Abstract
This study was conducted to describe the ultrasonographic appearance and technique for the evaluation of sciatic nerve (ScN) in cats. An anatomical study was carried out using six feline cadavers to determine anatomic landmarks. An ultrasonographic ‘in vitro’ study was performed using eight pelvic limbs from four fresh feline cadavers to assess the ScN in three regions (glutea, femoralis and popliteal) using a 4–13 MHz linear transducer. Five healthy adult experimental cats were employed for an ‘in vivo’ ultrasonographic study of the ScN using the same protocol described for the ‘in vitro’ study. The ultrasonographic images of the ScN were well correlated with those obtained in the anatomical study. The ScN was easily identified in all the approaches. The ScN was observed as a hypoechoic structure with internal echoes, outlined by hyperechoic lines. This study shows the usefulness of ultrasound to evaluate the entire pathology of the ScN in the cat.
The sciatic nerve (ScN) is the largest peripheral nerve of the body. It originates in the truncus lumbosacralis and is composed of the peroneus communis and tibialis nerves. 1 It is the most frequently injured nerve in the lower extremity. Injuries can be the result of pelvic, sacral and femoral fractures which may cause partial lacerations or complete transections of the ScN. Iatrogenic lesions are the result of intramuscular injections which may produce a direct traumatic injury or a neurotoxic injury due to the injected product. 1 Inadverted transections or compressions of the ScN can also be produced during the surgical repair of pelvic and femoral fractures as well as during perineal herniorrhaphies. 1 Primary tumours of peripheral nerves such as neurofibromas, schwannomas and lymphoma are infrequent but should also be considered in the differential diagnosis of peripheral neuropathies. 2
Clinical evaluation remains the mainstay in the diagnosis of disorders affecting peripheral nerves. In most patients, the clinical history and physical examination are helpful to orientate the diagnosis. Imaging techniques such as magnetic resonance imaging (MRI) and ultrasound (US) can be useful to detect the injured nerve providing information on its size, internal structure and vascularity. Standard radiographs and computed tomography are also useful to visualise structures adjacent to the injured nerve. 3
In recent years, the new development of high resolution, electronic broadband transducers has allowed US to become an optimal imaging technique to evaluate peripheral nerves in humans. 4 The advantages of US in this field are: the ability to realise a dynamic examination; it is a non-invasive and low cost technique; a quick, detailed image of the entire length of the major peripheral nerves can be obtained. 3,5 To the authors’ knowledge, information regarding the appearance and ultrasonographic approaches of peripheral nerves in the cat has not been documented. The aim of the present study was to determine the ultrasonographic appearance and approaches to assess the ScN in cats.
Material and methods
Animals
The project was approved by the Animal Care and Ethics Committee of the University of Murcia. It was carried out in three phases. In the first phase 12 pelvic limbs from six fresh feline cadavers with a mean weight of 4.6 kg (range 3.5–4.8 kg) were used for an anatomic study. In the second phase, eight pelvic limbs from four fresh feline cadavers were used for an ‘in vitro’ ultrasonographic study. These cats were adults (seven males and three females) with a mean weight of 3.8 kg (range 2.8–4.9 kg). The cats were obtained from the Local Zoonoses and Public Health Service and were humanely euthanased for reasons unrelated to pelvic limb lameness. In the third phase of the study, five healthy adult experimental cats (males) with a mean weight 3.8 kg (range 3.15–4.3 kg) were used to perform an ‘in vivo’ ultrasonographic study. The animals did not present signs of musculoskeletal or neurological disorders related to the pelvic limb and were handled following the guidelines for humane care of experimental animals.
Phase I: anatomic study
Gross dissection of the ScN
Eight limbs from four feline cadavers were used. The wings of the os ilium, trochanter major of the os femoris, tuber ischiadicum, and the caudal margin of the biceps femoris muscle were the landmarks employed to dissect the ScN. The skin of the lateral aspect of the pelvic limb was reflected. Then, the gluteous superficialis, gluteous medius and biceps femoris muscles were elevated from their insertion and the ScN identified (Fig 1).
Fig 1.
