Abstract
Ronidazole (RDZ) is an effective treatment for feline Tritrichomonas foetus infection, but has produced neurotoxicity in some cats. An understanding of the disposition of RDZ in cats is needed in order to make precise dosing recommendations. Single-dose pharmacokinetics of intravenous (IV) RDZ and immediate-release RDZ capsules were evaluated. A single dose of IV RDZ (mean 9.2 mg/kg) and a 95 mg immediate-release RDZ capsule (mean 28.2 mg/kg) were administered to six healthy cats in a randomized crossover design. Plasma samples were collected for 48 h and assayed for RDZ using high pressure liquid chromatography (HPLC). Systemic absorption of oral RDZ was rapid and complete, with detection in the plasma of all cats by 10 min after dosing and a bioavailability of 99.64 (±16.54)%. The clearance of RDZ following IV administration was 0.82 (±0.07) ml/kg/min. The terminal half-life was 9.80 (±0.35) and 10.50 (±0.82) h after IV and oral administration, respectively, with drug detectable in all cats 48 h after both administrations. The high oral bioavailability of RDZ and slow elimination may predispose cats to neurotoxicity with twice-daily administration. Less frequent administration should be considered for further study of effective treatment of T foetus-infected cats.
Tritrichomonas foetus is a flagellated protozoan parasite that colonizes the feline colon and distal ileum, causing colitis and a chronic foul smelling diarrhea. 1,2 While spontaneous remission of the diarrhea may occur, this can take as long as 2 years and evidence suggests that cats may remain infected for life. 3 The only known effective treatment for T foetus is ronidazole (RDZ). 4 Treatment of T foetus-infected cats with other drugs such as fenbendazole, paromomycin, tinidazole, metronidazole, and furazolidone improves fecal consistency during treatment, but the organism is not eradicated and diarrhea returns after the drugs are discontinued. 3–6
RDZ is in the 5-nitroimidazole class of drugs which includes the more familiar metronidazole. 7 RDZ is reduced by anaerobic organisms such as T foetus to autotoxic free radicals which in turn cause destabilization of organism DNA and organism death. 8 RDZ kills T foetus in vitro (RDZ≥1 μg/ml) 9 and eliminates T foetus from experimentally infected cats at doses ranging from 30 to 50 mg/kg orally q 12 h for 14 days. 2 Lower doses of RDZ (10 mg/kg PO orally q 12 h for 14 days), have been ineffective at eliminating infection. 2
RDZ is widely used for the treatment of canker (Trichomonas gallinae), a common disease in racing pigeons. 10,11 Currently, RDZ is not approved by the US Food and Drug Administration for use in companion animals and there is no FDA-approved available pharmaceutical product in the United States. However, the chemical-grade powder is available and, when prescribed, many pharmacies will compound the drug into tablets or capsules. 1,12
Despite its efficacy in T foetus treatment, RDZ has been reported to cause neurotoxicity in some cats. 12 Reported side effects include lethargy, ataxia, trembling of the extremities, facial tremors, agitation and hyperesthesia. 12,13 Reported neurologic signs begin a minimum of 3 days into treatment and resolve 1–4 weeks after discontinuation of RDZ. 12 Cats may require supportive veterinary care until the adverse effects resolve. Similar clinical signs have been observed from high doses of metronidazole and the mechanism of neurotoxicity is believed to be similar for both drugs. 7,14–17
The purpose of this study was to characterize the pharmacokinetics of RDZ after single-dose administration of IV RDZ solution and oral immediate-release RDZ capsules to cats. It was hypothesized that rapid and complete absorption of RDZ might cause high plasma concentrations and predispose cats to neurotoxicity. It was also hypothesized that a long half-life may contribute to RDZ accumulation and neurotoxicity with twice-daily administration.
Materials and methods
Solutions for intravenous (IV) infusion
RDZ [(1-methyl-5-nitroimadazol-2-yl) methyl carbamate] (Spectrum, Gardena, CA, USA) was dissolved in 5% dextrose in water (D5W) (5% dextrose, Baxter, Deerfield, IL, USA) to a concentration of 3.2 mg/ml. The solution was sonicated (FS5 Ultrasonic Cleaning System, Fisher Scientific, PA, USA) for 10 min and sterilized by membrane filtration using a 25 mm syringe filter with a 0.2 μm Supor membrane filter (Pall PharmAssure; Pall Medical, Port Washington, NY, USA), followed by aseptic filling into 50 ml sterile vials (Greer, Lenoir, NC, USA). Solutions were made a maximum of 24 h before administration to cats, and sonicated again for 10 min just before drawing into syringes for administration. All IV solutions were stored frozen at −80°C after administration. The potency of the administered solution was later verified via high pressure liquid chromatography (HPLC) and compared against a freshly-prepared reference standard solution. The actual IV dose recorded for each cat was based on the true concentration determined by the HPLC assay of the IV solution.
