Abstract
Inflammatory polyps of the nasal turbinates (IPNT) in cats are benign growths that are histologically distinct from feline nasopharyngeal polyps. Most cats with IPNT are presented at less than 1 year of age with sneezing, noisy breathing and epistaxis, but without mucoid or mucopurulent nasal discharge. Histologically, IPNT are characterised by the presence of woven bone as part of the proliferating stroma and erythrocyte-filled spaces. These unique histological features are analogous to nasal hamartomas (NH) of children, specifically chondromesenchymal hamartoma (NCMH) and sinonasal fibro-osseous hamartoma (SFOH), which also result in signs of nasal obstruction, sneezing and epistaxis. In our study, clinical and histopathological features in five cats with IPNT were compared with published descriptions of NH in children. We conclude that the terminology ‘feline mesenchymal nasal hamartoma’ provides a more accurate description of the disease currently termed IPNT, and has the added advantage of being consistent with its human counterpart.
The terminology ‘inflammatory polyps of the nasal turbinates’ (IPNT) has been used to describe a rare, benign disease of the nasal passages of young cats. 1–3 Cats with IPNT are usually younger than 1 year old, and are presented with epistaxis, paroxysmal sneezing and stertorous breathing. A unique clinical feature of this nasal disease is the absence of mucoid or mucopurulent nasal discharge. Tissue protruding from the nostril, open-mouth breathing and frontonasal deformation has been reported less frequently. 1–3
Inflammatory polyps of the nasal turbinates (IPNT) should not be confused with nasopharyngeal polyps (NPP). IPNT arise in the nasal passages and occasionally extend into the rhinopharynx, while NPP originate from the eustachian tube and can grow into the rhinopharynx, the middle ear or both. 4 Although both IPNT and NPP occur most often in young cats, to the authors’ knowledge, epistaxis has not been reported in cats with NPP. Macroscopically, IPNT are firm to cystic, and reddish-grey in colour, while NPP are usually solid, and white to pink in colour. These two lesions are also distinct histologically, with NPP characterised by a loose connective tissue stroma surrounded by an often ulcerated pseudostratified epithelium and infiltrated in some submucosal areas by macrophages, lymphocytes and plasma cells. 3–5
Nasal hamartomas (NH) are an uncommon, benign lesion in people that have striking similarities to IPNT in cats. Also arising in the nasal passages, NH are consistent with the excessive growth of tissues indigenous to the site of origin and can be composed of mesenchymal or epithelial tissue. 6,7 Mesenchymal hamartomas are the more common NH and have been termed lipomatous, chondroid, angiomatous, or chondromesenchymal, depending on the nature of the mesenchymal elements. Nasal chondromesenchymal hamartoma (NCMH) and sinonasal fibro-osseous hamartoma (SFOH) of children have clinical and pathological features that are strongly analogous to those of IPNT of cats. 6,8 Thus, in our study, the clinical and histopathological findings in five cats with IPNT are compared with published descriptions of NH in children in order to determine if IPNT in cats would be more appropriately designated as ‘feline mesenchymal nasal hamartoma’.
Materials and methods
Five cats were identified that had a histological diagnosis of IPNT and that had undergone nasal radiography or computed tomography (CT) and rhinoscopy. Cats were presented between May 2005 and February 2008 to the School of Veterinary Medicine, Università degli Studi di Milano, Italy. Medical records were reviewed for: signalment, history and physical examination, radiology reports from nasal radiography and CT, treatment and outcome. Results from the complete blood count (CBC), serum biochemistry panels, urinalysis, and feline leukaemia virus (FeLV) and feline immunodeficiency virus (FIV) testing were also reviewed. Outcome was based on a combination of one or more of the following: clinical re-evaluation, repeated rhinoscopy or telephone interviews.
