Abstract
The purpose of this study was to evaluate endosonography (EUS) as a potential diagnostic tool for feline pancreatitis. Eleven healthy cats and six cats diagnosed with pancreatitis based on an increased serum feline pancreatic lipase immunoreactivity (fPLI) concentration were included. Transabdominal ultrasound (AUS) and EUS were performed in all cats. The widths of both pancreatic limbs and echogenicity and homogenicity were assessed by AUS and EUS. Finally, findings from both modalities were subjectively compared. In the healthy cats, the right pancreatic limb was significantly smaller on EUS compared to AUS. Also, subjectively, general visualization of the normal pancreas was superior with EUS and, the pancreatic margins and parenchyma could be resolved better with EUS in all sick patients. In this study, EUS findings did not alter the diagnosis in six cats with pancreatitis when compared to AUS. However, EUS may be useful in cases where AUS fails due to obesity, hyperechoic mesentery, or excessive intestinal gas.
Feline pancreatitis has been shown to occur relatively frequently with a prevalence at necropsy ranging from 1.3% to 3.5%. 1,2 Also, in a recent study of 115 cats submitted for necropsy for a variety of reasons at the University of California, Davis, 75.7% showed lesions consistent with acute and/or chronic pancreatitis, suggesting that as in humans and dogs, pancreatitis is more common than previously suspected. 3 In contrast to histopathology, the disease often remains undiagnosed clinically as many cats experience subclinical disease or show only vague clinical signs. 4 Currently, a variety of diagnostic tools are being used for the diagnosis of feline pancreatitis, and all are associated with variable clinical usefulness. Changes in complete blood count (CBC) and serum chemistry profile are often non-specific and mild. They are mainly related to concurrent diseases or systemic complications of pancreatitis. 4 Amylase and lipase activities have been shown to be of no clinical value for the diagnosis of feline pancreatitis. 5 Abdominal radiographic findings are non-specific and thus of little use. 6–8 Abdominal ultrasonography has been shown to lack sensitivity, 5,7,8 with the following findings associated with feline pancreatitis: enlarged pancreas, hypoechoic (pancreatic necrosis) or hyperechoic parenchyma (fibrosis), surrounded by a hyperechoic mesentery (peripancreatic fat necrosis), peripancreatic fluid accumulation, and pancreatic duct dilation. 8–10 Enlargement of the pancreas, at least in dogs, has also been described in patients with hypoalbuminemia and portal hypertension, and is thus not sufficient as a sole finding for diagnosing pancreatitis. 11 The sensitivity of abdominal ultrasonography in feline pancreatitis has been reported to range between 11% and 35%, 7,8 and to be highly operator dependent. 8 A more recent study found a sensitivity of 80% in cats with moderate and severe pancreatitis and a specificity of 88% in healthy cats. However, in cats with clinical signs compatible with pancreatitis but no evidence of pancreatic disease on biopsies, the specificity of ultrasound was only 33%. 12 Additionally, a normal abdominal ultrasound examination does not rule out pancreatitis. 8,10 Feline serum trypsin-like immunoreactivity (fTLI) assay has been shown to be highly specific for exocrine pancreatic insufficiency but is not very sensitive for the diagnosis of feline pancreatitis. 13 One study found an overall sensitivity of 28% and a specificity of 82%. 12 An initial study of feline pancreatic lipase immunoreactivity (fPLI) concentration in serum suggested that this new test is both sensitive and specific for the diagnosis of feline pancreatitis. The study showed a sensitivity of 100% in cats with moderate to severe pancreatitis and 54% in mild pancreatitis with an overall sensitivity of 67%. The specificity was 100% in healthy cats and 91% for all cats without histological evidence of pancreatitis 12 using pancreatic biopsies as a gold standard. Histological analysis of pancreatic biopsies is often regarded as a gold standard for the diagnosis of pancreatitis in cats. 4 A big disadvantage of this method lies in the fact that pancreatic inflammation in cats may frequently be localized, and therefore, easily be missed on a single or even multiple biopsies. 3 Finally, computed tomography does not appear to be useful for the diagnosis of feline pancreatitis. 7,12
Based on the promising reports from the human literature 14 and preliminary clinical experience with endosonography for evaluation of the pancreas at the Small Animal Hospital of the University of Berne, Switzerland, this paper evaluates endosonography (EUS) as a diagnostic tool for feline pancreatitis. The echoendoscope is similar to a conventional endoscope but is equipped with an ultrasound transducer at its tip. When the transducer is positioned within the lumen of the stomach, there are only a few millimeters of tissue separating it from the pancreas and there is no gas interference or loss of signal due large penetration depths. 15 Therefore, very detailed images can be obtained and imaging of small structures is optimized.