Immediate-release capsules
The North Carolina State University College of Veterinary Medicine (NCSU CVM) pharmacy compounded 95 mg of RDZ (Spectrum, Gardena, CA, USA) into gelatin capsules (Gelatin capsules #3; Eli Lilly and Company, IN, USA). Immediate-release capsules were subjected to a dissolution test in a simulated gastric environment in 400 ml of 0.1 N hydrochloric acid (HCl) dissolution medium (Fisher Scientific, Fair Lawn, NJ, USA) (pH 2) in a 500 ml beaker between 37 and 40°C using a stir bar rotating at 50 rpm. The HCl solution for simulated gastric conditions was prepared as specified by the standards of the United States Pharmacopeia. 18 Drug release was monitored every 15 min using a spectrophotometer (Spectronic Genesys 2, Spectronic Instruments, Rochester, NY, USA) set at a wavelength of 313 nm. RDZ concentration in the beaker solution was determined by comparing absorbance of a 1 ml 1:4 dilution of the beaker solution compared to a calibration curve of absorbance plotted against RDZ concentration generated from six RDZ dilutions (range, 0–100 μg/ml) in the same HCl solution. The calibration curve was linear with an r2 value of greater than 0.99.
Cats
Six healthy 6-month-old intact male cats (Liberty Research, Waverly, NY, USA) were used in the study (3.42 ±0.24 kg body weight). Cats were housed in the institutional laboratory animal facility and at study completion all cats were either adopted as pets or became NCSU CVM blood donors, after which they were adopted. All protocols were approved by the Institutional Animal Care and Use Committee of North Carolina State University.
RDZ administration and sample collection
A randomized two-way crossover study was performed with each cat treated with immediate-release RDZ capsules (PO) and the RDZ solution (IV). A randomization scheme was generated by use of an Internet website (Randomization.com, www.randomization.com). 19 A washout period of at least 14 days was allowed between the IV and oral dosing.
Twelve hours prior to drug administration, a 19-gauge catheter (BD Intracath; Becton Dickinson, Sandy, UT, USA) was placed in the jugular or medial saphenous vein of each cat. If the cat was to receive IV RDZ, a 22-gauge cephalic catheter (BD Insyte; Becton Dickinson) was also placed at this time. To minimize the stress of catheter placement, anesthesia was induced with a combination of intramuscular (IM) ketamine (Fort Dodge Laboratories, Fort Dodge, IA, USA) and midazolam (Bedford Laboratories, Bedford, OH, USA) or medetomidine (Dormitor; Pfizer Animal Health, Exton, PA, USA). If cats received medetomidine, they were reversed with atipamezole (Antisedan; Pfizer Animal Health) on completion of catheter placement. Food was withheld for 19 h prior to, and 8 h after, drug administration. Water was available at all times.
RDZ solution (3.2 mg/ml) was administered intravenously at a mean dose of 9.18 mg/kg, (range 8.65–9.49 mg/kg) by slow bolus via the cephalic catheter. In the crossover, each cat received one 95 mg immediate-release capsule (mean dose 28.23 mg/kg, range 26.69–30.84 mg/kg) followed by 6 ml of tap water orally to ensure that the medication was swallowed. The lower IV dose was selected to decrease the risk of neurotoxicity.
Blood samples (3 ml each) were collected via the central catheter prior to, and 0.17, 0.33, 0.67, 1, 2, 4, 6, 8, 10, 12, 24, 30 and 48 h after drug administration by either route. Volume was returned to cats in the form of heparinized saline catheter flush (at least 2.5 ml/blood sample acquired). Blood was immediately transferred to tubes containing lithium heparin (BD Vacutainer, Becton Dickinson, Franklin Lakes, NJ, USA) and centrifuged to harvest plasma. Plasma was frozen and stored at −80°C until analysis. Cumulative blood collection volumes were within published guidelines to avoid adverse hemodynamic effects. 20 Packed cell volumes (PCVs) and total blood solids were measured in each cat prior to drug administration and at 12, 24, and 48 h after drug administration by either route.
RDZ assay
Feline plasma samples were analyzed by HPLC using a method developed in the authors' laboratory. The reference standard of RDZ was obtained from the same source as that used for drug formulations (Spectrum, Gardena, CA, USA) and confirmed to be 101% pure by supplier certificate of analysis. RDZ was dissolved in methanol (Sigma Aldrich, St Louis, MO, USA) to make up a 1 mg/ml stock solution. From this stock solution, further dilutions were made in distilled water to use as fortifying solutions for feline plasma in order to generate calibration curves in plasma and to generate quality control samples (0.1 μg/ml, 5 μg/ml, and 50 μg/ml RDZ in feline plasma). The stock solution was kept at 4°C in a tightly sealed dark vial. This reference standard solution (1 mg/ml) was verified to be stable and potent for 13 months in the refrigerator compared to a freshly-prepared reference standard solution. RDZ spiking solutions were added to blank (control) feline plasma to make up seven calibration standards (range 0.025–50 μg/ml).
