Abstract
Two closely related British Shorthair cats, which died after surgery performed in the dorsal position, were presented for dissection. In both, a thin, flaccid, enlarged transparent tendinous diaphragmatic portion protruded cranially into the thoracic cavity, forming a cupola in which left, right medial and quadrate hepatic lobes were encased in both cats and the stomach in one cat. Microscopically, no muscle fibres were observed in the membrane, but numerous hepatocytes and bile ducts were incorporated in its central part. The anomaly was diagnosed as a congenital diaphragmatic eventration.
Diaphragmatic eventration is a thinning of the diaphragmatic tissue, resulting in a more cranial position of the intact diaphragm 1 that forms a sac which may contain some of the abdominal organs. 2 The extent of thinning can range from a small discrete region to virtually the entire half of the diaphragm (hemidiaphragm). 3 This anomaly was first reported and named in a man in 1790 by Petit. 2 The uninterrupted continuity of the diaphragm differentiates diaphragmatic eventration from diaphragmatic hernia, in which the abdominal viscera protrudes through the diaphragm into the thoracic cavity. 4
Diaphragmatic eventration can be congenital or acquired. Congenital eventration is characterised by muscular paralysis, atrophy 5 or aplasia (undermuscularisation) due to incomplete ingrowth of muscle fibres between the pleural and peritoneal sheets of the diaphragm during the embryonic period. 6 Acquired eventration, on the other hand, is the consequence of trauma, inflammation 7 or neoplastic invasion of the phrenic nerve. 8
Diaphragmatic eventration is a rare anomaly in humans, occurring in about 0.001–0.003% of live births. 9 This anomaly is even less common in animals, and has been described only in Golden Lion tamarins (Leontopithecus rosalia), 10,11 in a leopard (Panthera pardus) 1 and in a foal. 12
Here we report a diaphragmatic eventration found at dissection in two closely related cats, which both died after surgical operation. To the best of our knowledge, this is the first report of diaphragmatic eventration in this animal species.
Case 1 was a 5-month-old, 3600 g, male neutered British Shorthair cat that died 1 day after castration. One hour prior to death, it became apathetic and dyspnoeic and died with signs of a severe dyspnoea and cyanosis.
Case 2 was a 7-month-old, 2940 g, female neutered British Shorthair cat that died 2 days after neutering. On the day of death, it became apathetic and dyspnoeic; it died 3 h later with signs of severe dyspnoea. The two cats were closely related – the male neutered cat was the uncle of the female neutered cat. Both cats were submitted for dissection at the Institute of Pathology, Forensic and Administrative Veterinary Medicine at the Veterinary Faculty, Ljubljana.
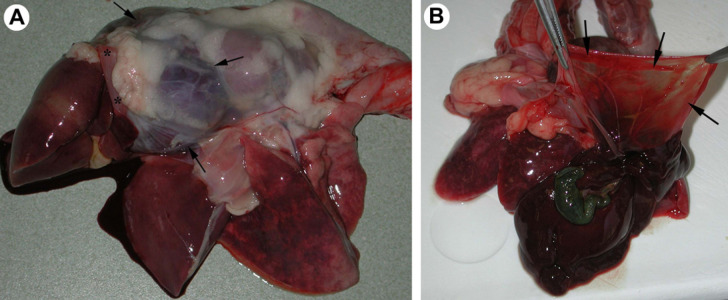
On dissection of the female neutered cat, a thin, flaccid, transparent tendinous portion of the diaphragm was observed to protrude into the thoracic cavity, forming a cupola and encasing the left medial, right medial and quadrate hepatic lobes, which compressed a severely dilated and pale heart (Fig 1). Twenty millilitres of yellow, translucent transudate was observed in the thoracic cavity and 10 ml in the pericardial sac. The lungs were severely congested, emphysematous and oedematous, with tracheal lumen filled with reddish, foamy liquid. The liver, kidneys, spleen and pancreas were moderately to severely congested.
Fig 1.
(A) Female neutered cat; lateral view at the exenterated organs of thoracic cavity. Left medial, right medial and quadrate hepatic lobes are encased in a tendinous portion of the diaphragm, which forms a cupola (arrows show the edges of the cupola). Asterisks mark the muscular part of the diaphragm. The hepatic lobes, which remained in the abdominal cavity, are in the leftmost position. (B) Female neutered cat; enlarged, flaccid central tendinous portion of the diaphragm (arrows) in the cat with diaphragmatic eventration.
Samples of the diaphragm of the female neutered cat were taken for histopathological examination, fixed in 10% buffered formalin and embedded in paraffin. Then, 4 μm thick tissue sections were deparaffinised, stained with haematoxylin and eosin (HE) and examined under a light microscope.