Nasal biopsy specimens were prepared as follows. Tissues were fixed in 10% buffered formalin, embedded in paraffin, and cut to 3 μm thickness. In addition to routine staining with haematoxylin and eosin, immunohistochemical studies were performed using the avidin–biotin peroxidase complex method. Antibodies were chosen according to the tissues found in the feline lesion and in human NH. 9–13
Sections from all specimens were stained with vimentin, S-100 protein, factor VIII, α-smooth muscle actin (SMA) and pancytokeratin (PC) (DaKo, CA, USA; clones are shown in Table 2). All immunostaining was performed according to laboratory standardised protocols, with appropriate tissue sections as controls. Control tissues used were normal skin for PC, subcutaneous fibrosarcoma for vimentin, tissue fragments from nerve sheath tumour for S-100 protein, normal muscular tissue for SMA and endothelial cells from intestinal tissue for factor VIII. The slides were examined by the same pathologist (DO).
Table 2.
Results of immune staining.
| Case | Vimentin (DAKO clone V9, 1:100) | S-100 (DAKO clone Zy44, 1:1000) | Factor VIII (DAKO, 1:100) | SMA (DAKO HHF35, 1:50) | PC (AE1–AE3 DAKO, 1:100) |
|---|---|---|---|---|---|
| 1 | S+, C+, O | IC | EFS+, S | ++ | LE |
| 2 | S+, C | IC | EFS+, S | + | LE |
| 3 | S+, C+, O | IC | EFS | + | LE |
| 4 | S+, C+, O | C+, S | EFS+, S | ++ | LE |
| 5 | S+, C+, O | IC | EFS+, S | + | LE |
SMA=smooth muscle actin; PC=pancytokeratin; S=fibrous stromal component; C=chondrocytes; IC=immature chondrocytes; O=osteocytes; EFS=endothelial cells lining the erythrocytes-filled spaces; LE=lining epithelium. Staining scale: 1+ indicates weak intensity; 2+, moderate intensity; and 3+, strong intensity.
Results
Signalment and clinical presentation
Of the five cats, two were female domestic shorthair (DSH), two were male DSH and one was female Holy Birman breed. Mean age was 10.8 months (range, 6–18 months). The main clinical signs are shown in Table 1. On physical examination, there was no air passage through the nasal cavity involved (right side in two cats, left side in four cats). Mean duration of clinical signs was 3.5 months (range, 1–8 months). None of the cats had a history of upper airway infections and all cats tested negative for FeLV and FIV (SNAP Combo FeLV antigen/FIV antibody; Idexx laboratories, ME, USA). CBC, blood chemistry and urinalysis were unremarkable in all cats.
Table 1.
Signalment, clinical signs, duration of clinical signs and lesion localization of the five cases of the study.
| Case | Breed | Age (month) | Sex | Duration (month) | Clinical signs | Localization | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | DSH | 6 | F | 1 | Paroxysmal sneezing, epistaxis, stertorous breathing | Monolateral right | Per-endoscopic removal |
| 2 | DSH | 7 | M | 1 | Paroxysmal sneezing, snoring, bilateral serous nasal discharge | Bilateral | No therapy |
| 3 | DSH | 18 | Fs | 8 | Stertorous breathing, open mouth breathing | Monolateral left and rhinopharynx | Dorsal rhinotomy |
| 4 | Holy Birman | 16 | F | 5 | Paroxysmal sneezing, epistaxis, nasal stridor, mass protruding from the nostril | Monolateral left | Per-endoscopic removal |
| 5 | DSH | 7 | Mn | 2.5 | Paroxysmal sneezing, epistaxis | Monolateral left | Per-endoscopic removal |
DSH = domestic short hair, F = female, Fs = female spayed, M = male, Mn = male neutered.
Imaging and rhinoscopic findings
All cats had radiographically identified lesions on the ventrodorsal open mouth view which were described as opalescent radiodensity and loss of the turbinate pattern within the nasal cavity (Fig 1). The changes were unilateral in four cats and bilateral in one. Partial opacification of the frontal sinus on the rostro-caudal view was present in one cat. CT was performed on one cat and showed the presence of a soft tissue density together with the loss of the turbinate pattern in the left nasal cavity and almost total occlusion of the rhinopharynx. The frontal sinuses were unremarkable. Minimal contrast enhancement was observed (data not shown).