The hypotheses of the current study were that endosonography will increase the sensitivity for detection of pancreatic changes in cats with pancreatitis and improve the image quality of the feline pancreas compared to conventional transabdominal ultrasound (AUS).
Material and Methods
The study protocol was evaluated and approved by the Canton of Berne Animal Experimentation Commission (authorization number 9/03).
Symptomatic Cats
Six client-owned cats with clinical signs consistent with pancreatitis were prospectively evaluated at the University of Berne Small Animal Hospital between November 2002 and February 2005 after their owners had given informed consent.
Healthy Control Cats
Eleven cats from a feline colony were evaluated during the same period. None of the healthy control cats had any previous history of gastrointestinal disease and all underwent the same evaluation as the symptomatic cats.
A complete history, physical examination, CBC, serum chemistry profile, serum amylase and lipase activities, and urinalysis were evaluated in all cats. Serum was collected for measurement of fTLI and fPLI concentration. AUS and EUS were performed in all cats.
Determination of Serum fPLI Concentration
Serum was batched and stored at −70°C before overnight shipping on dry ice to the Gastrointestinal Laboratory at Texas A&M University for analysis. Serum fPLI concentrations were determined by radioimmunoassay validated for use in the cat. 13 The reference range for fPLI was 2.0–6.8 μg/l, with concentrations ≥12 μg/l considered diagnostic of pancreatitis.
Transabdominal Ultrasound and Endosonography
All cats were examined with both AUS and EUS. All affected cats underwent AUS examination as part of their initial clinical workup, performed by one of three board-certified radiologists, which served as a screening tool for recruiting patients for this study. General anesthesia was induced in all affected and healthy cats after being held off food for at least 12 h within 36 h of the initial examination. The protocol used was based on methadone (Methadon Streuli; Streuli Pharma, Uznach, Switzerland) 0.2 mg IV, midazolam (Dormicum; Roche Pharma, Reinach, Switzerland) 0.3 mg/kg IV, ketamine (Ketanarcon; Streuli Pharma, Uznach, Switzerland) 3–5 mg/kg IV, tracheal intubation and maintenance of anesthesia with isoflurane (Isofluran Baxter; Volketswil, Switzerland). At that time AUS was repeated and EUS examination of the abdomen was performed. Both examinations were performed by the same board-certified radiologist (LG) who was aware of the diagnosis that had previously been made by one of two other board-certified radiologists or herself. However, only results for the AUS and EUS examinations performed by LG were used for analysis in this study.
For AUS, the ventral abdomen was clipped and ultrasound gel was applied. An Aloka Prosound ultrasound machine (SSD-5500 ultrasound unit, Aloka holding Europe AG, Zug, Switzerland) with multifrequency (5–10 MHz), microconvex array transducer was used for all transabdominal examinations. The subjective liver size was recorded as well as hepatic echostructure and echogenicity in comparison to the spleen. The left and right limbs of the pancreas were examined and the mean of three measurements of the width of both limbs was recorded. The echogenicity of the peripancreatic mesentery was recorded as well as the width of the duodenal papilla, bile duct, pancreatic duct, and gall bladder wall.
The EUS examinations were performed with a multifrequency (5–10 MHz) Olympus video ultrasound gastroscope supported by the Aloka Prosound SSD-5500 (Olympus Optical, Europa, GmbH, Hamburg, Germany) as previously described. 15 After inserting the endoscope into the stomach, the liver, pancreas, and contiguous structures were examined and the same parameters were recorded as during the AUS examination.
The width of the left and right pancreatic limbs as measured by AUS and EUS was compared. Furthermore, subjective comparisons were made as to the ability to identify the pancreas with AUS versus EUS in both groups of cats.
Statistical Analysis
Data were assessed for normality by the Kurtosis test. Data that were normally distributed are reported as mean±standard deviation (SD), data that were not normally distributed are reported as medians (ranges). A Mann–Whitney U test or Wilcoxon rank-sum test was used to evaluate differences between the two groups of cats, and a Wilcoxon signed-rank test was used to compare measurements of pancreatic limb width of the same animals. Statistical significance was set at P<0.05. For groups with less than five animals, only descriptive statistics were applied.
