Abstract
Two unrelated Ragdoll cat mothers in Norway were found dead from renal disease. The histopathology was consistent with oxalate nephrosis with chronic or acute-on-chronic underlying kidney disease. Both cats had offspring and relatives with signs of urinary tract disease, including a kitten dead with urethral gravel. Eleven living Ragdoll cats, including nine relatives of the dead cats and the male father of a litter with similarly affected animals, were tested for primary hyperoxaluria (PH) type 1 and 2 by urine oxalate and liver enzyme analysis. Renal ultrasound revealed abnormalities in five living cats. One of these was azotaemic at the time of examination and developed terminal kidney disease 9 months later. A diagnosis of PH was excluded in 11 cats tested. The inheritance and aetiological background of the renal disease present in the breed remains unresolved at this point in time.
During 2006 two apparently unrelated Ragdoll cat mothers in Norway died from renal disease. The autopsy reports described findings consistent with oxalate nephrosis, suggesting ethylene glycol poisoning. Both cats were indoor cats not exposed to antifreeze solutions. Adult offspring of one mother and a relative of the other mother developed azotaemia and uraemia. While one cat went back to non-azotaemic kidney disease IRIS stage 1, the other cat remained asymptomatic but mildly azotaemic with non-hypertensive, non-proteinuric IRIS stage 2 chronic kidney disease (CKD) (http://www.iris-kidney.com/guidelines/en/staging_ckd.shtml). Ultrasound screening of related cats by the local veterinarian revealed variable hyperechoic ultrasound abnormalities in a few cats.
Ragdolls in Sweden and Finland are enrolled in a voluntary ultrasound based screening programme for kidney disease, and informal web-based discussions from the USA (www.vin.com) support the concept of familial renal dysplasia and/or nephrocalcinosis in this breed. The information at this point in time seemed consistent with an inherited problem, such as primary hyperoxaluria (PH) type 1 or type 2.
The PHs are genetic disorders leading to accumulation of glyoxalate, a precursor of oxalate, and subsequent development of hyperoxaluria. Two types of PH are well characterised by enzymology and molecular genetics. Type 1 (PH1) arises from mutations in the AGXT gene affecting the structure and function of alanine:glyoxylate aminotransferase (AGT). Type 2 (PH2) is the result of mutations in the GRHPR gene which affect the structure and function of the enzyme glyoxylate reductase/hydroxypyruvate reductase (GR/HPR) (reviewed by Danpure and Rumsby 1 ). The clinical picture in humans is heterogeneous, with recurrent urolithiasis and/or nephrocalcinosis, renal failure and reduced life expectancy. The age of onset and rate of progression of renal pathology are also variable. 2
Both PH1 and PH2 have been described in dogs 3,4 and cats, 5–7 as well as other inherited conditions affecting the same gluconeogenic pathway although, in the case of d+-glyceric aciduria, without hyperoxaluria. 8–10 Chronic or acute oxalate nephrosis and renal failure were described in cats with PH2 with neurological involvement. 7
Against this background, affected cats and a group of relatives were referred to the Norwegian School of Veterinary Science (NCVS) for extensive testing of affected individuals and relatives with complete diagnostic workup and specific testing for PH1 and PH2.
The aim of the study was to define the nature of this disease in the Ragdolls available for study with respect to: (1) whether the observed disease is PH of a known type and (2) the possible mode of inheritance, based upon histopathological analysis, ultrasound and laboratory assessment of family members.
Materials and Methods
Cats
The presenting features of the animals used in this study are given in Table 1.
Table 1.