Fig 1.
Ventrodorsal open mouth view of case 5. Note the opalescent aspect of the left nasal cavity and partial radiopacity of the contralateral nasal cavity. Concurrent regions of radiolucency (black arrow) are detectable in the left nasal cavity and likely to represent the cystic spaces associated with the lesion.
On flexible retrograde rhinoscopy, the nasopharynx was involved in only one cat and appeared completely occluded by a firm to cystic mass. On anterograde rhinoscopy, a bluish, vascularised, firm to cystic lesion (Fig 2) associated with ipsilateral atrophy of the turbinates was detected unilaterally in four cats and bilaterally in one cat.
Fig 2.

Endoscopic appearance of the lesion on anterograde rhinoscopy. The lesion is a red-bluish vascularised, firm to cystic mass totally filling the left nasal cavity of case 4.
Treatment and outcome
Treatment included per-endoscopic removal of the mass and visibly abnormal turbinates in three cats. Severe nasopharyngeal extension necessitated dorsal rhinotomy and turbinectomy in one cat. Treatment was declined by the owner of the other cat. All cats were prescribed amoxicillin and clavulanic acid (Synulox, Pfizer; 15 mg/kg q12 h for 7 days), and prednisone (Deltacortene, Bruno Farmaceutici; 1 mg/kg q12 h for 7 days, 1 mg/kg q24 h for 7 days and 1 mg/kg q48 h for 6 days).
The clinical outcome was assessed for a period of 12–44 months (mean 25.5 months). Three cats were considered cured based on subsequent clinical examination and telephone interviews; one of these was confirmed to be disease free by rhinoscopy. The untreated cat showed spontaneous resolution of clinical signs 4 months after diagnosis. The Holy Birman cat was diagnosed with recurrent disease 12 months after treatment. This cat was re-presented after an acute episode of epistaxis following sneezing. On rhinoscopy, the lesion had recurred on the same side as before (left side) but this time also had extension of disease into the left choana. The visibly abnormal tissue was again removed per-endoscopically and the cat had no further clinical signs as of 4 months after removal.
Pathology
On gross examination (Fig 3), the tissue from each cat consisted of multiple fragments of different sizes (0.5–2.3 cm). All tissue was firm and white to tan in colour. The tissues were concurrently covered by regular to irregular mucosa, which was often ulcerated. Variably sized transparent to dark red cystic lesions were visible (Fig 4). Many others cystic spaces with interposed stroma were detectable on cut surface. The cyst content was haemorrhagic, serosanguineous or serous.
Fig 3.

Macroscopic appearance of the lesion at necropsy in a 9-month-old male castrated DSH cat presenting epistaxis and stertorous breathing. Note the severe extension of the lesion in the sinonasal cavity and the haemorrhagic content of the several cystic spaces. On physical examination the cat exhibited severe nasofrontal deformation. Source: Hedlund, Taboada, eds. Clinical atlas of ear, nose and throat diseases in small animals, the case-based approach. Hannover, Germany: Schlütersche, 2002.
Fig 4.