Results
A total of 17 cats were included in this study. There were six client-owned cats with pancreatitis confirmed by an increased fPLI concentration, and 11 healthy control cats with a serum fPLI concentration in the reference range. Six cats were male (one intact and five neutered), and 11 female (10 intact and one spayed). Thirteen cats were domestic shorthair cats, including all cats of the healthy control group. Other breeds included one Egyptian Mau, one Abyssinian, one Somali, and one Persian. The cats' ages ranged from 7 to 17 years (mean 11.3 years, SD 4.4) in the symptomatic group and from 2 to 12 years (mean 6.8 years, SD 3.2) in the healthy control group, which was significantly different (P=0.02). Clinical signs on presentation as well as onset and duration of disease of the cats in the symptomatic group are listed in Table 1.
Table 1.
Clinical signs and onset/duration of illness, and results of cytologic or histopathologic evaluation of the pancreas in six cats with clinical signs compatible with pancreatitis
| Cat | Clinical signs | Onset | Cytology/histopathology |
|---|---|---|---|
| 1 | Lethargy | Chronic with acute episode | Histopathology: WNL |
| Dehydration, icterus | |||
| 2 | PU/PD, vomiting | Chronic with acute episode | Cytology: neutrophilic and mononuclear inflammation |
| Lethargy, anorexia, tachypnea | |||
| 3 | Anorexia, lethargy, abdominal pain | Acute | N/A |
| 4 | PU/PD, tenesmus | Chronic with acute episode | Histopathology: WNL |
| Anorexia, lethargy | |||
| 5 | Vomiting | Chronic with acute episode | N/A |
| Anorexia | |||
| 6 | Anorexia, fever | Intermittent bouts with acute episode | N/A |
| Abdominal pain, lethargy, dehydration |
Necropsy performed in cats 1 and 4. N/A=not available, WNL=within normal limits.
CBC, chemistry panel, urinalysis and serum amylase activities were not different between both cat groups. Serum lipase was significantly higher (P=0.004) in sick cats (median 37.1 U/l, range 27.6–89.8 U/l) compared to healthy (median 23.6 U/l, range 4.0–32.0 U/l) cats (reference range: 0–83 U/l). fPLI was significantly higher (P=0.001) in sick cats (median 27.2 μg/l, range 18.3–95.9 μg/l) compared to the healthy control group (median 4.5 μg/l, range 3.4–6.4 μg/l). The reference range for fPLI is 2.0–6.8 μg/l and the cut-off value for fPLI is >12 μg/l.