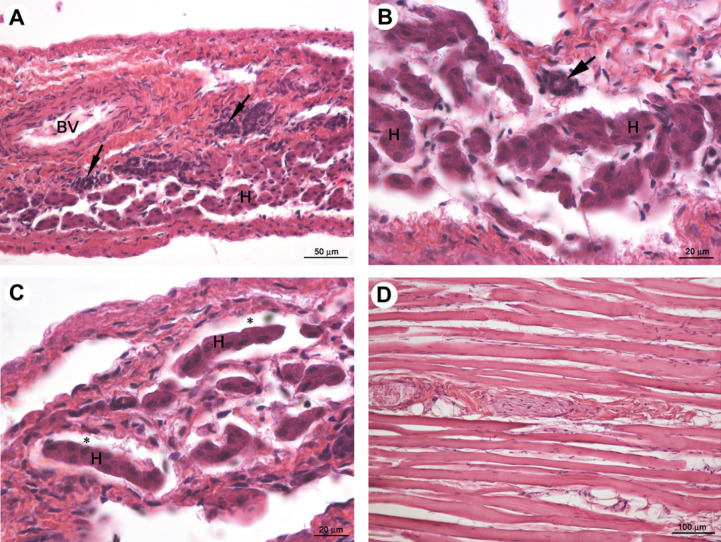
Microscopically, the transparent cupola of the diaphragm was composed of irregular tufts of fibrocytes associated with small, thin collagen bundles and small blood vessels. No muscle fibres were present. The tissue of the cupola was slightly oedematous with mild multifocal infiltrates of lymphocytes. At its very centre, numerous hepatocytes, arranged in small groups or rows, and a few bile ducts were observed (Fig 2A–C). The intact muscular part forming the rim of the diaphragm was microscopically unchanged (Fig 2D).
Fig 2.
Female neutered cat; light micrographs of transparent cupola of diaphragm (A–C) and intact muscular part (D). H and BV indicate the hepatocytes arranged in small groups or rows and the blood vessel. Black arrows and asterisks indicate bile ducts and sinusoidal-like spaces, respectively. HE staining.
Based on the gross and histological findings, a diagnosis of congenital diaphragmatic eventration was made in the female neutered cat.
In the male neutered cat, an enlarged, thin, flaccid transparent tendinous portion of the diaphragm protruded cranially into the thoracic cavity, forming a cupola in which left, right medial and quadrate hepatic lobes and stomach were encased. Both caudal pulmonary lobes and the heart were compressed by displaced abdominal viscera and consequently pale. Seventy-five millilitres of yellow, translucent transudate was observed in the thoracic cavity and 150 ml in the abdomen. The right ventricular chamber was severely dilated; cranial pulmonary lobes, kidneys and spleen were severely congested. The male neutered cat was diagnosed with congenital diaphragmatic eventration.
In both cats an enlarged, thin, flaccid transparent tendinous diaphragmatic portion formed a cupola, which protruded cranially into the thoracic cavity. Microscopically, no muscle fibres were present in the diaphragm of the female neutered cat, but numerous hepatocytes and some bile ducts were incorporated in its central part. The anomaly was diagnosed as congenital diaphragmatic eventration. Congenital diaphragmatic eventration is a result of incomplete muscle formation – the ingrowth of muscle fibres between the pleural and peritoneal sheet of the diaphragm during the embryonic period is incomplete 6 due to a failure of myoblasts to migrate between the two leaves of the pleuroperitoneal membrane during fetal life, or to its non-innervation. 5 The anomaly can be seen as a translucent flaccid membrane, contiguous with the diaphragm, which protrudes into the thoracic cavity. 10 Anshu et al 5 reported that the transverse septum is primordial in the development of the central part of diaphragm; its cranial part is involved in formation of the diaphragm and the liver develops in its caudal part. The cranial part normally separates from the developing liver and differentiates into a tendinous structure composed mainly of fibroblasts and collagen fibres. 13 Another portion of diaphragm anlage, called the post-hepatic mesenchymal plate which is involved in the closure of the diaphragm, was described. If there is a failure of normal growth of the post-hepatic mesenchymal plate, its cells become disorganised and can display the typical morphology of liver cells and cause liver heterotopias. 14 Hepatocytes and bile ducts, found in the very centre of diaphragmatic eventration in the female neutered cat, were possibly the consequence of a developmental anomaly or just an ectopic liver tissue (choristia) in the diaphragm.
Diaphragmatic eventration is an uncommon anomaly in animals, 1,10–12 and has never been described microscopically.
