Abstract
A percutaneous technique to place oesophageal feeding tubes in cats is described. Nineteen traumatised cats underwent different surgical procedures for facial and/or oral trauma and subsequent oesophagostomy. The tube placement was easy to perform, requiring a 14 G over-the-needle vascular catheter, a curved forceps and a 1.2 mm feeding tube. During insertion, the tube was directed aborally (normograde direction) to its definitive position in a one-step procedure. The tube was removed when the patient started to eat voluntarily. A major limit of this technique was represented by the small diameter of the feeding tube, requiring the use of a liquid diet. However, considering the absence of complications, the rapidity of the procedure and its cost efficacy, it was considered as an effective alternative to more complicated and invasive techniques, in particular if a short period of starvation is predictable.
Management of traumatised and critical patients should be considered as a multidisciplinary speciality, in which first assessment, end-points of resuscitation, time of surgical intervention, postoperative care as well as nutritional support are particularly important. Many traumatised patients are unable to voluntarily consume food, and both parenteral and enteral feeding methods may be used to maintain an adequate protein and caloric intake, thereby reducing morbidity and possibly a prolonged hospitalisation. 1–3 While the parenteral route involves intravenous administration of nutritional products through a central or peripheral vein 4,5 ; the enteral route relies on the insertion of a feeding tube. 6 Enteral nutrition should be preferred over the parenteral nutrition if the gastrointestinal tract is functional. 6 In addition to preserving the intestinal mucosal barrier and thereby reducing the risk of bacterial translocation and potential septic complications, 7,8 enteral delivery of nutrients is more physiological and cost effective. 4,9–11 Further, patients receiving parenteral nutrition may experience catheter-related infections, catheter obstructions and metabolic or electrolyte imbalances. 12,13 Multiple techniques are available for enteral placement of feeding tubes (nasoesophageal, oesophagostomy, gastrostomy, jejunostomy and transpyloric feeding tubes), the decision of which method to use depends on the site of disease, the approximate time the animal is expected to starve as well as early and late potential complications regarding tube insertion and position. 10,11 All but naso-oesophageal/gastric tubes require general anaesthesia, thereby limiting the use of most of them in critically ill patients. An oesophagostomy tube allows delivery of food or water to patients unable or unwilling to eat. It can be used immediately after insertion, provides for short and long time patient nutrition and can be safely removed if the patient starts to eat spontaneously. 11,14 Since the first report describing the use of an oesophagostomy tube in critically ill patients, several methods of placement have been described. 15–19
The aim of this report is to describe a rapid, safe and minimally invasive technique to place an oesophagostomy feeding tube in cats with oral and facial trauma.
Materials and Methods
Patient Selection
A group of traumatised cats with facial trauma requiring an oesophagostomy feeding tube were retrospectively evaluated. No age, breed, or sex restrictions were applied. Required follow-up was from surgery to removal of the oesophagostomy tube. Patients dying immediately after surgery or with an incomplete signalment or follow-up were excluded from the study group.
Data Collection
Data on breed, age, gender or sexual status, body weight, surgical procedure other than oesophagostomy tube insertion, complications and time of tube removal were collected.