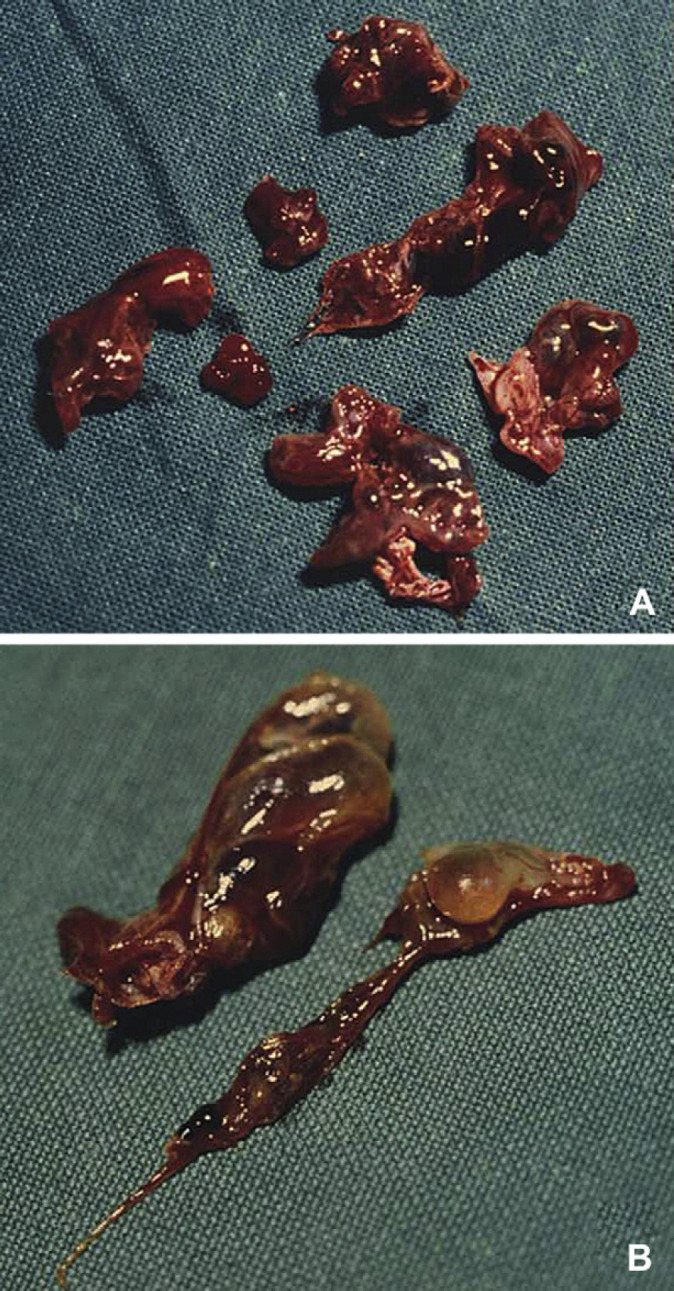
Macroscopic features: (A) several cystic spaces filled with serous to serosanguineous variably sized cystic spaces (black arrow); (B) cystic spaces containing transparent to haemorrhagic fluid.
Histologically, the tissues were composed of various mesenchymal components, mainly fibrous connective tissue and woven bone, and erythrocyte-filled spaces; the tissue fragments were covered by ciliated columnar epithelium (Fig 5A–C). The stroma was mainly composed of spindle cells with variable cellularity. The areas with less cellularity showed a myxomatous appearance; in the more cellular areas the spindle cells were organised in fascicles with variable amounts of interweaving collagen, which in some areas, formed large eosinophilic bundles that merged with woven bone. The spindle cells were uniform in appearance, had elongated nuclei with fine chromatin, visible nucleoli, and small to moderate amounts of eosinophilic cytoplasm. Rare mitotic figures were present but without atypia. The bony trabeculae were of variable thickness and composed of woven bone (Fig 5D). The osteocytes had no signs of atypia and no mitoses were detected. Scattered foci of new bone formation and calcification were seen. Several erythrocyte-filled spaces observed were surrounded by spindle cells, amorphous collagen and endothelial cells. The lumen was filled with erythrocytes and serosanguineous material. Areas of haemorrhages and haemosiderin were also noted.
Fig 5.
Microscopic features: (A) Haematoxylin and eosin (HE), 2.5×: Erythrocyte-filled spaces (black arrow) and fibrous stroma are seen along with areas of cartilage and woven bone (thick black arrow). Note the normal overlying respiratory epithelium. (B) HE 10×: Presence of small cartilage areas, congested vessels and fibrous stroma. (C) HE 40×: Spindle cells interweaving among a moderate deposition of myxoid matrix, woven bone areas, erythrocyte-filled space (black arrow), and osteoclast-like giant cells (arrowhead) are seen. (D) HE 65×: Woven bone with interweaving spindle cells. The osteocytes do not show signs of atypia.