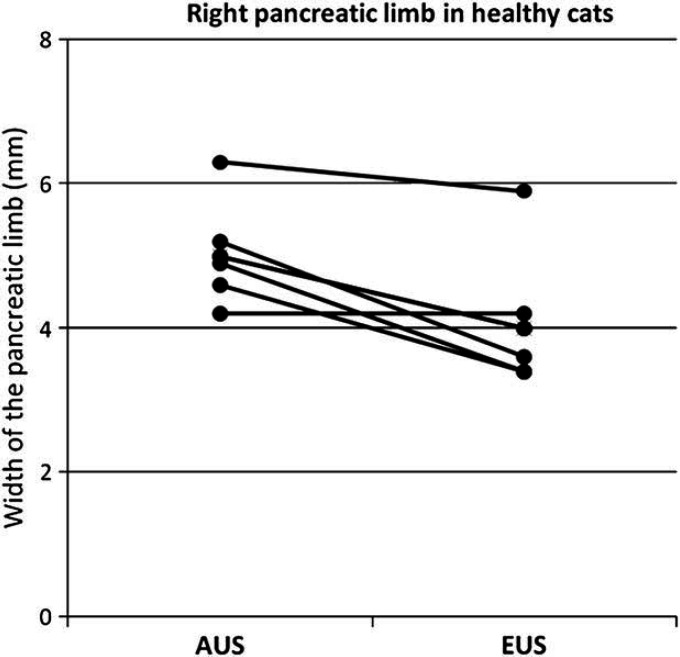
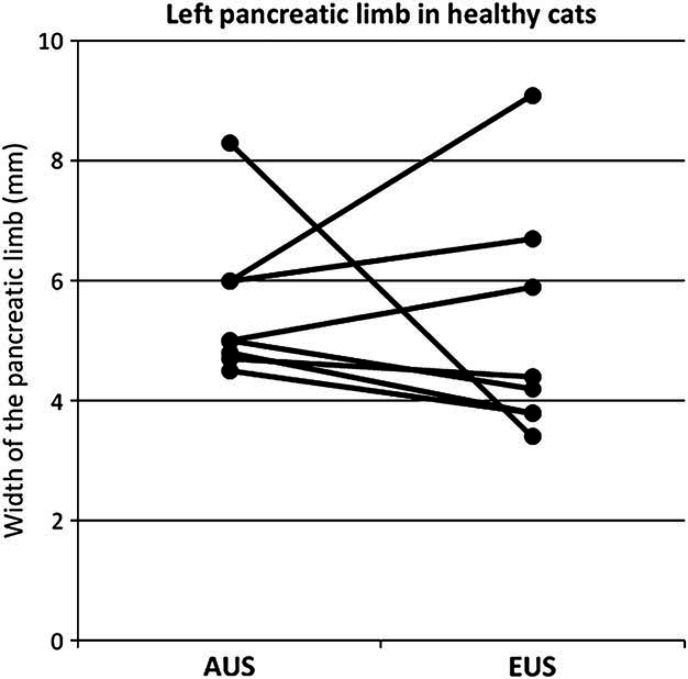
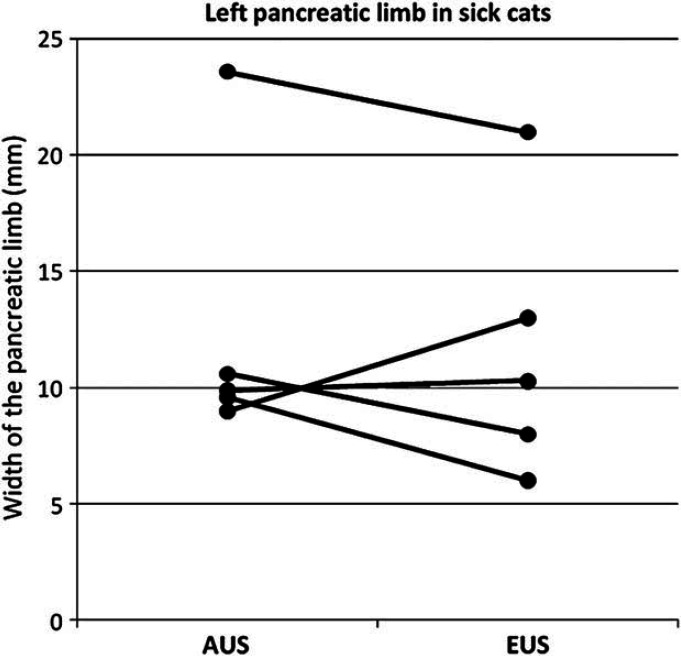
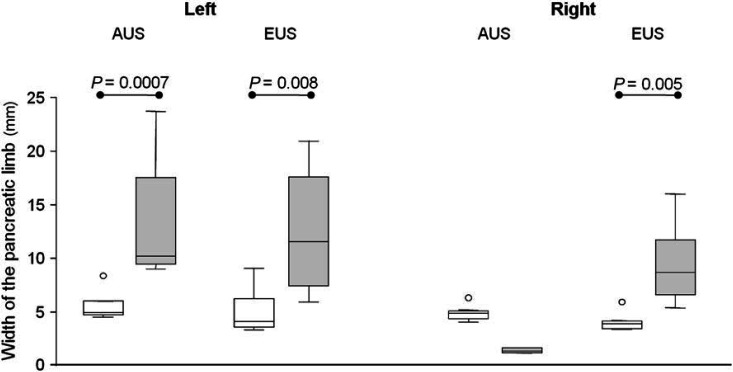
Results of the median width of the left and the right pancreatic limb measured by EUS and AUS, compared between the two radiologic modalities as well as between the sick and the healthy cats are shown in Figs 1–4. For the width of the right pancreatic limb, the number of sick cats was too small for comparison between EUS and AUS. Echogenicity of the pancreas for healthy and sick cats is summarized in Table 2. The pancreas appeared isoechoic to the mesentery and was homogeneous in 9/11 healthy control cats on both AUS and EUS. Subjectively, however, the borders of the pancreas were easier to be delineated, subjectively better margination of the left limb of the pancreas in 5/6 sick cats was possible, and the hilus of the liver and the portal vein were easier to identify with EUS compared with AUS. This was considered to be important as the portal vein was used as a landmark for identifying the pancreas. Figure 5 shows the improved delineation of the pancreatic margins in a normal cat with EUS versus AUS. Figure 6 shows a similar situation in the left pancreatic limb in an affected cat. The pancreatic duct could only be identified in 2/11 healthy control cats by AUS and was 1.0 and 1.2 mm in diameter, respectively. It could be identified in four healthy control cats by EUS and ranged between 1.0 and 2.0 mm in diameter. Using EUS the pancreatic duct could be detected in 4/6 cats with pancreatitis and diameters ranged between 1.0 and 2.0 mm. In contrast, the duct could only be seen in 2/6 affected cats by AUS and was 2.0 and 2.7 mm in diameter. Although the pancreatic duct could be identified and measured more often by EUS, the number of cats is too low for valid statistical comparison. The duodenal papilla could only be detected in 2/11 healthy control cats with AUS and 3/11 cats with EUS. In affected cats, the papilla could be detected in 4/6 cats with both methods. Thus, the ability to identify the duodenal papilla was not improved with EUS in either healthy or affected cats.
