in the cat has recently been emphasised (Thomas 1998).
Unfortunately the long-term outlook for cats with vegetative endocarditis in the present series was generally poor, with only two cats surviving for a reasonable length of time. In the first four cats this largely reflected the existence of irreversible, haemodynamically important cardiac pathology at the time the animals were presented for echocardiography and death was most likely due to cardiac decompensation rather than sepsis. In Cases 5 and 6, septicaemia and the effects of disseminated pyogenic disease combined with congestive heart failure to contribute to the cats' demises. The poor prognosis in cats with vegetative endocarditis is in accord with what has been reported previously for dogs, particularly when there is aortic valve involvement (Lombard & Buergelt 1983, Elwood et al 1993, Sisson & Thomas 1984). However, not all affected cats have a poor prognosis and a recent report demonstrated a favourable response to treatment in a cat with a small vegetative lesion on the aortic valve (Caney 1998).
There are probably insufficient cats in the present series, and indeed in the published literature, to make generalisations concerning the signalment and physical findings in cats with bacterial endocarditis. The present series consisted mainly of neutered young to middle aged adult crossbred cats of both sexes. Unlike dogs, males were not overrepresented. In two cats the development of bacterial endocarditis may have been a sequela of infected cat bite injuries. There is some suggestion from our report and previous studies (Shouse & Meier 1956, Yamaguchi et al 1983, Noakes 1988, Liu 1988, Bonagura 1989, Malik et al 1992, Goodwin & Miller 1997, Caney 1998, De Jonghe et al 1998) that the aortic valve is affected most commonly, although the mitral valve was also frequently involved. In this respect the cat is similar to both humans and dogs (Calvert 1982, Lombard & Buergelt 1983, Calvert et al 1985, Sisson & Thomas 1984, Kasari & Roussel 1989, Elwood et al 1993). Pre-existing congenital heart disease or myocardial disease was not identified historically, echocardiographically or at necropsy in any of our patients. A recent research abstract from the UK reported briefly on findings concerning six cats with valvular endocarditis: five of the cats were purebreds (including four Siamese), males were overrepresented (four cats), and either the aortic valve (four cats) or mitral valve (two cats) were infected. Furthermore there was a high prevalence of concurrent systemic disease, such as feline infectious peritonitis, lymphoma, mammary carcinoma and megacolon (Caney et al 1997).
Interestingly, clinical features of aortic insufficiency (bounding arterial pulses and diastolic murmurs) were usually not appreciated in our cases, even with the benefit of hindsight after echocardiography revealed aortic valvular lesions. The echocardiographic diagnosis of bacterial endocarditis in cats is generally more straightforward than in canine patients, as endocardiosis is not an entity in the cat and normally myocardial diseases rather than valvular disease are prominent in the differential diagnosis of cats with murmurs, arrhythmias and gallop rhythms. However, a diagnosis of bacterial endocarditis was not even considered antemortem in the last case, presumably because the murmur of tricuspid regurgitation was too soft to be detected during auscultation. This emphasises that bacterial endocarditis should be considered in the differential diagnosis of cats with severe systemic disease even when a murmur is absent.
We are loath to make any recommendations concerning diagnosis and therapy based on this series, which consisted mainly of cases referred specifically for echocardiography, rather than for ongoing management. The isolation of a Bartonella species in pure culture from two of the cats subjected to blood culture suggests that special methods that favour isolation of this pathogen should be utilised as part of a microbiology evaluation in cats with vegetative lesions. The current recommendation for treating bacterial endocarditis in dogs and cats using a β-lactam antibiotic (penicillin, amoxycillin or a first generation cephalosporin) combined with an aminoglycoside (gentamicin or amikacin) or fluoroquinolone (Goodwin & Miller 1997) is likely to be successful in eradicating organisms so far reported to cause bacterial endocarditis in cats, such as Bartonella spp, E coli, Streptococcus spp and Staphylococcus spp, although less commonly used antibiotics such as doxycycline and azithromycin may have a place in long-term follow-up therapy, for example, in cats with bartonellosis. Ideally, antibiotics should be administered intravenously for the first part of therapy, although this is not always practicable, and therapy should be continued for at least 6–8 weeks. In the present series, the best outcomes were obtained in Cases 3 and 4, which had the benefit of antimicrobial therapy and also responded to symptomatic management of their congestive heart failure.
Acknowledgements
Richard Malik is supported by the Valentine Charlton Bequest administered by the Post Graduate Foundation in Veterinary Science of The University of Sydney. The authors wish to thank George Kaplun, Harry Pisk, Caryn Wun and Simon Whitaker for referring cases and permitting use of their case records and diagnostic images. Malcolm France and Terry Rothwell kindly provided the histological descriptions of Cases 3 and 6, respectively.
References
- Bonagura JD. (1989) Cardiology. In The cat: diseases and clinical management, Scherding RG, (ed.) New York, Churchill Livingstone, pp. 730–731. [Google Scholar]
- Breitschwerdt EB. (1995) Bartonellosis and culture negative endocarditis. Proceedings of the American College of Veterinary Internal Medicine Forum pp. 311–313.