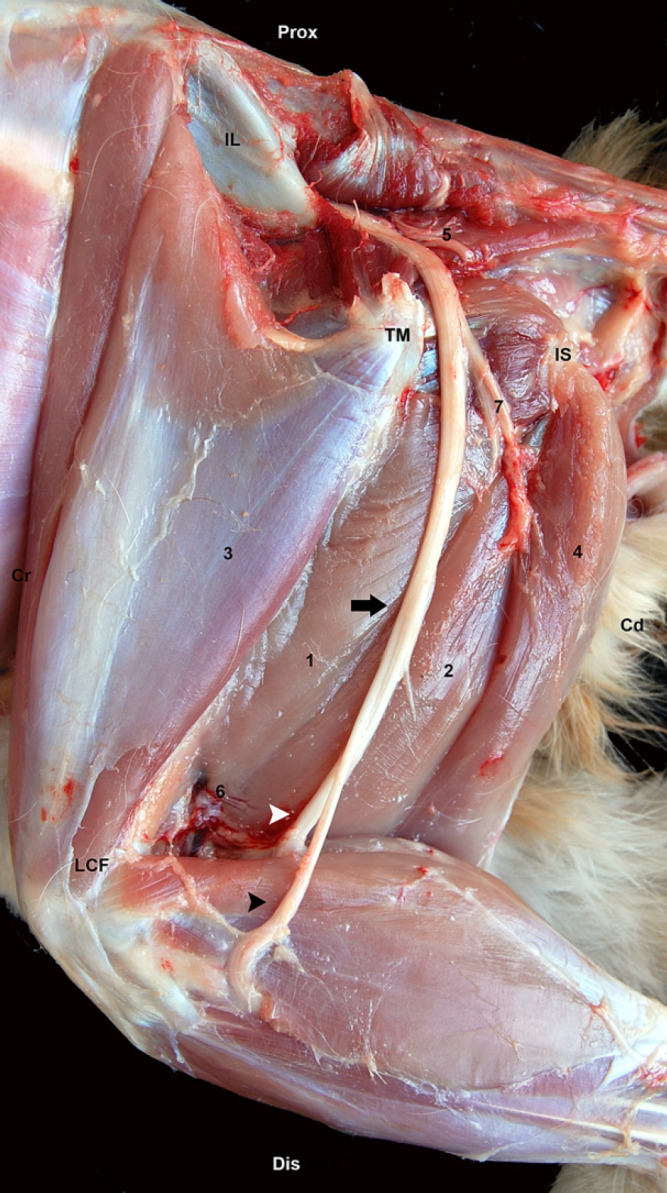
Gross anatomy of the ScN at the lateral aspect of the thigh. Biceps femoris, gluteous superficialis, medious, profundus and gluteous femoralis muscles are reflected. Os ilium (IL), trochanter major of os femoris (TM), tuber ischiaticum (IS). ScN (black arrow), peroneous communis nerve (black arrow head), tibialis nerve (white arrow head), (1) adductor magnus muscle, (2) semimembranosus muscle, (3) vastus lateralis muscle (4) semitendinosus muscle, (5) gluteal caudalis artery and vein, (6) caudalis femoris media artery and vein, (7) rami musculares. Prox=proximal; Dis=distal; Cr=cranial; Cd=caudal.
Vascular injection and section
Red coloured latex was introduced through the thoracic aorta in two fresh feline cadavers. The cats were then frozen at −20°C for 24 h and then moved at −80°C for another 24 h. Later, transverse cryosections were made of the pelvic limbs using a high-speed band saw at a thickness of 0.5 cm from the sacrum to the stifle. Photographs of each slide were taken to facilitate the interpretation.
Phase II: ultrasonographic study in vitro
Ultrasonographic scans of ScN were carried out on eight limbs from four fresh cadavers. The US scans were performed immediately after euthanasia. The cadavers were positioned on lateral recumbency. The hair was clipped from the sacroiliac region to just below the stifle. The skin was cleaned and coupling gel applied. The limbs were scanned using a 4–13 MHz linear transducer (MyLab 70, Esaote, Barcelona, Spain).