Presenting features in 15 Ragdoll cats
| Cat number (see Fig 1) | Gender, age | Clinical signs | Ultrasound abnormalities | Serum urea, mmol/l (<10) | Serum creatinine, μmol/l (<180) | HCT % (0.24–0.45) | USG (<1.035) | Urine dipstick or sediment findings |
|---|---|---|---|---|---|---|---|---|
| 1 | M, 2 y | No | No | 7 | 151 | 0.44 | >1.050 | Normal |
| 2 | M, 1 y | No | No | 9 | 171 | 0.37 | >1.050 | Normal |
| 3 | F, 1.5 y | No | No | 6 | 92 | 0.31 | 1.048 | Normal |
| 4 | M, 1.5 y | No∗ | Yes | 10 | 123 | 0.37 | 1.039 | Normal |
| 5 | F, 1.5 y | No | No | 8 | 95 | 0.31 | >1.050 | Normal |
| 6 | F, 3 y | No | Yes | 9 | 95 | 0.23 | >1.050 | Normal |
| 7 | M, 2 y | No | Yes | 12 | 129 | 0.32 | >1.050 | Normal |
| 8 | M, 3.5 y | No | No | 8 | 102 | 0.37 | 1.034 | Normal |
| 9 | M, 3.5 y | No | Yes | 9 | 117 | 0.33 | >1.050 | Normal |
| 10 | F, 3 y | No | No | 8 | 134 | 0.32 | 1.047 | Normal |
| 11§ | F, 2 y | Anorexia | Yes | 18.4 | 212 | 0.44 | 1.047 | Renal cells‡ |
| 13 | M, 6 w∥ | Urolithiasis† | – | – | – | – | – | White “gravel” |
| 14 | F, 3 y∥ | Uraemia | Yes | 49 | 640 | 0.37 | 1.015 | – |
| 15 | F, 2 y∥ | Uraemia | Yes | 46 | 741 | 0.35? | – | Uroliths |
| 16 | M, 2 y∥ | Intestinal foreign body | No | 10 | 113 | – | 1.045 | – |
M=male, F=female, y=years, w=weeks, HCT=haematocrit, USG=urine specific gravity, laboratory reference values in parentheses. Cat 12: not shown on above table is a DSH used as a control. Cats 1–11 were screened for PH (Table 3).
Six months earlier had had clinical depression and azotaemia after jaw fracture (creatinine up to 1061 μmol/l).
– = Not examined, or data not available at the time of examination.
Euthanased at 6 weeks of age due to recurrent obstructive urolithiasis.
A few renal epithelial cells observed in urine sediment.
Alive at the time of the testing for PH; euthanased 10 months later; renal histopathological examination available.
Dead at the time of the study.
Eleven Ragdolls (cats 1–11) between 1 and 3.5 years of age were tested for PH1 and PH2.
Renal tissue was available from five other cats (four related and one unrelated, cats 13–16). Material was available from one control cat; a domestic shorthair (DSH) treated after head trauma with subsequent euthanasia for animal welfare reasons (cat 12).
Pathological Methods
Autopsy material was available from two mothers (cats 14 and 15), while a detailed clinical history was available from a kitten sibling with recurrent urinary tract obstruction by white gravel at the age of 6 weeks (cat 13). Cat 6 had a wedge biopsy collected from the caudal pole of the left kidney during ovariohysterectomy 7 months after testing for PH. One littermate of the tested cats (cat 16) developed multiorgan failure after surgery for an intestinal foreign body and was euthanased; an autopsy made kidney tissue available for examination. Ten months after testing for PH, one azotaemic cat (cat 11) developed clinical signs of uraemia and was euthanased. A histopathological examination of kidney tissue was performed. Only a part of the left kidney was available for histopathological examination from cats 11 and 15.
Kidney tissue was fixed in phosphate buffered 4% formaldehyde, dehydrated in graded alcohol, and embedded in paraffin according to standard methods. The sections were stained with haematoxylin–eosin (HE), elastin, van Gieson (vG) and periodic acid-Schiff (PAS). Besides light microscopical examination, the sections were studied in a Leica DM 2500 microscope equipped with a polarisation filter.
Clinical and Sampling Procedures for PH Testing
The cats were sedated by simultaneous subcutaneous injections of medetomidine at 75 μg/kg and buprenophine 25 μg/kg before blood sampling and installation of intravenous catheters. An ultrasound examination of the abdomen was performed. The kidneys were scanned in longitudinal, transverse, and dorsal planes. Urine was collected by ultrasound guided cysteocentesis. Under brief propofol anaesthesia, liver biopsy was collected by a semi-automatic biopsy needle (Temno Evolution biopsy needle, 16 G 10 mm, Cardigan Health, Ohio, USA). Biopsies were not obtained from three cats for the following reasons: one was pregnant (cat 6), in another the liver was located too deep within the thoracic cavity for safe biopsy (cat 9) and the third was azotaemic (cat 11). In eight cats one biopsy was collected, producing 2–8 mg liver tissue per cat. If the sample was small a second attempt was not performed for safety reasons. The amount of tissue was sufficient for enzyme analysis in 5/8 cats (cats 2, 4, 5, 7, 8 and control cat, cat 12). Two cats bled from the biopsy site after the biopsy and were followed by ultrasound until recovery from anaesthesia, which was uneventful without any detectable compromise in clinical condition.