- Breitschwerdt EB, Kordick DL. (1995) Bartonellosis. Journal of the American Veterinary Medical Association 206, 1928–1931. [PubMed] [Google Scholar]
- Breitschwerdt EB, Kordick DL, Malarkey DE, et al. (1995) Endocarditis in a dog due to infection with a novel Bartonella subspecies. Journal of Clinical Microbiology 33, 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert CA, Greene CE, Hardie EM. (1985) Cardiovascular infections in dogs: Epizootiology, clinical manifestations, and prognosis. Journal of the American Veterinary Medical Association 187, 612–616. [PubMed] [Google Scholar]
- Calvert CA. (1982) Valvular bacterial endocarditis in the dog. Journal of the American Veterinary Medical Association 180, 1080–1084. [PubMed] [Google Scholar]
- Caney S. (1998) Aortic valvular endocarditis in a Siamese cat. Feline Advisory Bureau Journal 36, 60–63. [Google Scholar]
- Caney SM, Wotton PR, Sparkes AH, et al. (1997) Valvular endocarditis in six cats. Proceedings of the American College of Veterinary Internal Medicine Forum, Lake Buena Vista, Florida p. 689.
- Chomel BB, Abbot RC, Kasten RW, et al. (1995) Bartonella henselae prevalence in domestic cats in California: Risk factors and association between bacteremia and antibody titers. Journal of Clinical Microbiology 33, 2445–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonghe S, Ducatelle R, Devriese LA, et al. (1998) Verrucous endocarditis due to Escherichia coli in a Persian cat. Veterinary Record 143, 305–307. [DOI] [PubMed] [Google Scholar]
- Elwood CM, Cobb MA, Stepien RL. (1993) Clinical and echocardiographic findings in 10 dogs with vegetative bacterial endocarditis. Journal of Small Animal Practice 34, 420x–427. [Google Scholar]
- Goodwin J-K, Miller MW. (1997) Infective endocarditis. In Consultations in Feline Internal Medicine 3, August JR. (ed.), Philadelphia: WB Saunders, pp. 273–278. [Google Scholar]
- Kasari TR, Roussel AJ. (1989) Bacterial endocarditis. Part 1. Pathophysiologic, diagnostic and therapeutic considerations. Compendium on Continuing Education for the Practising Veterinarian 11, 655–659. [Google Scholar]
- Kordick DL, Breitschwerdt EB. (1997) Relapsing bacteremia after blood transmission of Bartonella henselae to cats. American Journal of Veterinary Research 58, 492–497. [PubMed] [Google Scholar]
- Liu SK. (1988) Cardiovascular pathology. In Canine and Feline Cardiology, Fox PR. (ed.), New York: Churchill Livingstone, p. 650. [Google Scholar]
- Lombard CW, Buergelt CD. (1983) Vegetative bacterial endocarditis in dogs: Echocardiographic diagnosis and clinical signs. Journal of Small Animal Practice 24, 325–339. [Google Scholar]
- Love DN. (1979) Blood culture—an aid to diagnosis in equine practice. Australian Veterinary Practitioner 9, 109–111. [Google Scholar]
- Malik R, Church D, Allan GS, Hunt GB. (1992) What is your diagnosis? Journal of the American Veterinary Medical Association 200, 1391–1393. [PubMed] [Google Scholar]
- Noakes ME. (1988) What is your diagnosis? Journal of the American Veterinary Medical Association 193, 719–721. [Google Scholar]
- Raoult D, Fournier PE, Drancourt M, et al. (1996) Diagnosis of 22 New Cases of Bartonella Endocarditis. Annals of Internal Medicine 125, 646–652. [DOI] [PubMed] [Google Scholar]
- Sisson DD, Knight DH, Helinski C, et al. (1991) Plasma taurine concentrations and M-mode echocardiographic measures in healthy cats and cats with dilated cardiomyopathy. Journal of Veterinary Internal Medicine 5, 232–238. [DOI] [PubMed] [Google Scholar]
- Sisson D, Thomas WP. (1984) Endocarditis of the aortic valve in the dog. Journal of the American Veterinary Medical Association, 184, 570–577. [PubMed] [Google Scholar]
- Shouse CL, Meier H. (1956) Acute vegetative endocarditis in the dog and cat. Journal of the American Veterinary Medical Association 129, 278–289. [PubMed] [Google Scholar]
- Stepien RL, Bonagura JD. (1991) Aortic stenosis: Clinical findings in six cats. Journal of Small Animal Practice 32, 341–350. [Google Scholar]
- Thomas WP. (1998) ‘Silent’ valvular disease in dogs and cats. Proceedings of the American College of Veterinary Internal Medicine Forum, San Diego, CA, p. 90.
- Yamaguchi RA, Pipers FS, Gamble DA. (1983) Echocardiographic evaluation of a cat with bacterial vegetative endocarditis. Journal of the American Veterinary Medical Association 183, 118–120. [PubMed] [Google Scholar]