Oesophagostomy Tube Insertion Technique
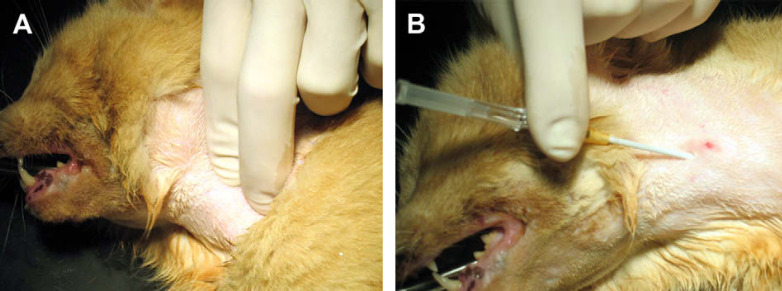
Anaesthetised and intubated patients were positioned in right lateral recumbence; the lateral cervical area was clipped and aseptically prepared for tube insertion. A Kelly forceps or, alternatively, others angled or curved forceps of appropriate length was introduced through the mouth into the proximal oesophagus; forceps jaws were slightly open and the tips palpated under the skin in the middle cervical area dorsally to the jugular vein. A 14 G over-the-needle catheter, 64 mm long, was inserted following a normograde direction (ie, towards the stomach) in between the forceps tips directly into the oesophagus (Fig 1). The needle was promptly removed right after the oesophagus was penetrated, whereas the external catheter sheath was pushed caudally. A feeding tube fitting the internal diameter of the 14 G vascular catheter (outer diameter 2.15 mm) was subsequently selected. The tube length was measured from the entrance site in the neck to the eighth intercostal space and marked with a permanent marker. The feeding tube was then inserted through the catheter sheath until the desired depth was reached. The catheter sheath was then withdrawn and the feeding tube secured in place by a Chinese finger trap suture at its egress from the skin; alternatively a butterfly tape glued to the tube next to the entrance was sutured to the skin with a skin stapler; a second tacking suture involving the fascia of the atlas wing prevented tube dislodgement (Fig 2). The oesophagostomy tube cap prevents the catheter sheath from being completely removed; the catheter sheath is left to cover the proximal end of the oesophagostomy tube and its hub is glued to the cap. Once the procedure was complete, placement was confirmed by radiography, and a circumferential sterile bandage applied.
Fig 1.
(A) Angled or curved forceps are introduced through the mouth into the proximal oesophagus; forceps jaws are open and the tips palpated under the skin in the middle cervical area dorsally to the jugular vein. (B) A 14 G over-the-needle catheter is inserted following a normograde direction in between the forceps tips directly into the oesophagus.
Fig 2.
(A) and (B) The needle is removed and a 1.2 mm feeding tube is introduced through the sheath and pushed into the distal third of oesophagus. (C) The catheter sheath is withdrawn from the oesophagus running over the feeding tube. (D) and (E) The feeding tube is secured in place by a Chinese finger trap suture. (F) A second suture involving the fascia.
Results
Data obtained from 25 cats with facial and oral trauma were analysed, and 19 met the inclusion criteria (Table 1). Six cats were excluded from the study: two because of incomplete signalment and four for inadequate description of facial lesions and surgical technique. There were 16 domestic shorthair cats, two domestic longhair cats and one Persian cat. Ten cats were males (five castrated) and nine were female (five neutered); the age varied from 5 months to 10 years (mean 2.9 years; median 3 years). In each cat, the oesophagostomy tube was inserted at the end of the main surgical procedure. Tube feeding began 4–12 h from full anaesthesia recovery (mean 6.6 h; median 6 h); spontaneous feeding started 1–5 days postoperatively (mean 2 days; median 2 days). The feeding tube was removed 2–7 days after placement (mean 3.5 days; median 3 days). After tube removal, the oesophagostomy stoma healed by second intention in each case. Three cats (15.8%) experienced minor complications. In two cases, the tube was misplaced in the stomach, whereas in one case pharyngeal oedema developed during the anaesthesia recovery, requiring a new tracheal intubation; the oedema partially resolved with furosemide and methylprednisolone sodium succinate in 20 min. Patients were fed with a polymeric diet composed by 42% protein, 25% oil, 6.2% ash, 2.7% fibre, 4% moisture, vitamin A 10 000 IU/kg, vitamin D 1000 IU/kg, vitamin E 600 mg/kg, total copper 16 mg/kg; caloric density about 1.2 kcal/ml (Covalescence Support, Instant Diet, Waltham Veterinary Diet) at their resting energy requirement (RER=[30 ×body weight kg]+70). 20 Cats were fed either by bolus administration (five cases) or by peristaltic intermittent pump (14 cases); when using peristaltic intermittent pump, the administration rate was obtained dividing the RER volume (ml) in 24 h while the time period was selected automatically. None of cats vomited or had diarrhoea.
Table 1.