Few nodules of mature and immature hyaline cartilages were detected in each specimen. Immature chondrocytes were blended with the fibrous stroma. The nodules were occasionally hypercellular but the overall histological appearance was considered normal.
The epithelium was often organised in hyperplastic papillae with polypoid-like configuration and focal areas of ulceration were seen.
Osteoclast-like multinucleated giant cells, haemosiderin deposits and foamy macrophages were occasionally observed interposed between the spindle cells. Perivascular hyalinisation was also observed. In all cases rare neutrophils, lymphocytes and plasma cells were detected between the woven bones.
On immune staining, the mesenchymal cells and chondrocytes were positive for vimentin (Fig 6A); the immature and mature chondrocytes and osteocytes were positive for S-100 protein (Fig 6B). The spindle cells demonstrated multi-focal immunoreactivity for SMA; the expression of factor VIII demonstrated the presence of many vessels in the stroma and few vessels irregularly associated to the haemorrhagic cavities. Only the lining respiratory epithelium was positive for PC. Details for immune staining results are listed in Table 2.
Fig 6.
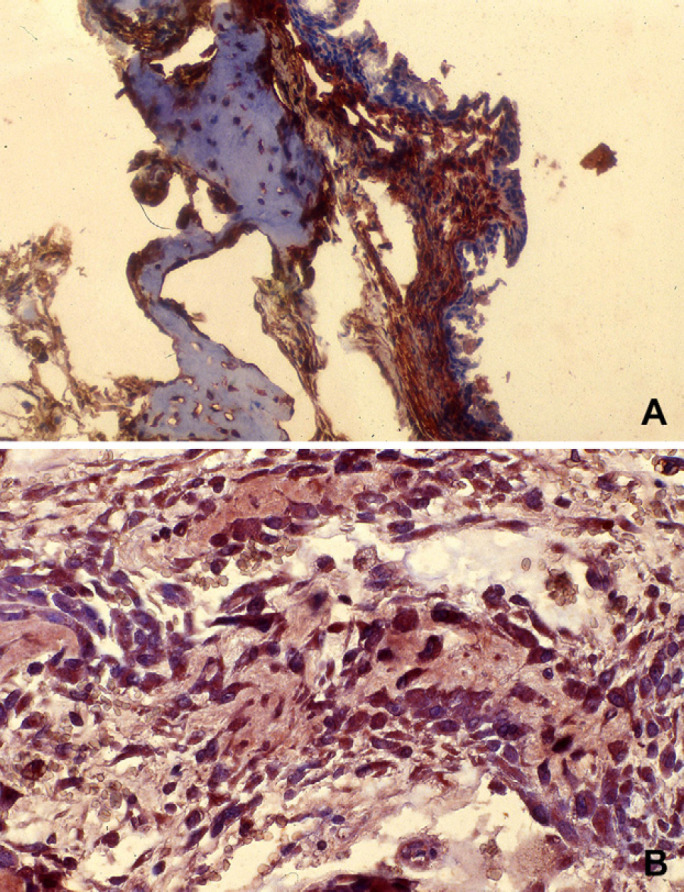
Immune staining. (A) Vimentin staining, 10×: Positive staining is limited to the spindle cells present in this section that includes woven bone trabecula and fibrous stroma. (B) S-100 staining: Positive staining is limited to osteocytes.
Discussion
The feline lesion IPNT has been reported in the veterinary literature as haemangiomas, IPNT, fibrous dysplasia, ossifying fibroma and aneurysmal bone cyst. 1–3,14 None of these terms accurately represents the features of IPNT. It is clear that this lesion is not an haemangioma or a nasopharyngeal polyp because the first is a neoplasm of vascular origin and the latter consists of a core of loosely fibrovascular tissue, with interposed inflammatory cells, covered by a stratified squamous to ciliated columnar epithelium. 4,5 Fibrous dysplasia is considered a genetic disease affecting bones; it is composed of fibrous tissue and woven bone without osteoblastic rimming. 8,15 Ossifying fibroma is a benign tumour composed of fibrous stroma and deposition of lamellar bone with osteoblastic rimming. 8,16 Fibrous dysplasia and ossifying fibroma are not characterised by erythrocyte-filled spaces, do not usually affect the nasal passages, and are not diagnosed in infants. Aneurysmal bone cysts have cavernous spaces filled with blood but without a lining endothelium; the spaces are separated by collagenous tissue containing fibroblast and multinucleated giant cells; normal to woven bone trabeculae and scattered foci of cartilage are also detectable. 17,18 Though microscopically similar to the feline lesion, aneurysmal bone cysts usually affect long bone and rarely arise in the nasal passages. In cats, it has been reported only affecting the long bones. 19,20 Osseous and chondroid metaplasia were not considered likely because the tissues involved in the feline lesion here described are indigenous to the site of origin.