The mobile phase for HPLC analysis consisted of acetonitrile (15%) (Fisher Scientific, Fair Lawn, NJ, USA) and 0.1% trifluoroacetic acid (85%) (Fisher Scientific). The HPLC system consisted of a quaternary solvent delivery system (Agilent Technologies, Wilmington, DE, USA) set at a flow rate of 1 ml/min, an autosampler (1100 Series Autosampler, Agilent Technologies), and ultraviolet detector set at a wavelength of 313 nm (1100 Series UV–Visible Detector, Agilent Technologies). The chromatograms were integrated with a computer program (1100 Series Chemstation software, Agilent Technologies). A C8 reverse-phase column (Zorbax RX-C8, 4.6×150 mm reverse-phase column, Agilent Technologies) was used for separation and kept at a constant temperature of 40°C.
All experimental plasma samples, calibration samples, quality control samples, and blank (control) feline plasma samples were prepared identically. Solid phase extraction cartridges (Oasis 1 cc extraction cartridges, Waters Corporation, Milford, MA, USA) were attached to a vacuum manifold and initially conditioned with 1 ml methanol followed by 1 ml distilled water. Each plasma sample was then extracted (0.5 ml), and the cartridge was washed with 1 ml of a 95:5 (vol:vol) mixture of distilled water:methanol. Samples were then eluted by use of 1 ml of 100% methanol into clean glass tubes. The resulting eluate was evaporated under a stream of air at 40°C for 20 min. Evaporated samples were reconstituted with 200 μl of mobile phase and vortexed. Then, 25 μl of each reconstituted solution was injected into the HPLC system. Retention time for peak of interest was 4.06–4.19 min. A fresh set of calibration and blank samples were prepared for each day's run. All calibration curves were linear with an r2 value of 0.99 or greater. Limit of quantification for RDZ in feline plasma was 0.025 μg/ml, which was determined from the lowest point on a linear calibration curve that produced an acceptable signal-to-noise ratio. Sample concentrations were calculated from peak heights and a linear calibration curve from each day's analysis. Quality control samples were prepared and analyzed with each day's run and compared against the calibration curve. The laboratory used guidelines published by the United States Pharmacopeia for method validation. 21
Pharmacokinetic calculations
Plasma drug concentrations of RDZ following IV and oral administration were plotted on linear and semi-logarithmic graphs for analysis. The average of each cat's normalized RDZ concentration (cat's RDZ concentration at a given time point divided by the ratio of the cat's dose to the mean dose) was calculated and used for plotting on concentration vs time graphs. Analysis of plasma concentration–time curves and pharmacokinetic modeling after IV and oral administrations were performed using a commercial pharmacokinetic program (WinNonlin Version 5.0.1, Pharsight Corporation, Mountain View, CA, USA). Appropriate compartmental models for data analysis after both the IV and oral administration were selected on the basis of visual inspection of the plasma concentration curves, observation of observed vs predicted time points, and minimum Akaike's information criterion (AIC). 22 A one-compartment analysis was determined to be the most appropriate model.
For IV administration, the following equation for a one-compartment open model was used:
where CC t is the plasma concentration at time t, C0 is the y-axis intercept, e is the base of the natural logarithm, t is time after dosing, and K is the terminal rate constant.
A weighting factor of 1/(predicted Y) 2 was used for pharmacokinetic curve fitting, where Y is the plasma concentration. From the primary parameters listed in the equation above, secondary parameters of systemic clearance (CL), area under the curve from zero to infinity (AUC0–∞), apparent volume of distribution (Vd), and mean residence time (MRT) were calculated. The terminal half-life (T1/2) was calculated using ln(2)/K, where ln(2) is the natural logarithm of 2.0. The initial plasma concentration at time zero (C0) was calculated from the model.
For the oral dose, absorption rates were calculated using the following formula:
where C t is the plasma concentration at time t, Ka is the non-IV absorption rate, assuming first-order absorption, K is the terminal rate constant, Vd is the apparent volume of distribution, F is the fraction of drug absorbed, and D is the oral dose. In this model, it is assumed that Ka>>> K, or that there is no ‘flip-flop’ effect caused by slow absorption from the gastrointestinal tract. A lag-time was added to the model to account for dissolution of capsule and stomach emptying (average lag-time 0.17±0.06 h). Secondary parameters calculated from the model included the peak concentration (CMAX), time to peak concentration (TMAX), clearance per fraction absorbed (CL/F), and the area under the plasma concentration vs time profile from zero to infinity (AUC0–∞). The absorption and terminal half-lives (T1/2) were calculated using ln 2.0/Ka and ln 2.0/K, respectively.