Injuries and response to oesophagostomy tube feeding in 19 cats included in the study
| Breed | Sexual status | Age | Injury | Surgical procedure | Start feeding (h) | Spontaneous feeding (days) | Removal of tube (days post op) | Compli-cations | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | DSH | M | 3 yo | Mandibular fracture (body) and soft tissues degloving injury | AESF and suture of soft tissues (reinforced by polypropylene button) | 6 | 2 | 2 | None |
| 2 | DSH | F | 5 mo | Mandibular symphysis separation | Two cross pins | 4 | 2 | 3 | Gastric position (repositioned) |
| 3 | DSH | M | 18 mo | Mandibular soft tissues degloving injury | Suture reinforced with polypropylene button | 4 | 1 | 3 | None |
| 4 | DSH | F | 6 mo | Mandibular shiphysis fracture | Two cross pins | 8 | 1 | 2 | None |
| 5 | DSH | F | 10 mo | Mandibular soft tissues degloving injury | Suture reinforced with polypropylene button | 8 | 1 | 3 | None |
| 6 | DSH | F | 1 yo | Mandibular shiphysis fracture | Two cross pins | 4 | 2 | 4 | Gastric position (not repositioned) |
| 7 | DSH | nM | 4 yo | Fracture of mandibular synphysis and right dental arch of maxilla | 1.2 mm cross pins (mandibula) 1.0 mm cross pins (maxilla) | 4 | 4 | 6 | None |
| 8 | DLH | nM | 8 yo | Mandibular fracture (horizontal ramus) | AESF | 4 | 2 | 3 | None |
| 9 | DSH | nF | 4 yo | Head trauma, mandi-bular fracture and cleft of the hard palate | Two cross pins | 6 | 3 | 4 | None |
| 10 | DSH | nF | 3 yo | Mandibular symphysis separation | Two cross pins | 6 | 1 | 2 | None |
| 11 | DSH | M | 2 yo | Mandibular symphysis separation | Two cross pins | 6 | 2 | 3 | None |
| 12 | DSH | nF | 3 yo | Mandibular symphysis separation | Two cross pins | 10 | 2 | 3 | None |
| 13 | DSH | nF | 10 mo | Mandibular symphysis separation | Two cross pins | 4 | 3 | 4 | None |
| 14 | DSH | nM | 3 yo | Mandibular symphysis separation | Two cross pins | 6 | 2 | 3 | None |
| 15 | DSH | M | 3 yo | Mandibular fracture (body) | AESF | 8 | 1 | 2 | None |
| 16 | DLH | nF | 10 yo | Mandibular symphysis separation and cleft of the hard palate | Cerclage wire and tracheostomy | 8 | 5 | 7 | None |
| 17 | Persian | nM | 5 yo | Mandibular soft tissues degloving injury | Suture reinforced with polypropylene button | 12 | 1 | 3 | None |
| 18 | DSH | nM | 18 mo | Mandibular fracture (body) | AESF | 6 | 1 | 4 | None |
| 19 | DSH | M | 8 mo | Mandibular symphysis separation | Cerclage wire | 12 | 2 | 4 | Pharingeal oedema (reintubated) |
DSH=domestic shorthair, DLH=domestic longhair, M=male, F=female, nM=neutered male, nF=neutered female, Yo=years old, mo=months old, AESF=acrylic external skeletal fixator.
Discussion
The metabolic response to trauma and surgery, characterised by altered metabolism and starvation, has been well described both in veterinary and human medicine, 20,21 and is considered as an important source of morbidity and mortality. One of the most important metabolic alterations is an increasing protein turnover rates associate with a decrease use of carbohydrated. 20,22 Indeed, in the absence of exogenous intake of proteins and other nutrients, this metabolic change leads to malnutrition, loss of lean tissue mass, increased risk of sepsis and ultimately to multiple-organ failure. 7,8,23 Enteral nutrition has been shown to have clinical advantages over parenteral nutrition in critically ill patients, and early nutritional support may attenuate the body response to trauma or surgery. 22 The technique for oesophagostomy feeding tube placement described here seems to be rapid, effective and safe.