We believe our findings, and those of previously reported cases of cats with IPNT, support the use of the nomenclature from human medicine of NH as a more accurate description of the feline lesion. The feline disease involves tissues normally present in the nasal cavities and, as described for mesenchymal hamartomas, is composed of two or more mesenchymal components, in addition to fibrous tissue. 6,21 In both species, the main clinical findings are signs of respiratory occlusion, epistaxis and less frequently facial deformation. 1,3,6–10,22–26
The aetiopathogenesis of both NCMH and SFOH is obscure, but authors have suggested the possibility of a congenital disease 6 ; in the few cases reported in adults a proper hormonal or environmental stimulus could have led the lesion present since birth to grow. 9,22,23,25
In cats, the early age of diagnosis (usually between 6 and 12 months) suggests the possibility of a hereditary or congenital cause. In support of a genetic predisposition, IPNT have been diagnosed in brothers and in a kitten related to a previously affected male cat. 27 Upper airway infections have been proposed as possible inciting factors, but no evidence of this has been demonstrated. 1,3 The disease has a relatively high incidence in Italy (personal observation), which could be a result of either genetic or environmental factors.
NH in people and cats are benign lesions, but they can be locally aggressive showing expansive and destructive clinical and radiological features suggestive of malignancy (Fig 3). In both species, a good prognosis is warranted; complete surgical excision has been reported to be curative but recurrences due to incomplete removal have been reported. 1–3,6,8,28 Spontaneous regression of the lesions has also been described and occurred in one of the five cats in this study. 26,29 Per-endoscopic removal hereby reported was a new minimally invasive approach which could potentially represent an alternative technique for the treatment of this feline disease in cases without severe nasopharyngeal extension or frontonasal deformation.
Gross and histopathological similarities between IPNT in cats and NH of the children are clearly noticeable. Macroscopically, NCMH appears white to light tan, of fibrous consistency, with foci of calcification and multiple cystic spaces. Microscopically, NCMH is a collage of histological features mainly characterised by demarcated nodules of cartilage with some variation in the cellular density and maturation of the chondrocytes, a myxoid to spindle cell stroma, the presence of woven bone and focal osteoclast-like giant cells within the stroma, and erythrocyte-filled spaces. NCMH are often lined by respiratory epithelium which occasionally shows a polypoid configuration. 6,7,9,10,22,25 The presence of inflammatory cells has been seldom reported. 6,9,23 Cases of NCMH lacking a cartilage component have been reported and the need for cartilage as a prerequisite for the diagnosis of NCMH requires further evaluation. 8,30
Reported cases of IPNT in cats have shown the entire collage of NCMH, but in cats the predominant component is bone rather than cartilage. Hence, our recommendation is for the more general terminology of nasal mesenchymal hamartoma, rather than NCMH. The tissue from the cats in our study had immunostaining properties consistent with NH. In both species, the spindle cells expressed vimentin and SMA indicating a myofibroblastic phenotype which might undergo maturation or differentiation into a vascularised matrix producing osteocartilaginous areas as in the fetus. 7,9,29,30 Immunoreactivity of chondrocytes to S-100 may indicate a functional role of chondrocytes for bone formation in the lesion. 9,31
In conclusion, we propose that the benign, tumefactive disease of the nasal cavity of young cats, most recently termed IPNT, be referred to in the future as ‘feline mesenchymal nasal hamartoma’. The terminology ‘feline mesenchymal nasal hamartoma’ provides a more accurate description of the disease and has the additional advantage of being consistent with its human counterpart.