Fig 1.
Width of the right pancreatic limb in millimeters measured by AUS compared to EUS in healthy cats (n=5, P=0.014).
Fig 2.
Width of the left pancreatic limb in millimeters measured by AUS compared to EUS in healthy cats (n=7, P=0.62).
Fig 3.
Width of the left pancreatic limb in millimeters measured by AUS compared to EUS in sick cats (n=5, P=0.75).
Fig 4.
Width of the left and the right pancreatic limb in millimeters measured by AUS and EUS in healthy cats (white bars) compared to sick cats (gray bars).
Table 2.
Evaluation of pancreatic echogenicity using AUS and EUS in 11 healthy cats and in six cats with clinical signs compatible with pancreatitis
| Pancreas | Normal cats (n=11) | Sick cats (n=6) | ||||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | |||||
| AUS | EUS | AUS | EUS | AUS | EUS | AUS | EUS | |
| Normal | 9 | 11 | 9 | 10 | – | – | – | 1 |
| Abnormal | 2 | – | 2 | – | 6 | 6 | 2 | 4 |
| Not found | – | – | – | 1 | – | – | 4 | 1 |
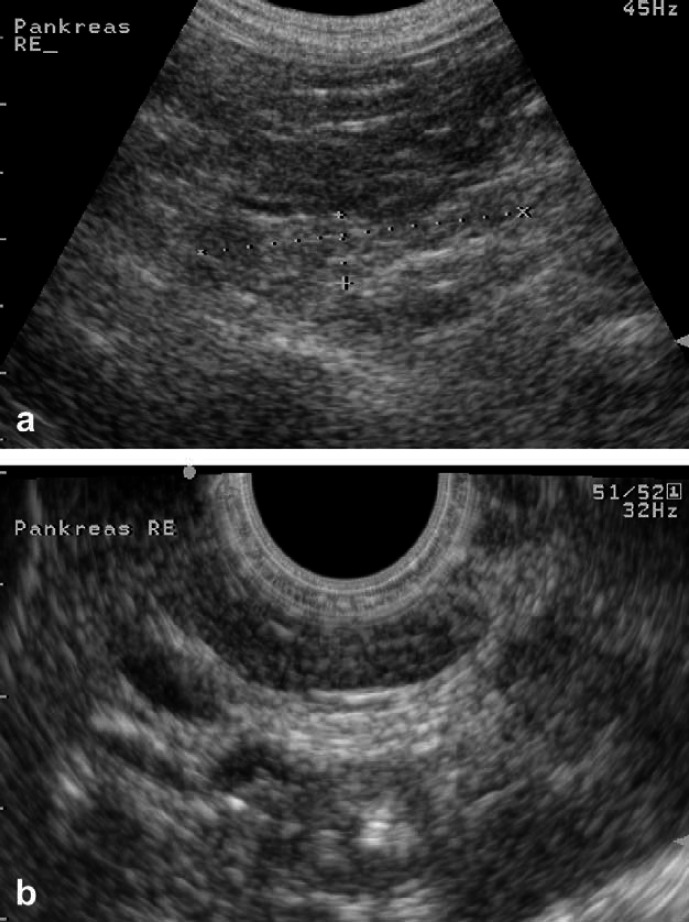
Fig 5.
Right pancreatic limb in a normal cat examined with AUS (a) and EUS (b). Note the better delineation with EUS and the discrepancy in size between the two methods.
Fig 6.