Assuming linearity, systemic availability (%F) from the oral dose was calculated from the following formula:
A simulation was performed for oral administration in cats on a computer program (WinNonlin Version 5.0.1, Pharsight Corporation, Mountain View, CA, USA). The simulation plots projected plasma RDZ concentrations in an average cat following once- vs twice-daily dosing at 30 mg/kg by use of derived mean pharmacokinetic parameters reported in this study.
Results
Drug formulation analysis
All IV RDZ solutions were determined to be within 16% of 3.2 mg/ml (range 2.9 −3.7 mg/ml). Under simulated gastric conditions, immediate-release capsules achieved 74% dissolution within 30 min and 99% dissolution within 45 min.
RDZ assay
All quality control samples back-calculated to be within ±15% of their nominal concentrations. Accuracy of the HPLC assay was within 5.33±3.62% of the true value, and precision was within 5.14±2.57% of the mean. Mean recovery of RDZ from plasma following extraction was 92.70±5.78%.
RDZ remained stable and potent in stored solutions. The 1 mg/ml reference standard solution of RDZ in methanol maintained its potency over 13 months of storage in the dark at 4°C.
Single-dose pharmacokinetics
Mean plasma RDZ concentrations are reported in Table 1, with accompanying linear and semi-logarithmic graphs of plasma concentration vs time shown in Fig 1. RDZ was not detected in any of the time 0 samples for either phase of the crossover study. One-compartmental mean pharmacokinetic parameters are reported in Table 2. Systemic absorption of oral RDZ was rapid and complete with an absorption half-life of only 8.4 (±4.8) min. RDZ appeared in the plasma of all six cats by 10min after administration with a mean bioavailability after oral administration of 99.64 (±16.54) %. CMAX following oral administration was 35.37 (±3.03) μg/ml and TMAX was 1.02 (±0.40) h. The terminal half-life was 9.80 (±0.35) and 10.50 (±0.82) h following the IV and oral dose, respectively, with RDZ remaining in the plasma of all cats 48h after both oral and IV administration. Mean systemic clearance was 0.82 (±0.07) ml/kg/min. The apparent volume of distribution (Vd) was 700 (±60) ml/kg.
Table 1.
Mean±standard deviation (SD) plasma RDZ concentrations after oral and IV administration of a single dose of RDZ to six cats.
| Time (h) | Oral dose (mean 28.2±1.4 mg/kg) | |
|---|---|---|
| Mean±SD* | Median (range)* | |
| RDZ (μg/ml) | ||
| 0.17 | 2.81±2.16 | 3.05 (0.30, 5.44) |
| 0.33 | 21.18±10.29 | 22.44 (6.52, 31.88) |
| 0.67 | 32.68±3.58 | 32.86 (28.79, 36.99) |
| 1 | 33.67±2.88 | 33.30 (29.49, 37.15) |
| 2 | 31.44±1.83 | 31.68 (28.51, 33.36) |
| 4 | 30.43±3.95 | 29.22 (26.09, 36.78) |
| 6 | 27.12±3.74 | 26.11 (22.98, 32.34) |
| 8 | 23.08±2.59 | 23.17 (18,94, 26.88) |
| 10 | 19.56±2.34 | 19.07 (16.83, 22.66) |
| 12 | 16.95±2.19 | 16.74 (14.51, 20.63) |
| 24 | 8.10±1.20 | 8.59 (6.01, 9.19) |
| 30 | 5.35±0.80 | 5.34 (4.40, 6.17) |
| 48 | 1.57±0.32 | 1.63 (1.10, 1.86) |
| Time (h) | IV dose (mean 9.2±0.4 mg/kg) | |
| Time (h) | IV dose (mean 9.2±0.4 mg/kg) | |
|---|---|---|
| Mean±SD* | Median (range)* | |
| RDZ (μg/ml) | ||
| Mean±SD* | Median (range)* | |
| RDZ (μg/ml) | ||
| 0.17 | 13.61±1.67 | 13.45 (11.63, 15.63) |
| 0.33 | 12.54±1.33 | 12.64 (10.74, 14.18) |
| 0.67 | 12.41±1.39 | 12.68 (10.69, 14.39) |
| 1 | 11.83±0.99 | 11.50 (10.81, 13.44) |
| 2 | 11.28±1.10 | 11.25 (9.49, 12.83) |
| 4 | 10.29±1.09 | 9.92 (9.13, 12.17) |
| 6 | 8.71±1.07 | 8.81 (7.05, 10.07) |
| 8 | 7.60±0.82 | 7.40 (6.94, 9.21) |
| 10 | 6.50±0.87 | 6.13 (5.81, 7.88) |
| 12 | 5.67±0.77 | 5.63 (4.77, 6.79) |
| 24 | 2.49±0.28 | 2.43 (2.16, 2.95) |
| 30 | 1.57±0.14 | 1.53 (1.43, 1.83) |
| 48 | 0.43±0.05 | 0.44 (0.34, 0.50) |
Concentrations are reported as normalized, meaning the cat's plasma RDZ concentration at each time point is divided by the ratio of the cat's RDZ dose to the mean dose.