Oesophageal feeding is more physiological than other enteral feeding, as the food traverses the most part of gastrointestinal tract. In the case of patients with facial or oral trauma, it is possible to choose between pharyngostomy and oesophagostomy feeding tubes. Despite the easiness of naso-oesophageal feeding tube placement in conscious animals, disadvantages include patient discomfort during the procedure and the requirement of an Elizabethan collar in most cases, often leading to limited voluntary food intake. Moreover, it is not advisable to insert the tube through the nasal meatus in the case of facial trauma, as the normal facial anatomy can be disrupted, potentially causing misplacement of the tube (such as an airway) and promoting the development of sinusitis. 11,24 Moreover, pre-existing epistaxis and sneezing may worsen. Pharyngostomy tube represents a valid alternative in patients undergoing oral surgery, because many of them already have a pharyngotomy for endotracheal intubation. However, pharyngostomy feeding tube may interfere with laryngeal movements (especially in cats), causing dysphagia and precluding spontaneous feeding during recovery. 15 The main disadvantage of oesophagostomy feeding tube placement is general anaesthesia. 11,14,19 However, in our series, patients were already under general anaesthesia and intubated for the main surgical procedure. The technique described here presents several advantages, such as no need for specialised instruments, readily available intravenous cannulas, easy penetration of the needle with minimal tissue trauma and undermining. The immediate advantage of providing calories is a faster recovery with fewer complications. Moreover, with this technique the tube is placed in its definitive distal position without being exteriorised and redirected aborally. Redirecting the tube back through the oro-pharynx into the oesophagus could be difficult as it is anatomically narrow. 19 A relative disadvantage of this procedure is represented by an obligate small diameter feeding tube and the use of a liquid diet; this may cause diarrhoea, 11,25 especially if human products are used (personal observation). However, none of the patients in this group showed gastrointestinal problems; either with bolus administration or with delivery by peristaltic pump, possibly due to the relatively short term of artificial feeding or to the composition of the diet used, which is intended formulated for animals. Common complications, such as misplacement (mediastinum or airways), infection at the tube-skin interface and dislodgement by vomit, 11,17 were not observed here. Possible explanations are that the small number of patients did not allow for complication recognition, or the short period during which the tube was left in place. In two cases the tube reached the stomach because of incorrect measurement. In one case the tube was left in the stomach with no obvious negative consequences, while in the other it was repositioned in the distal oesophagus. According to the literature, if the tip of the tube is placed in the stomach, gastric reflux can occur, potentially damaging the distal oesophagus. 26 For this reason, it is advisable to pre-measure the oesophagostomy tube carefully before its placement. 19 In the third case, pharyngeal oedema developed, leading to marked respiratory (inspiratory) distress during recovery from anaesthesia. Although causality could not be demonstrated, it can be speculated that both procedures (surgical and tube placement) could have contributed to the development of pharyngeal swelling and obstruction.
Although the technique described in this paper seems to be efficacious in cats, its use is limited to short term enteral nutrition, because of the small diameter of the feeding tube (more likely to obstruct than larger diameter oesophagostomy tubes) and, in turn, the obligate use of specialised liquid diet. In particular, the diet used in this study can be difficult to manage by the owner as, if not properly dissolved in water, it tends to coagulate obstructing the feeding tube. This could limit its use at home. In the future it could be opportune to use liquid diet (ie, non-instant diet) in order to assess this technique for long term (at home) nutritional support.
In conclusion, the easiness of the procedure, the availability of the materials, the low degree of invasiveness and the cost efficacy of the procedure, may increase the use of this method of feeding in the surgical, traumatised or critically ill patients needing a short period of artificial nutrition and in which the facial trauma precludes the use of naso-oesophageal feeding tubes.