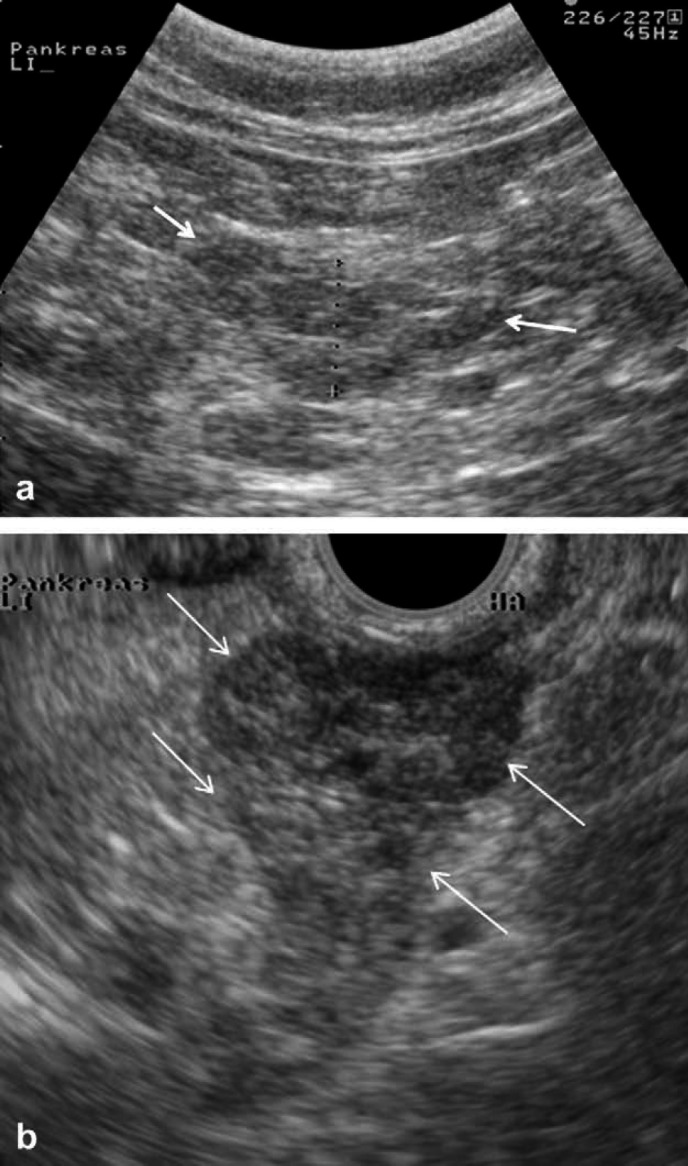
Left pancreatic limb of an affected cat examined with AUS (a) and EUS (b). Arrows mark the improved delineation of the pancreatic borders. Improved visualization of the nodules is evident.
In four affected cats, enlarged and rounded peri-portal lymph nodes were only identified during the EUS examination. In one affected cat, a fluid-filled intra-abdominal cavity was falsely assessed as a nodule during the AUS examination. With EUS, the structure was recognized to be fluid filled and was aspirated afterwards under AUS guidance and confirmed to be fluid filled by cytology. In two cats, pancreatic nodules were identified on EUS that could not be appreciated during AUS (Fig 6).
The common bile duct could be identified in three healthy control cats (diameter: median 1 mm, range 0.7–1.3 mm) by AUS and in four healthy control cats by EUS (median 2.5 mm, range 1.2–3.0 mm). The gall bladder wall thickness measured 1.0 mm in all healthy control cats with EUS whereas it ranged between 0.8 and 1.7 mm when measured by AUS.
The liver was found to be hyperechoic and enlarged in 5/6 sick cats and was normally sized and of normal echogenicity (hypoechoic to the spleen) in all healthy control cats. In one sick cat, the liver was inhomogeneous. There was no difference in the ability to examine the liver between the two ultrasound methods.
Histopathology of the pancreas was performed in three cats of the healthy control group and revealed no abnormalities. Results of histopathology in the sick cats are summarized in Table 1.