Fig 1.
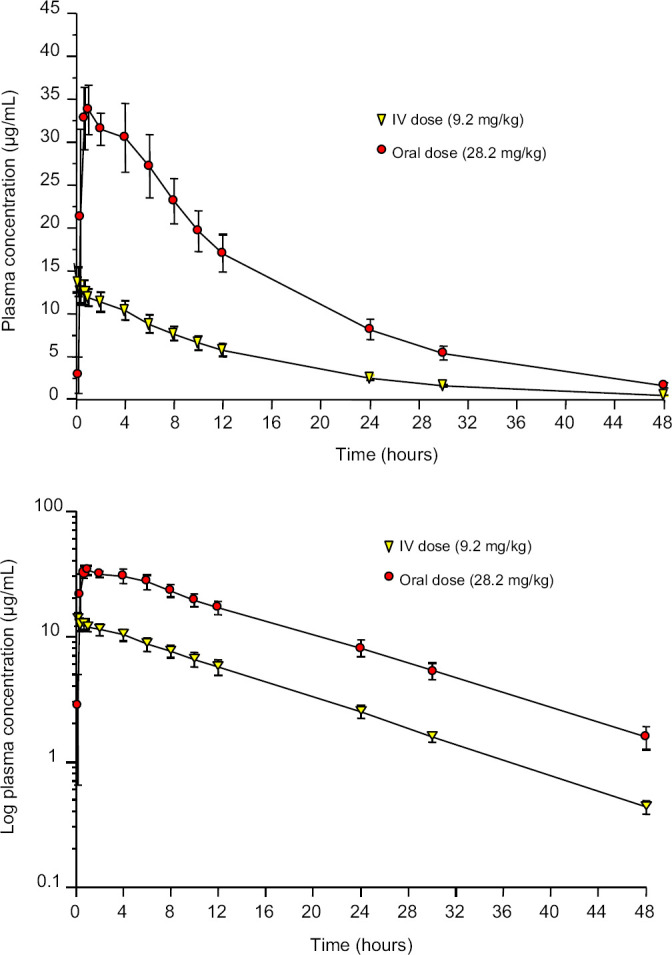
Plasma concentration of RDZ (mean±SD) vs time curves in six cats after administration of a single dose of RDZ IV (9.2±0.4 mg/kg) or orally (28.2±1.4 mg/kg) as immediate-release capsules. Top panel is a linear axis, bottom panel is a semi-logarithmic axis.
Table 2.
Mean±standard deviation (SD) one-compartmental pharmacokinetic parameters after administration of a single dose of RDZ IV (9.2±0.4 mg/kg) or orally (28.2±1.4 mg/kg) as immediate-release capsules in six cats.
| Parameter | Drug administration | ||
| Units | IV | Capsule PO | |
| Dose | mg/kg | 9.175±3.81 | 28.23±1.38 |
| T 1/2 (K) | h | 9.80±0.35 | 10.50±0.82 |
| K | h | 0.071±0.003 | 0.066±0.004 |
| T 1/2 (K a) | h | NA | 0.14±0.08 |
| K a | h | NA | 7.16±5.76 |
| C 0 | μg/ml | 13.27±1.72 | NA |
| AUC0–∞ | h×μg/m | 187.30±21.50 | 566.98±60.04 |
| Vd or Vd/F | ml/kg | 700±60 | 770±60 |
| Clearance or CL/F | ml/kg/min | 0.82±0.07 | 0.84±0.08 |
| MRT | h | 14.14±0.50 | NA |
| T MAX | h | NA | 1.02±0.40 |
| C MAX | μg/m | NA | 35.37±3.03 |
| F (%) | % | NA | 99.64±16.54 |
T1/2 (K)=terminal half-life; K=terminal rate constant; T1/2 (Ka)=absorption half-life; Ka=absorption rate; C0=initial plasma concentration after IV dose (y-axis intercept); AUC0–∞=total area under the plasma concentration vs time curve from zero to infinity; Vd=apparent volume of distribution (Vd/F for oral dose); CL=systemic clearance (CL/F for oral dose); MRT=mean residence time; CMAX=maximum plasma concentration after oral administration; TMAX=time to maximum plasma concentration after oral dose; F (%)=fraction of total oral dose absorbed systemically (%) or bioavailability; SD=standard deviation; IV=intravenous; PO=per os.
Adverse effects associated with RDZ administration by either route were not observed. The lowest recorded PCV and total solids measurements ranged from 25% to 40% and 5.0 to 8.0g/dl, compared with the normal ranges from the Clinical Pathology Laboratory at NCSU CVM of 32–48% for PCV and 5.8–8.2g/dl for total solids. None of the cats showed clinical signs of anemia, such as tachycardia, lethargy, pale mucus membranes, or increased respiratory rate.