References
- 1.Galloway P.E., Kyles A., Henderson J.P. Nasal polyps in a cat, J Small Anim Pract 2, 1997, 78–80. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter J.L., Andrews L.K., Holzworth J. Tumors and tumor-like lesions. Holzworth J. Diseases of the cat, 1987, WB Saunders: Philadelphia, 406–596. [Google Scholar]
- 3.Mortellaro C.M. The nasal cavity and paranasal sinuses. Hedlund C.S., Taboada J. Clinical atlas of ear, nose and throat diseases in small animals, 1st edn, 2002, Schlütersche: Hannover, 61–102. [Google Scholar]
- 4.Anderson D.M., Robinson R.K., White R.A.S. Management of inflammatory polyps in 37 cats, Vet Rec 147, 2000, 684–687. [PubMed] [Google Scholar]
- 5.Kudnig S.T. Nasopharyngeal polyps in cats, Clin Tech Small Anim Pract 4, 2002, 174–177. [DOI] [PubMed] [Google Scholar]
- 6.McDermott M.B., Ponder T.B., Dehner L.P. Nasal chondromesenchymal hamartoma: an upper respiratory tract analogue of the chest wall mesenchymal hamartoma, Am J Surg Pathol 4, 1998, 425–433. [DOI] [PubMed] [Google Scholar]
- 7.Hsueh C., Hsueh S., Gonzalez-Crussi F., Lee T., Su J. Nasal condromesenchymal hamartoma in children, Arch Pathol Lab Med 3, 2001, 400–403. [DOI] [PubMed] [Google Scholar]
- 8.Boudewyns A.N., van Dinther J.J., Colpaert C.G. Sinonasal fibro-osseous hamartoma: case presentation and differential diagnosis with other fibro-osseous lesions involving the paranasal sinuses, Eur Arch Otorhinolaryngol 3, 2006, 276–281. [DOI] [PubMed] [Google Scholar]
- 9.Ozolek J.A., Carrau R., Barnes E. Leon, Hunt J.L. Nasal condromesenchymal hamartoma in older children and adults, Arch Pathol Lab Med 11, 2005, 1444–1450. [DOI] [PubMed] [Google Scholar]
- 10.Kato K., Ijiri R., Tanaka Y., Hara M., Sekido K. Nasal chondromesenchymal hamartoma of infancy: the first Japanese case report, Pathol Int 8, 1999, 731–736. [DOI] [PubMed] [Google Scholar]
- 11.Joling P., Broekhuizen R., de Weger R.A., Rottier P.J., Egberink H. Immunohistochemical demonstration of cellular antigens of the cat defined by anti-human antibodies, Vet Immunol Immunopathol 53, 1996, 115–127. [DOI] [PubMed] [Google Scholar]
- 12.Couto S.S., Griffey S.M., Duarte P.C., Madewell B.R. Feline vaccine-associated fibrosarcoma: morphologic distinctions, Vet Pathol 39, 2002, 33–41. [DOI] [PubMed] [Google Scholar]
- 13.Fuji R.N., Patton K.M., Steinbach T.J., et al. Feline systemic reactive angioendotheliomatosis: eight cases and literature review, Vet Pathol 42, 2005, 608–617. [DOI] [PubMed] [Google Scholar]
- 14.Cheli R., Mortellaro C.M., Andreola S. Su di una particolare turbinopatia nel gatto. About a nasal cavities disease of the cat, SISVet 38, 1984, 310–313. [Google Scholar]
- 15.Al Kaissi A., Klaushofer K., Grill F. Outward bulging of the right parietal bone in connection with fibrous dysplasia in an infant: a case report, Cases J 1, 2008, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelbrecht V., Preis S., Hassler W., Lenard H.G. CT and MRI of congenital sinonasal ossifying fibroma, Neuroradiol 7, 1999, 526–529. [DOI] [PubMed] [Google Scholar]