Discussion
The findings of our study suggest that the left pancreatic limb is significantly wider in sick than in healthy cats when evaluated by either AUS or EUS. The same applies to EUS findings for the right pancreatic limb while the number of cats where the right pancreatic limb was visible during AUS was too small for statistical analysis. This increase in size of the pancreas is likely due to inflammation and this finding was consistent with other studies. 8 The right pancreatic limb appears significantly wider when measured by AUS in healthy cats. One possible explanation for this is that, subjectively, the margins of the right pancreas were easier to be delineated with EUS, whereas, cursor placement at the more poorly defined pancreatic margin may have been less exact. This may have led to an overestimation of measurements of the right limb of the pancreas by AUS. In contrast, in sick cats the right pancreatic limb appeared narrower on AUS than on EUS. However, it was only visible in 2/6 sick cats on AUS, while it was identified in all six cats using EUS. Unfortunately, these numbers were too small to perform a valid statistical analysis. However, subjectively, the authors believed that the right pancreatic limb could be better evaluated and measured with EUS than with AUS.
Results from our study suggest that the pancreatic margins and parenchyma can be better resolved with EUS than with AUS. This appears to be due mostly to the fact that penetration depths are much smaller with EUS, preventing much of the artifacts that can arise from surrounding structures. This factor is important, especially in obese or large animals, or those with a hyperechoic mesentery. Most affected cats in this study did have a hyperechoic mesentery and the pancreatic margins could not be clearly identified transabdominally. However, due to the nature of the changes in the cranial abdomen (hyperechoic mesentery, enlarged pancreas), the diagnosis of pancreatitis could be made transabdominally in all affected cats. In a number of cases, details of the parenchyma were easier to see by EUS. This included a better appreciation of the pancreatic margins and parenchyma, regional lymph node enlargement, and of the presence of nodules or fluid-filled cavities. However, EUS findings did not provide additional help for the diagnosis of pancreatitis when compared to AUS. Nevertheless, the improved visualization of nodules and cavities changed the diagnostic strategies in one case (cat 3 with pancreatitis), and tissue aspiration with cytological evaluation of the specimen (cat 3), was performed. Pancreatic nodules found on EUS in two cats were not verified surgically as no surgical intervention was deemed necessary clinically. Additionally, cats with pancreatitis often have concurrent hepatic lipidosis, a disease that may require aggressive treatment including placement of a feeding tube, which may be performed during the same anesthesia as the EUS examination.
Disadvantages of EUS include anesthetic risk, as cats with severe pancreatitis may be poor anesthesia candidates. Moreover, in cats with mild pancreatitis, elective anesthesia may seem rather invasive. EUS requires special equipment and a successful examination requires a well-trained operator.
A significant age difference was noted between symptomatic and healthy cats. It is possible that cats with pancreatitis are older than healthy cats. However, more likely, selecting healthy colony cats resulted in a younger population of cats compared with the hospital symptomatic population. There was no correlation of pancreatic width with age, and no correlation of pancreatic echogenicity with age or body weight in a previous study, 16 which leads the authors to the belief that the age difference did not influence the imaging results in this study.
Serum lipase was significantly higher in sick cats than in healthy cats (P=0.004). This is in contrast to a previous study that did not report such a difference. 17 However, only one cat had a serum lipase activity that was above the upper limit of the reference range, limiting a potential diagnostic utility of this parameter.
fPLI was chosen as a gold standard in this study as pancreatic biopsies were not attainable in either the client-owned cats or the control cats. Necropsy results were included if available. Ideally, both histopathology and fPLI would have been assessed, and the authors are aware of the fact, that the current gold standard is still considered to be pancreatic histopathology, 4 but results of initial studies concerning the sensitivity and specificity of fPLI 12 should justify this choice. Histology of the pancreas had been performed in cats 1 and 4 and showed no abnormalities. Unfortunately, only single sections of the pancreas were evaluated, which represents a major limitation, as pancreatic inflammation can be localized. However, with the highly suggestive results of fPLI and both AUS and EUS together with the clinical picture, the authors feel confident concerning the diagnosis in these two cases. Hepatic changes on both AUS and EUS may raise the suspicion of concurrent hepatopathy, possibly cholangiohepatitis. However, even though this may not be excluded, serum chemistry changes compatible with hepatopathy were not noted.
Another limitation is the fact that the radiologist performing EUS (LG) was not blinded to AUS findings as well as the clinical suspicion. As EUS requires anesthesia, the authors did not believe that EUS could have been justified without a prior suspicion of pancreatitis. However, an AUS examination was performed by this same radiologist (LG) as the EUS examination, and thus allowed direct comparison of the two modalities by the same sonographer, which was one of the main points of interest in this study.
In summary, in control cats, both AUS and EUS show subjectively similar results for the feline pancreas in terms of size and echogenicity. However, EUS may be superior for imaging the pancreas with increased resolution in obese cats when AUS fails due to large penetration depths or air artifacts.