The results of the simulated plasma concentration of RDZ comparing once- vs twice-daily oral administration of 30mg/kg are depicted in Fig 2.
Fig 2.
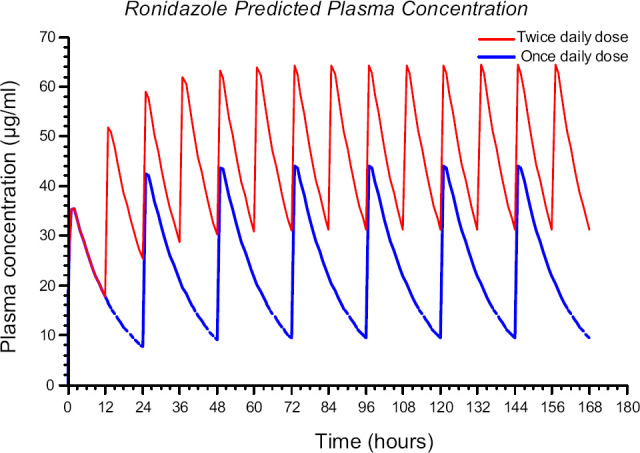
Simulated plasma concentration of RDZ vs time curves in cats after 30 mg/kg oral of immediate-release capsules q 24 h (blue line) and 30 mg/kg q 12 h (red line).
Discussion
This is the first published report of RDZ pharmacokinetics in cats. Oral absorption of immediate-release RDZ capsules is rapid and complete, producing high plasma RDZ concentrations shortly after the dose was administered. The half-life of RDZ was long and there was still drug remaining in the plasma 48h after a single dose. Systemic clearance of RDZ in cats (0.82±0.07ml/kg/min) was slower than either feline glomerular filtration (about 2.7ml/kg/min) or hepatic plasma flow (about 18ml/kg/min). 23,24 The reason for the slow clearance is not known. The only other species for which RDZ clearance data could be found was pigeons, in which the clearance was 0.93(±0.15) ml/kg/min. 10 It is suspected that the long half-life of RDZ may produce drug accumulation with administration q 12 h. Collectively, the rapid absorption, high bioavailability, and slow elimination of RDZ may explain the occurrence of neurotoxicity in some cats with twice-daily drug administration regimens.
Adverse effects associated with single-dose RDZ administration were not observed in this study. However, the reported neurotoxicity of RDZ appears to be associated with repeated dosing and may be attributed to drug accumulation. Neurologic signs in clinical cases of T foetus-infected cats treated with RDZ occur a minimum of 3 days after initiating treatment with a dosage of ≥30 mg/kg PO q 12 h. 12,13 In those cases, if RDZ was discontinued, neurotoxicity resolved within 1–4 weeks. 12 In contrast, continuation of RDZ therapy after neurologic signs began resulted in progression of neurologic signs until the drug was discontinued. 12
While the mechanism of RDZ neurotoxicity is unknown, formation of superoxide and semiquinone radicals from catecholamine-RDZ oxidation–reduction reactions under aerobic conditions is proposed. 16 In research dogs, neurotoxicity was observed in animals treated with ≥10 mg/kg per day for 1–2 years. 25 On post-mortem exam, dogs receiving ≥20 mg/kg/day RDZ for 2 years had gross central nervous system lesions such as slight hydrocephalus, subdural hemorrhage, and pale yellowish discoloration of the brain and histopathologic changes including focal hemorrhage of the cerebellum, leukomalacia, and neurophagia. 25 Neuropathologic findings in cats have not been reported.
While the maximum tolerated and minimum effective dose of RDZ needed to clear T foetus infection in cats are not known, 10 mg/kg PO q 12 h for 2 weeks did not eliminate T foetus from experimentally-infected cats, whereas 30 mg/kg PO q 12 h for 2 weeks was effective. 2 It is not possible to use the plasma RDZ concentrations reported in this study to determine the minimum effective RDZ dose and administration frequency because RDZ concentrations in the colon are more likely to predict efficacy than plasma concentrations. The RDZ mean lethal concentration (MLC) of 1 μg/ml required to kill T foetus in vitro 9 can be achieved in the plasma with a dose of approximately 10 mg/kg (Fig 1), however, as previously stated, this dose is not effective at eliminating infection. 2
High systemic RDZ concentrations – particularly if they are achieved rapidly after enteral absorption from the proximal small intestine – may not be sufficient to achieve effective levels in the distal ileum and colon of infected cats, where T foetus is confined. 1 In this study, oral absorption of the immediate-release formulation was rapid and complete and likely occurred in the upper small intestine soon after stomach emptying. Therefore, RDZ delivery to the distal intestinal tract occurs through diffusion from the systemic circulation.