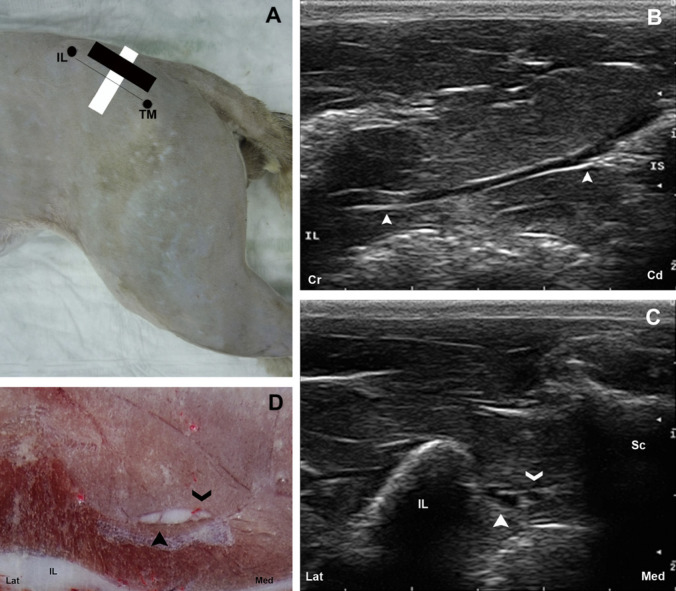
The ScN was imaged from its origin on the truncus lumbosacralis to the point where the peroneous communis and tibialis nerves diverge near the stifle. The ScN was divided in three regions: glutea, femoris and poplitea to standardise the examinations. The glutea zone was divided in two parts: cranialis and caudalis. The landmarks employed to approach the gluteal cranialis zone were the ala ossis ilii and the trochanter major. A line was drawn between these landmarks using a skin marking pen. The transducer was positioned parallel to this line and directed dorsally to obtain a longitudinal scan of the ScN (Fig 2A, B). Transverse images of the nerve were obtained by rotating the probe 90° (Fig 2A, C) and compared with the corresponding transverse cryosections (Fig 2D). The trochanter major and the tuber ischiaticum were used as landmarks to approach the caudalis part of the glutea area. The transducer was positioned between these points with its orientation marker directed dorsally (Fig 3A) to obtain the longitudinal view (Fig 3B). Transverse images of the zone were obtained by rotating the transducer 90° anticlockwise (Fig 3A, C) and compared with the corresponding transverse cryosections (Fig 3D).
Fig 2.
(A) The anatomic landmarks for the glutea cranial approach are outlined by black dots. The position of the transducer is represented by a black rectangle for the longitudinal and a white rectangle for the transversal approaches. (B) Longitudinal US image of the ScN (arrow heads). (C) Transverse US image of the ScN (full arrow head). Another small oval structure with the same appearance corresponding to the gluteous caudalis nerve is observed (open arrow head). (D) Cross-sectional anatomical image of the ScN (full arrow head). The gluteous caudalis nerve, a branch from the truncus lumbosacralis (open arrow head) is visible. IL=os ilium; TM=trochanter major of os femoris; IS=tuber ischiadicum; Sc=os sacrum; Lat=lateral; Med=medial; Cr=cranial; Cd=caudal.
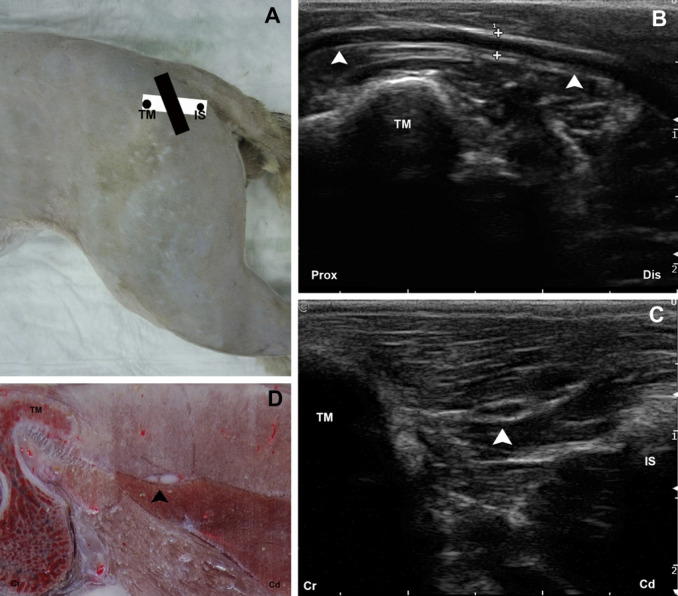
Fig 3.