Acknowledgments
The authors would like to thank Patrick Kircher, Division of Radiology, Vetsuisse Faculty, University of Berne, Switzerland, for his valuable help.
References
- 1.Owens J.M., Drazner F.H., Gilbertson S.R. Pancreatic disease in the cat, J Am Anim Hosp Assoc 11, 1975, 83–89. [Google Scholar]
- 2.Hänichen T., Minkus G. Retrospektive Studie zur Pathologie der Erkrankungen des exokrinen Pankreas bei Hund und Katze, Tierarztl Umsch 45, 1990, 363–368. [Google Scholar]
- 3.De Cock H.E., Forman M.A., Farver T.B., Marks S.L. Prevalence and histopathologic characteristics of pancreatitis in cats, Vet Pathol 44, 2007, 39–49. [DOI] [PubMed] [Google Scholar]
- 4.Steiner J.M., Williams D.A. Feline exocrine pancreatic disorders, Vet Clin North Am Small Anim Pract 29, 1999, 551–575. [PubMed] [Google Scholar]
- 5.Steiner J.M. Diagnosis of pancreatitis, Vet Clin North Am Small Anim Pract 33, 2003, 1181–1195. [DOI] [PubMed] [Google Scholar]
- 6.Hill R.C., Van Winkle T.J. Acute necrotizing pancreatitis and acute suppurative pancreatitis in the cat. A retrospective study of 40 cases (1976–1989), J Vet Intern Med 7, 1993, 25–33. [DOI] [PubMed] [Google Scholar]
- 7.Gerhardt A., Steiner J.M., Williams D.A. Comparison of the sensitivity of different diagnostic tests for pancreatitis in cats, J Vet Intern Med 15, 2001, 329–333. [PubMed] [Google Scholar]
- 8.Saunders H.M., VanWinkle T.J., Drobatz K., Kimmel S.E., Washabau R.J. Ultrasonographic findings in cats with clinical, gross pathologic, and histologic evidence of acute pancreatic necrosis: 20 cases (1994–2001), J Am Vet Med Assoc 221, 2002, 1724–1730. [DOI] [PubMed] [Google Scholar]
- 9.Wall M., Biller D.S., Schoning P., Olsen D., Moore L.E. Pancreatitis in a cat demonstrating pancreatic duct dilatation ultrasonographically, J Am Anim Hosp Assoc 37, 2001, 49–53. [DOI] [PubMed] [Google Scholar]
- 10.Hecht S., Henry G. Sonographic evaluation of the normal and abnormal pancreas, Clin Tech Small Anim Pract 22, 2007, 115–121. [DOI] [PubMed] [Google Scholar]
- 11.Lamb C.R. Pancreatic edema in dogs with hypoalbuminemia or portal hypertension, J Vet Int Med 13, 1999, 498–500. [DOI] [PubMed] [Google Scholar]
- 12.Forman M.A., Marks S.L., De Cock H.E., et al. Evaluation of serum feline pancreatic lipase immunoreactivity and helical computed tomography versus conventional testing for the diagnosis of feline pancreatitis, J Vet Intern Med 18, 2004, 807–815. [DOI] [PubMed] [Google Scholar]
- 13.Steiner J.M., Wilson B.G., Williams D.A. Development and analytical validation of a radiommunoassay for the measurement of feline pancreatic lipase immunoreactivity in serum, Can J Vet Res 68, 2004, 309–314. [PMC free article] [PubMed] [Google Scholar]
- 14.Tandon M., Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis, Am J Gastroenterol 97, 2002, 1244–1246. [DOI] [PubMed] [Google Scholar]
- 15.Gaschen L., Kircher P., Lang J. Endoscopic ultrasound instrumentation, applications in humans, and potential veterinary applications, Vet Radiol Ultrasound 44, 2003, 665–680. [DOI] [PubMed] [Google Scholar]
- 16.Larson M. Moon, Panciera D.L., Ward D.L., Steiner J.M., Williams D.A. Age-related changes in the ultrasound appearance of the normal feline pancreas, Vet Radiol Ultrasound 46 (3), 2005, 238–242. [DOI] [PubMed] [Google Scholar]
- 17.Parent C., Washabau R.J., Williams D.A., et al. Serum trypsin-like immunoreactivity, amylase and lipase in the diagnosis of feline acute pancreatitis [abstract], J Vet Intern Med 9 (3), 1995, 194. [Google Scholar]