The rapid, complete absorption profile of RDZ from immediate-release capsules may also increase the risk of neurotoxicity. An additional concern is that RDZ is currently only provided by compounding pharmacists, and various dosage forms (capsules, solutions, suspensions, and medicated treats) may have significantly different oral absorption profiles, with solutions and suspensions potentially achieving more rapid and complete absorption than the immediate-release capsules used in this study. A modified-release oral formulation that targets the colonic epithelium may be more suitable for T foetus treatment in cats. Preliminary evidence from the authors' laboratory indicates that such a formulation could be potentially successful. 26 It is also not known if it is the peak concentration (CMAX) above the MLC, or the duration above the MLC that is predictive of efficacy for T foetus elimination. Colon-targeted drug delivery could provide a means for achieving a longer duration of colonic drug concentrations above the MLC.
Previous work showed that a dose of 30 mg/kg was effective in eliminating T foetus infection in cats, but the dose was administered twice daily. To decrease the risk of drug accumulation that may produce neurotoxicity, and considering the long half-life of RDZ in cats, investigation of once-daily administration at this dose deserves study. The simulation of projected plasma concentrations from Fig 2 comparing once- vs twice-daily dosing at 30 mg/kg reveals the potential for 1.5 times greater steady-state plasma concentrations with twice-daily dosing.
It should be noted that with sonication, RDZ could be solubilized in D5W so that it could be administered as an IV solution. This is important, because RDZ is relatively water insoluble (2900 mg/l water solubility) 27 and previous IV solutions have only been prepared in relatively toxic solvents that would be undesirable to administer to cats (for example, dimethylacetamide or polyethylene glycol). 10,28,29 This D5W RDZ solution could be used safely in cats in situations where oral medication is not possible.
In conclusion, this pharmacokinetic study demonstrated rapid and complete absorption of RDZ following oral administration. After absorption, the elimination profile appears similar for both IV and oral routes of administration, indicating that the route of administration does not affect distribution or clearance. The oral dose used in this study (approximately 30 mg/kg) produced plasma RDZ concentrations that were detectable for 48 h. It is speculated from the results of this first feline RDZ pharmacokinetics study that the same dose of RDZ given twice daily may result in drug accumulation that may consequently increase the risk of neurotoxicity. These pharmacokinetic findings support the need for studies to examine the efficacy of a reduced frequency of RDZ administration (for example, once daily) for treatment of T foetus-infected cats.
Acknowledgements
The authors thank Ms Delta Dise and Maria Stone for expert technical assistance. This study was funded through a gift account to support the investigator's (MGP) research program. There were no external sponsors.
References
- 1.Tolbert M.K., Gookin J. Tritrichomonas foetus: a new agent of feline diarrhea, Compend Contin Educ Vet 31, 2009, 374–381. [PubMed] [Google Scholar]
- 2.Gookin J.L., Copple C.N., Papich M.G., et al. Efficacy of ronidazole for treatment of feline Tritrichomonas foetus infection, J Vet Intern Med 20, 2006, 536–543. [DOI] [PubMed] [Google Scholar]
- 3.Foster D.M., Gookin J.L., Poore M.F., Stebbins M.E., Levy M.G. Outcome of cats with diarrhea and Tritrichomonas foetus infection, J Am Vet Med Assoc 225, 2004, 888–892. [DOI] [PubMed] [Google Scholar]
- 4.Gookin J.L., Stauffer S.H., Coccaro M.R., Poore M.F., Levy M.G., Papich M.G. Efficacy of tinidazole for treatment of cats experimentally infected with Tritrichomonas foetus, Am J Vet Res 68, 2007, 1085–1088. [DOI] [PubMed] [Google Scholar]
- 5.Gookin J.L., Breitschwerdt E.B., Levy M.G., Gager R.B., Benrud J.G. Diarrhea associated with trichomonosis in cats, J Am Vet Med Assoc 215, 1999, 1450–1454. [PubMed] [Google Scholar]
- 6.Kather E.J., Marks S.L., Kass P.H. Determination of the in vitro susceptibility of feline Tritrichomonas foetus to 5 antimicrobial agents, J Vet Intern Med 21, 2007, 966–970. [DOI] [PubMed] [Google Scholar]
- 7.Davis J., Gookin J. Antiprotozoan drugs. Riviere J., Papich M. Veterinary pharmacology and therapeutics, 9th edn, 2009, Wiley-Blackwell: Ames, IA, 1145–1179. [Google Scholar]