(A) The anatomic landmarks for the glutea caudal approach are outlined by black dots. The position of the transducer is represented by a black rectangle for the longitudinal and a white rectangle for the transversal approaches. (B) Longitudinal US image of the ScN (arrow heads). (C) Transverse US image of the ScN (arrow head). (D) Cross-sectional anatomical image of the ScN (arrow head). TM=trochanter major of os femoris; IS=tuber ischiaticum; Prox=proximal; Dis=distal; Cr=cranial; Cd=caudal.
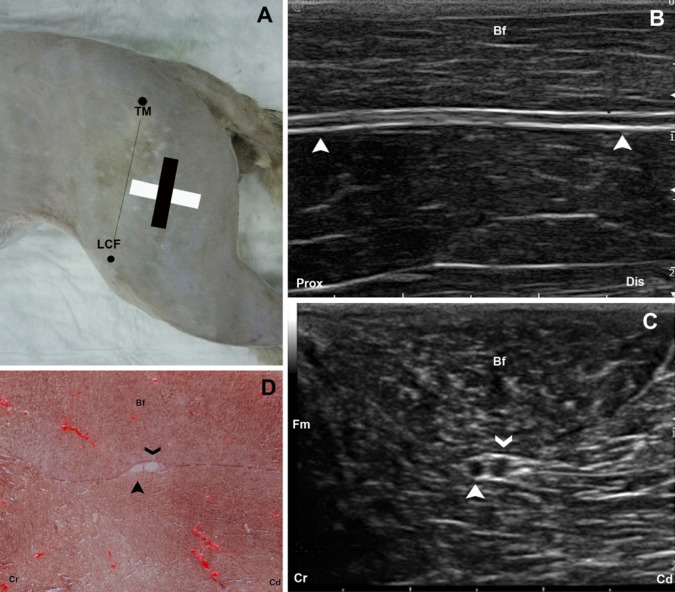
The femoris area was approached from the trochanter major to the lateral condyle of the os femoris. The transducer was positioned parallel to the femur at the middle of the thigh (Fig 4A) and longitudinal images were obtained (Fig 4B). Transverse images were obtained rotating the transducer 90° clockwise plane (Figs 4A, C) and compared with the corresponding transverse cryosections (Fig 4D).
Fig 4.
(A) The anatomic landmarks for the femoral window are outlined by black dots. The position of the transducer is represented by a black rectangle for the longitudinal and a white rectangle for the transversal approaches. (B) Longitudinal US image of the ScN (arrow heads). (C) Transverse US image of the ScN. The smaller and more cranial one, representing peroneous communis nerve (full arrow head) and the larger and more caudal the tibialis nerve (open arrow head). (D) Cross-sectional anatomical image of the ScN at this level. The peroneous communis (full arrow head) and tibialis nerve (open arrow head) components of the ScN are observed. TM=trochanter major of os femoris; LCF=condylus lateralis of os femoris (LCF); Bf=biceps femoris muscle; Prox=proximal; Dis=distal; Cr=cranial; Cd=caudal.
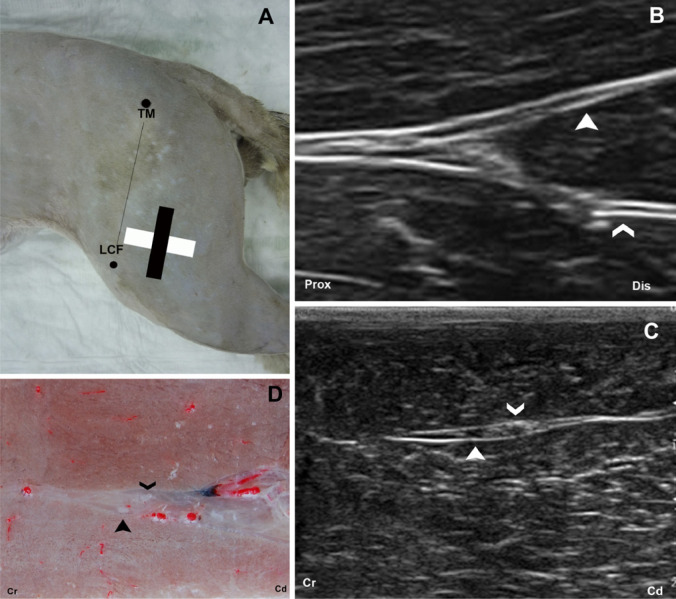
The poplitea approach was obtained at the os femoris distal epifisis in the area where the ScN is divided. The transducer was positioned on the femoris distal epifisis in a longitudinal (Fig 5A, B) and transverse planes (Fig 5A, C) and then moved towards the area where the division of the nerve takes place. The ultrasonographic images were compared with the corresponding transverse cryosections (Fig 5D).