The two cats were closely related – the male neutered cat was the uncle of the female neutered cat. Direct consanguinity was reported also in Golden Lion tamarins, where eventration was diagnosed in a great-granddaughter of a female with diaphragmatic hernia, so an autosomal recessive mode of inheritance was suggested. 11
Both cats died after surgical operations, during which they were put in a dorsal position, enabling the cranial displacement of some of the abdominal viscera, which severely compressed the lungs and heart. In both, a severe dyspnoea was observed prior to death. Jackson et al 12 reported that the foal with diaphragmatic eventration was stillborn, the leopard with both diaphragmatic eventration and hiatal hernia exhibited anorexia, emaciation and muscle wasting, 1 whereas one tamarin was without any clinical signs and the defect was diagnosed by radiography. 11 In humans, respiratory distress was often described in neonates with diaphragmatic eventration whereas, in older children and adults, the defect may be asymptomatic. 5 The tamarin and the leopard recovered completely after surgical obliteration of the defect. 1,11
To the best of our knowledge, this is the first report of diaphragmatic eventration in cats. The death of both cats was most probably a consequence of the thin, enlarged tendinous portion of the diaphragm without muscle fibres which, together with an abnormal body position during the surgical procedure, contributed to the displacement of abdominal viscera and caused a severe dyspnoea and asphyxiation. As the anomaly was found in two British Shorthair cats, ultrasound or radiography are advised before every surgical procedure in this breed.
Acknowledgements
The authors would like to thank Professor Roger Pain for English proofreading of the manuscript.
References
- 1.Kearns K.S., Jones M.P., Bright R.M., Toal R., DeNovo R., Orosz S. Hiatal hernia and diaphragmatic eventration in a leopard (Panthera pardus), J Zoo Wildl Med 31, 2000, 379–382. [DOI] [PubMed] [Google Scholar]
- 2.BMJ Publishing Group. Eventratio diaphragmatica, BMJ 1, 1924, 1064–1065, Available via: http://www.jstor.org/stable/20436954, Accessed 20 Oct 201020771634 [Google Scholar]
- 3.Pober B.R. Overview of epidemiology, genetics, birth defects and chromosome abnormalities associated with CDH, Am J Med Genet 145C, 2007, 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malone P.S., Brain A.J., Kiely E.M., Spitz L. Congenital diaphragmatic defects that present late, Arch Dis Child 64, 1989, 1542–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anshu S., Poonam S., Singh R.J., Veena S. Eventration of diaphragm – embryonal basis, J Anat Soc India 54, 2005, 39–41. [Google Scholar]
- 6.Dohler R., Heinemann G. Congenital diaphragmatic eventration, Fortschr Med 97, 1979, 1769–1771. [PubMed] [Google Scholar]
- 7.Hesselink J.R., Chung K.J., Peters M.E., Crummy A.B. Congenital partial eventration of the left diaphragm, Am J Rentgenol 131, 1978, 417–419. [DOI] [PubMed] [Google Scholar]
- 8.Moinuddeen K., Baltzer J., Zama N. Diaphragmatic eventration, Chest 119, 2001, 1615–1616. [DOI] [PubMed] [Google Scholar]
- 9.Urschel H.C., Disiere J., McQuirter W.M. Current therapy in cardiothoracic surgery in eventration and placation, Philadelphia Decker 18, 1989, 258–259. [Google Scholar]
- 10.Bush M., Beck B.B., Dietz J., et al. Radiographic evaluation of diaphragmatic defects in Golden Lion tamarins (Leontopithecus rosalia rosalia): implication for reintroduction, J Zoo Wildl Med 27, 1996, 346–357. [Google Scholar]
- 11.Bush M., Montali R.J., Kleiman D.G., Randolph J., Abramowitz M.D., Evans R.F. Diagnosis and repair of familial diaphragmatic defects in Golden Lion tamarins, J Am Vet Med Assoc 177, 1980, 858–862. [PubMed] [Google Scholar]
- 12.Jackson C., Collyer P. Borchers, Loynachan A. Congenital diaphragmatic eventration in a stillborn foal, J Vet Diagn Invest 18, 2006, 412–415. [DOI] [PubMed] [Google Scholar]
- 13.Liu J., Zhang L., Dongmei W., et al. Congenital diaphragmatic hernia, kidney agenesis and cardiac defects associated with Slit3-deficiency in mice, Mech Develop 120, 2003, 1059–1070. [DOI] [PubMed] [Google Scholar]
- 14.Patel Y., McNally J., Ramani P. Left congenital diaphragmatic hernia, absent pericardium, and liver hererotopia: a case report and review, J Pediatr Surg 42, 2007, E29–E31. [DOI] [PubMed] [Google Scholar]