- 8.Moreno S.N., Mason R.P., Muniz R.P., Cruz F.S., Docampo R. Generation of free radicals from metronidazole and other nitroimidazoles by Tritrichomonas foetus, J Biol Chem 258, 1983, 4051–4054. [PubMed] [Google Scholar]
- 9.Gookin J.L., Stauffer S.H., Dybas D., Cannon D.H. Documentation of in vivo and in vitro aerobic resistance of feline Tritrichomonas foetus isolates to Ronidazole, J Vet Intern Med 24, 2010, 1003–1007. [DOI] [PubMed] [Google Scholar]
- 10.Herman J., Vermeersch H., Remon J.P., De Backer P. Pharmacokinetics and bioavailability of ronidazole from a prolonged release tablet in the homing pigeon (Columba livia), J Vet Pharmacol Ther 12, 1989, 46–49. [DOI] [PubMed] [Google Scholar]
- 11.Franssen F.F., Lumeij J.T. In vitro nitroimidazole resistance of Trichomonas gallinae and successful therapy with an increased dosage of ronidazole in racing pigeons (Columba livia domestica), J Vet Pharmacol Ther 15, 1992, 409–415. [DOI] [PubMed] [Google Scholar]
- 12.Rosado T.W., Specht A., Marks S.L. Neurotoxicosis in 4 cats receiving ronidazole, J Vet Intern Med 21, 2007, 328–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pham D. Chronic intermittent diarrhea in a 14-month-old Abyssinian cat, Can Vet J 50, 2009, 85–87. [PMC free article] [PubMed] [Google Scholar]
- 14.Dow S.W., LeCouteur R.A., Poss M.L., Beadleston D. Central nervous system toxicosis associated with metronidazole treatment of dogs: five cases (1984–1987), J Am Vet Med Assoc 195, 1989, 365–368. [PubMed] [Google Scholar]
- 15.Evans J., Levesque D., Knowles K., Longshore R., Plummer S. Diazepam as a treatment for metronidazole toxicosis in dogs: a retrospective study of 21 cases, J Vet Intern Med 17, 2003, 304–310. [DOI] [PubMed] [Google Scholar]
- 16.Rao D.N., Mason R.P. Generation of nitro radical anions of some 5-nitrofurans, 2- and 5-nitroimidazoles by norepinephrine, dopamine, and serotonin. A possible mechanism for neurotoxicity caused by nitroheterocyclic drugs, J Biol Chem 262, 1987, 11731–11736. [PubMed] [Google Scholar]
- 17.Caylor K.B., Cassimatis M.K. Metronidazole neurotoxicosis in two cats, J Am Anim Hosp Assoc 37, 2001, 258–262. [DOI] [PubMed] [Google Scholar]
- 18.United States Pharmacopeial Convention – National Formulary (USP 33/NF28). General chapter 711 Dissolution. United States Pharmacopeia, 2010, United States Pharmacopeia: Rockville, MD. [Google Scholar]
- 19.randomization.com. http://www.tufts.edu/gdallal/assign.htm (accessed June 19, 2007).
- 20.Diehl K.H., Hull R., Morton D., et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes, J Appl Toxicol 21, 2001, 15–23. [DOI] [PubMed] [Google Scholar]
- 21.United States Pharmacopeial Convention – National Formulary (USP 33/NF28) General chapter 1225. United States Pharmacopeia, 2010, United States Pharmacopeia: Rockville, MD. [Google Scholar]
- 22.Yamaoka K., Nakagawa T., Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations, J Pharmacokinet Biopharm 6, 1978, 165–175. [DOI] [PubMed] [Google Scholar]
- 23.Haller M., Rohner K., Muller W., et al. Single-injection inulin clearance for routine measurement of glomerular filtration rate in cats, J Feline Med Surg 5, 2003, 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burczynski F.J., Pushka K.L., Sitar D.S., Greenway C.V. Hepatic plasma flow: accuracy of estimation from bolus injections of indocyanine green, Am J Physiol 252 (5 Pt 2), 1987, H953–H962. [DOI] [PubMed] [Google Scholar]
- 25.WHO 669 Ronidazole, WHO Food Additives Series 25, 1990, INCHEM. http://www.inchem.org/documents/jecfa/jecmono/v25je05.htm, (accessed Dec 26, 2009)
- 26.LeVine D., Papich M., Gookin J., et al. Ronidazole pharmacokinetics in cats after IV administration and oral administration of an immediate release capsule and a colon-targeted delayed release tablet [abstract], J Vet Intern Med 22, 2008, 745. [Google Scholar]
- 27.ChemIDplus, Advanced. Ronidazole. http://chem.sis.nlm.nih.gov/chemidplus/ProxyServlet?objectHandle=Search&actionHandle=getAll3DMViewFiles&nextPage=jsp%2Fcommon%2FChemFull.jsp%3FcalledFrom%3D&chemid=007681767&formatType=_3D (accessed Dec 25, 2009).
- 28.Baert L., Vermeersch H., Remon J.P. Effect of some organic vehicles on the rate of elimination of intravenously administered ronidazole in pigeons, J Vet Pharmacol Ther 14, 1991, 341–343. [DOI] [PubMed] [Google Scholar]
- 29.Baert L., Herman J., Remon J.P. Influence of feeding on the bioavailability of ronidazole prolonged-release formulations in pigeons, J Vet Pharmacol Ther 13, 1990, 304–308. [DOI] [PubMed] [Google Scholar]