Fig 5.
(A) The anatomic landmarks for the poplitea window are outlined by black dots. The position of the transducer is represented by a black rectangle for the longitudinal and a white rectangle for the transversal approaches. (B) Longitudinal US image of the ScN. The peroneous communis (full arrow head) and the tibialis nerve (open arrow head). (C) Transverse US image and (D) Cross-sectional anatomical image of the ScN at popliteal level. The two components of the ScN, the peroneous communis (full arrow head) located more cranial and tibialis nerve (open arrow head) more caudal. TM=trochanter major of os femoris; LCF=condylus lateralis of os femoris; Prox=proximal; Dis=distal; Cr=cranial; Cd=caudal.
The appearance, echogenicity and echotexture of the ScN and its surrounding structures were recorded. In transverse plane images, the orientation marker of the transducer was positioned cranially. In longitudinal plane images the marker was placed proximally.
The structure identified as the ScN was injected with 0.3 ml/kg of blue ink under US guidance to demonstrate the accuracy of the US nerve location. The needle insertion sites were marked on the skin and photographed to serve as reference points for the identification of the nerve. These limbs were immediately dissected and the ScN evaluated. The presence of blue ink staining the targeted nerve confirmed its accurate location.
Phase III: ultrasonographic examination ‘in vivo’
The ultrasonographic evaluation of the ScN was carried out on five cats. The animals were sedated by IM administration of medetomidine (Domtor; Pfizer, Barcelona, Spain) 30 μg/kg, butorphanol (Turbogesic; FortDodge, Barcelona, Spain) 0.2 mg/kg and ketamine (Ketased, FortDodge; Barcelona, Spain) 4 mg/kg. The ScN was assessed by US using the approaches described for the phase II study.
Results
Phase I: anatomic study
The gross dissection of the ScN was easily performed in all cases. The ScN leaves the pelvic cavity by crossing through the great sciatic notch. It passes caudally over the tendon of the obturator internus giving off a large muscular branch to the biceps femoris, semimembranosus and semitendinosus muscles. The caudal gluteal artery and vein pass along caudal of this part of the nerve and its muscular branch. The ScN continues distal and medial to the biceps femoris and lateral to the semimembranosus muscles and finally it is divided into two large branches, the tibialis and peroneous communis nerves, around the femoral caudal artery and vein. The lateral cutaneous sural nerve, arising from the peroneal component of the sciatic and the caudal cutaneous sural nerve, arising from the tibial component of the sciatic were also observed (Fig 1). The amount of connective tissue surrounding the nerve was considerably higher at the poplitea fossa area (Fig 5D).
Phase II: ultrasonographic study ‘in vitro’
The ScN was correctly identified by US in the three tested approaches: glutea, femoralis and poplitea. This was demonstrated by anatomical dissection observing the ScN successfully stained by ink in all cases. The caudal part of the truncus lumbosacralis and the origin of the ScN were observed in the glutea cranial approach (Fig 2B). In this approach, the ScN was visualised as the largest nerve structure in this area. Distally, this nerve was located caudal to the ala ossis ilii, between the trochanter major and tuber ischiadicum, passing over the dorsocaudal aspect of trochanter major (Fig 3B). The ScN was visible in the femoralis approach between the muscles of the thigh where it lay medial to the biceps femoris and caudal to the femur (Fig 4B–D). The two components of the ScN, the peroneous communis and the tibialis nerves, were readily distinguished as independent structures in the popliteal approach (Fig 5B–D). The peroneous communis nerve passes the lateral aspect and the tibialis nerve the medial aspect of the caudal femoral artery in the longitudinal view. The peroneous communis nerve always appeared cranially and slightly smaller in diameter than the tibialis nerve in the transverse view. In one cat, a third neural structure was visualised below the tibialis nerve at this level. This structure was identified as the caudal cutaneous sural nerve, a branch of the tibialis nerve (Fig 6).