References
- 1.Crowe D.T., Devey J.J. Clinical experience with jejunostomy feeding tubes in 47 small animal patients, J Vet Emerg Crit Care 7 (1), 1997, 7–19. [Google Scholar]
- 2.Gramlich L., Kichian K., Pinilla J., et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patient? A systematic review of the literature, Nutrition 20, 2004, 843–848. [DOI] [PubMed] [Google Scholar]
- 3.Michel K.E., Higgins C. Investigation of the percentage of prescribed enteral nutrition actually delivered to hospitalized companion animals, J Vet Emerg Crit Care 16 (S1), 2006, S2–S6. [Google Scholar]
- 4.Thomovsky E., Reniker A., Backus R., Mann F.A., Dodam J.R. Parenteral nutrition: Uses, indications, and compounding, Comp Cont Edu Pract Vet 29, 2007, 76–84. [PubMed] [Google Scholar]
- 5.Chandler M.L., Payne-James J.J. Prospective evaluation of a peripherally administered three-in-one parenteral nutrition product in dog, J Small Anim Pract 47, 2006, 518–523. [DOI] [PubMed] [Google Scholar]
- 6.Waddel L.S., Michel K.E. Critical care nutrition: Routes of feeding, Clin Tech Small Anim Pract 13, 1998, 197–203. [DOI] [PubMed] [Google Scholar]
- 7.Hannant D. Mucosal immunology: Overview and potential in veterinary species, Vet Immunol Immunopathol 87, 2002, 265–267. [DOI] [PubMed] [Google Scholar]
- 8.Kenneth A., Kudsk M.D. Current aspects of mucosal immunology and its influence by nutrition, Am J Surg 183, 2002, 390–398. [DOI] [PubMed] [Google Scholar]
- 9.Frost P., Bihari D. The route of nutritional support in the critically ill: Physiological and economical considerations, Nutrition 13 (9), 1997, 58S–63S. [DOI] [PubMed] [Google Scholar]
- 10.Prittie J., Barton L. Route of nutrient delivery, Clin Tech Small Anim Pract 19, 2004, 6–8. [DOI] [PubMed] [Google Scholar]
- 11.Han E. Esophageal and gastric feeding tubes in ICU patients, Clin Tech Small Anim Pract 19, 2004, 22–31. [DOI] [PubMed] [Google Scholar]
- 12.Woodcock N.P., Zeigler D., Palmer M.D., et al. Enteral versus parenteral nutrition: A pragmatic study, Nutrition 17, 2001, 1–13. [DOI] [PubMed] [Google Scholar]
- 13.Pyle S.C., Marks S.L., Kass P.H. Evaluation of complications and prognostic factors associated with administration of total parenteral nutrition in cats: 75 cases (1994–2001), J Am Vet Med Assoc 225, 2004, 242–250. [DOI] [PubMed] [Google Scholar]
- 14.Mazzaferro E.M. Esophagostomy tubes: Don't underutilize them!, J Vet Emerg Crit Care 1 (2), 2001, 153–156. [Google Scholar]
- 15.Crowe D.T. Nutritional support for the hospitalized patient: An introduction of tube feeding, Comp Cont Edu Pract Vet 12, 1990, 1711–1721. [Google Scholar]
- 16.Rawlings C.A. Percutaneous placement of a midcervical esophagostomy tube: New technique and representative cases, J Am Anim Hosp Assoc 29, 1993, 526. [Google Scholar]
- 17.Crowe D.T., Devey J.J. Esophagostomy tubes for feeding and decompression: Clinical experience in 29 small animal patients, J Am Anim Hosp Assoc 33, 1997, 393. [DOI] [PubMed] [Google Scholar]
- 18.Devitt C.M., Seim H.B. Clinical evaluation of tube esophagostomy in small animals, J Am Anim Hosp Assoc 33, 1997, 55–60. [DOI] [PubMed] [Google Scholar]
- 19.von Werthen C.J., Wess G. A new technique for insertion of esophagostomy tubes in cats, J Am Anim Hosp Assoc 37, 2001, 140–144. [DOI] [PubMed] [Google Scholar]
- 20.Chan D.L. Nutritional requirements of the critically ill patient, Clin Tech Small Anim Pract 19, 2004, 1–5. [DOI] [PubMed] [Google Scholar]
- 21.Biffl W.L., Moore E.E., Haenel J.B. Nutrition support of the trauma patient, Nutrition 18, 2002, 960–965. [DOI] [PubMed] [Google Scholar]
- 22.Ekingen G., Ceran C., Guvenc H., Tuzlaci A., Kahraman H. Early enteral feeding in newborn surgical patients, Nutrition 21, 2005, 142–146. [DOI] [PubMed] [Google Scholar]
- 23.Villeta S., Chiolerob R.L., Bollmannb M.D., et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients, Clin Nutrit 24, 2005, 502–509. [DOI] [PubMed] [Google Scholar]
- 24.Abood S.K., Buffington C.A. Enteral feeding in dogs and cats: 51 cases (1989–1991), J Am Vet Med Assoc 6, 1992, 19–22. [PubMed] [Google Scholar]
- 25.Michel K.E. Preventing and managing complications of enteral nutritional support, Clin Tech Small Anim Pract 19, 2004, 49–53. [DOI] [PubMed] [Google Scholar]
- 26.Proulx J. Nutrition in critically ill animals. Wingfield W.E., Raffe M.R. The veterinary ICU book, 1st edn, 2004, Teton NewMedia: Jackson, WY, 202–217. [Google Scholar]