- 17.Soyer T., Karnak I., Talim B., Tanyel F.C. Aneurysmal bone cyst of the rib in a child: report of a case, Surg Today 10, 2005, 886–889. [DOI] [PubMed] [Google Scholar]
- 18.Purohit A., Chopra S., Sinha V.D., Dharker S.R. Aneurysmal bone cyst of the temporal bone: a case report, Neurol India 4, 2002, 511–513. [PubMed] [Google Scholar]
- 19.Walker M.A., Duncan J.R., Shaw J.W., Chapman W.W. Aneurysmal bone cyst in a cat, J Am Vet Med Assoc 10, 1975, 933–934. [PubMed] [Google Scholar]
- 20.Biller D.S., Johnson G.C., Birchard S.J., Fingland R.B. Aneurysmal bone cyst in a rib of a cat, J Am Vet Med Assoc 9, 1987, 1193–1195. [PubMed] [Google Scholar]
- 21.Kim B., Park S.H., Min H.S., Rhee J.S., Wang K.C. Nasal chondromesenchymal hamartoma of infancy clinically mimicking meningoencephalocele, Pediatr Neurosurg 3, 2004, 136–140. [DOI] [PubMed] [Google Scholar]
- 22.Alrawi M., McDermott M., Orr D., Russell J. Nasal chondromesynchymal hamartoma presenting in an adolescent, Int J Pediatr Otorhinolaryngol 6, 2003, 669–672. [DOI] [PubMed] [Google Scholar]
- 23.Low S.E., Sethi R.K., Davies E., Stafford J.S. Nasal condromesenchymal hamartoma in an adolescent, Histopathology 3, 2006, 321–323. [DOI] [PubMed] [Google Scholar]
- 24.Silkiss R.Z., Mudvari S.S., Shetlar D. Ophthalmologic presentation of nasal chondromesenchymal hamartoma in an infant, Ophthal Plast Reconstr Surg 3, 2007, 243–244. [DOI] [PubMed] [Google Scholar]
- 25.Johnson C., Nagaraj U., Esguerra J., Wasdahl D., Wurzbach D. Nasal chondromesenchymal hamartoma: radiographic and histopathologic analysis of a rare pediatric tumor, Pediatr Radiol 1, 2007, 101–104. [DOI] [PubMed] [Google Scholar]
- 26.Mortellaro CM. Nasal polyps in cats. In: Proceedings of the XVII WSAVA Congress; 1992 September 24–27; Rome, Italy: 507–15.
- 27.Mortellaro CM. Cosiddetta poliposi nasale del gatto (displasia fibrosa dei turbinati del gatto): diagnosi e trattamento. In: Proceedings of the 29th SCIVAC Congress; 1995 Sept 27–30; Salsomaggiore, Italy: 49–54.
- 28.Norman E.S., Bergman S., Trupiano J.K. Nasal condromesenchymal hamartoma: report of a case and review of the literature, Pediatr Dev Pathol 5, 2004, 517–520. [DOI] [PubMed] [Google Scholar]
- 29.Amstalden E.M., Carvalho R.B., Pacheco E.M.B., Oliveira-Filho A., Stragea-Neto L., Rosenberg A.E. Chondromatous hamartoma of the chest wall: description of 3 new cases and literature review, Int J Surg Pathol 2, 2006, 119–126. [DOI] [PubMed] [Google Scholar]
- 30.Shet T., Borges A., Nair C., Desai S., Mistry R. Two unusual lesions in the nasal cavity of infants – a nasal chondromesenchymal hamartoma and an aneurysmal bone cyst like lesion. More closely related than we think?, Int J Pediatr Otorhinolaryngol 3, 2004, 359–364. [DOI] [PubMed] [Google Scholar]
- 31.Weiss A.P., Dorfman H.D. S-100 protein in human cartilage lesions, J Bone Joint Surg Am 4, 1986, 521–526. [PubMed] [Google Scholar]