Fig 6.
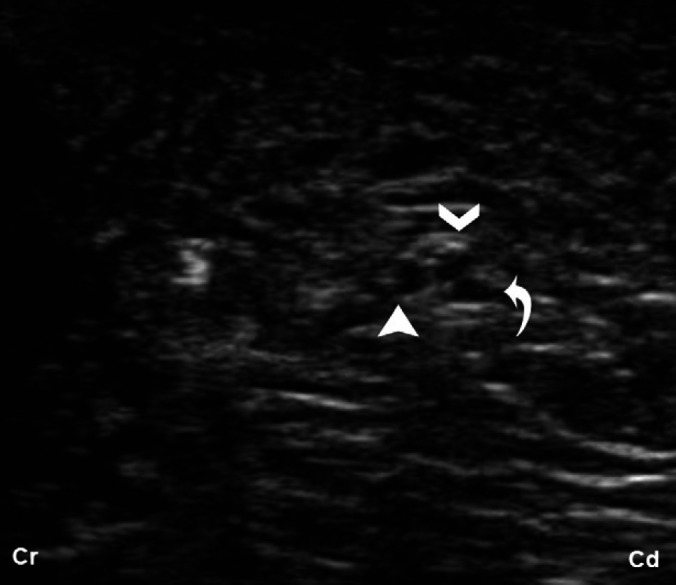
Transverse US image of the ScN at midfemoral approach. At this level, three hypoechoic round structures, surrounded by a hyperechoic halo are visible, representing the peroneous communis (full arrow head), tibialis nerve (open arrow head), components of the ScN and the third structure (turned arrow) corresponding with the caudal cutaneous sural nerve branch from the tibial nerve.
In the three approaches, the ScN appeared as a tubular hypoechogenic structure delimited by two hyperechoic lines on sagittal views. In the transverse images, its two components were readily distinguished and differentiated as oval-to-round hypoechogenic structures surrounded by a hyperechogenic thin rim. In the femoral distal approach (midfemoral diafisis), the ScN appeared with internal echoes in the longitudinal view (Fig 4B).
Phase III: ultrasonographic study ‘in vivo’
The ScN was easily identified in the three approaches: glutea, femoralis and poplitea in all the cats. There were no differences in either the ultrasonographic appearance or the location of the ScN between cadavers and living cats. The ScN was observed close to the caudal gluteal artery and vein, which were readily distinguished using colour flow, in the glutea approach. The caudal cutaneous sural nerve (a branch from tibialis) was also visualised ‘in vivo’ caudal to the tibialis nerve in the distal femoris area, in 2/5 cats (Fig 6).
Discussion
The introduction of high-frequency linear array transducers in humans has allowed US to become an optimal imaging technique to evaluate normal anatomy and disorders of peripheral nerves in humans. 4 To the authors’ knowledge, there is little information regarding the use of nerve sonography in routine clinical veterinary practice. This is mainly due to the lack of familiarity of the examiners with the local anatomy and typical appearance of normal and pathological nerve structures. The ScN was selected to perform this study because it is the most frequently nerve injured on the hind limb of cats. 1
The anatomical study was carried out to identify the ScN and its relevant associated structures as well as to determine the optimal acoustic windows to approach it.
The results of this study confirm that the course of the ScN can be clearly depicted by sonography in the cat. In all animals, the ScN was scanned at three locations: gluteal (cranial and caudal), femoralis and popliteal regions using a linear high-frequency transducer. The bony landmarks, ala ossis ilii, trochanter major, tuber ischiaticum provide a consistent guide to locate the ScN on the gluteal region. The trochanter major and the lateral condyle of the os femoris provided a consistent guide to locate the ScN on the femoral and popliteal regions.
The ultrasonographic appearance of the ScN in cats was similar to that described for the ScN of dogs. 5,6 There were no differences between the ultrasonographic appearance of the ScN in fresh cadavers and live cats as has also been documented for dogs. 6 In contrast, it has been reported 5 that the nerve appeared more uniformly hypoechoic in cadavers. They hypothesised that post-mortem degeneration of the nerve may be the responsible for the reduction in the internal echotexture of the nerve structures. These differences may be explained by the fact that in the present study the US scans were performed immediately after euthanasia while in the other report 5 thawed cadavers were employed. On transversal images, at gluteal location, the ScN appeared as an oval shaped hypoechoic surrounded by a hyperechoic rim. At midfemoral level it appeared as a bi-tubular structure until the nerve reached the poplitea zone. These structures were the peroneous communis component on the cranial aspect and the tibial nerve on the caudal aspect. The peroneous communis was smaller in diameter than the tibialis as previously reported. 5,6 On longitudinal images, the ScN appeared from proximal to distal as a tubular hypoechoic structure bordered by two hyperechogenic lines. The hyperechoic rim is likely to represent the epineurium, whereas the hypoechoic areas separated by the hyperechoic bands are likely to represent the neuronal fascicles and the epineurium. 3 At distal femoralis zone, the ScN appeared with an increase of internal echogenicity. This increase of echogenicity could be related to regional differences in the characteristics of the ScN as, the degree of hyperechogenicity of a peripheral nerve reflects the amount of connective tissue within the nerve. The echogenicity of a nerve varies as nerves pass through different tissues. 7 It has also been reported that the anatomic areas provided with an increased amount of connective tissue are those where peripheral nerves are more prone to be subjected to physiological compression or stretching. 3
At the glutea region, the ScN is localised close to the glutea caudalis vein and artery and it can be confused with these vascular structures. Any uncertainty can be addressed using colour-flow Doppler. 5 At the level of mid-femur, the ScN is not surrounded by these structures. The nerve can be easily identified, medial to the biceps femoris, observing its typical bi-tubular ultrasonographic appearance. 5,6 In three cats (one cadaver and two live) a third neural structure was visualised at the level of poplitea region, caudal to the tibialis nerve on the transversal plane. This structure was identified as a branch of tibialis nerve, the caudal cutaneous sural nerve. The inconsistency in visualising this branch may be attributed to the small diameter of this nerve. Hence, this finding should be investigated in future research.
Conclusions
This report provides a description of the ultrasonographic approaches and appearance of the feline ScN and validates the usefulness of the US to evaluate the normal anatomy ScN in the cat. This information can be useful for clinical purposes, but further studies are necessary to determine its potential applications.
Acknowledgements
This research was supported by ‘Fundación Seneca’ – Agencia Regional de Ciencia y Tecnología (08784/PPC/08) Murcia (Spain). CONACYT – Consejo Nacional de Ciencia y Tecnología, Mexico.
References
- 1.Forterre F., Tomek A., Rytz U., Brunnberg L., Jaggy A., Spreng D. Iatrogenic sciatic nerve injury in eighteen dogs and nine cats (1997–2006), Vet Surg 36, 2007, 464–472. [DOI] [PubMed] [Google Scholar]
- 2.Linzmann H., Brunnberg L., Gruber A.D., Klopfleisch R. A neurotrophic lymphoma in the brachial plexus of a cat, J Feline Med Surg 11, 2009, 522–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchi E. Ultrasound of the peripheral nerves, Joint Bone Spine 75, 2008, 643–649. [DOI] [PubMed] [Google Scholar]
- 4.Stuart R.M., Koh E.S.C., Breidal W.H. Sonography of peripheral nerve pathology, Am J Roentgenol 182, 2004, 123–129. [DOI] [PubMed] [Google Scholar]
- 5.Benigni L., Corr S.A., Lamb C.R. Ultrasonographic assessment of the canine sciatic nerve, Vet Radiol Ultrasound 48, 2007, 428–433. [DOI] [PubMed] [Google Scholar]
- 6.Echeveverry D.F., Gil F., Laredo F.G., et al. Ultrasound-guided block of the sciatic and femoral nerves in dogs. A descriptive study, Vet J 186, 2010, 210–215. [DOI] [PubMed] [Google Scholar]
- 7.Carty S., Nicholls B. Ultrasound-guided regional anaesthesia, Contin Educ Anaesth Crit Care Pain 7, 2007, 20–24. [Google Scholar]