Blood was submitted for biochemistry and haematology on the same day. Routine urinalysis was performed in all cats. Urine was titrated to pH 3 with HCl and frozen immediately, and stored at −20 °C. Liver tissue was frozen dry in aluminium foil within 30 min, stored at −80 °C and shipped on dry ice by overnight courier together with the urine samples.
Laboratory Analysis for PH
Urine oxalate was measured using an oxalate oxidase method (Trinity Biotech) on the Cobas Bio centrifugal analyser. Reference ranges were established in 10 cats using urine samples collected from healthy cats that during sedation for castration or ovariohysterectomy.
AGT, GR and HPR were measured in liver biopsies as previously described. 11,12
Results
Familial Relationships
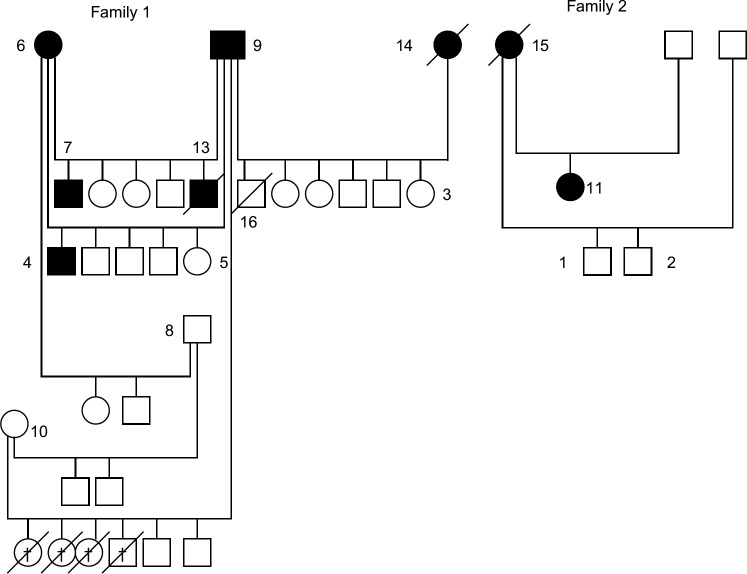
The familial relationship between the animals is shown in Fig 1. The litters which are offspring of cat 8 and cat 10 were only a few months old and thus are below the age that renal disease is commonly observed in these animals.
Fig 1.
Pedigree of cats. Open circle=healthy female, closed circle=female with abnormalities on ultrasound or biopsy. Open square=healthy male, closed square=male with abnormalities on ultrasound or biopsy. Overlaid /=deceased animal. † Dead following caesarean section.
Clinicopathological Findings
The presenting features of all cats are given in Table 1. Further details regarding the cats with azotaemia and/or histopathologically confirmed kidney disease or ultrasound abnormalities are as follows: The two probands (cats 14 and 15) developed weight loss during lactation and clinical depression 2–3 months after gestation. Both animals had small and irregular kidneys and abnormalities on ultrasound. Blood samples revealed azotaemia (IRIS stage 4). Intensive treatment failed to produce an improvement in clinical condition and the animals were euthanased. The texture of the renal cortex was moderately increased in cat 14, and mildly increased in cat 15. Cat 15 also had a dilated pelvis with several uroliths (Table 2, Fig 2).
Table 2.
Ultrasound and histopathological findings on 15 Ragdoll cats
| Cat | Ultrasound findings | Histopathological findings | |
|---|---|---|---|
| Size | Appearance | ||
| 1 | Normal | Normal | ND |
| 2 | Normal | Normal | ND |
| 3 | Normal | Normal | ND |
| 4 | Normal | Distinct corticomedullary definition. Medulla appeared normal. Cortical hyperechoic patchy areas – especially in both poles. | ND |
| 5 | Normal | Normal | ND |
| 6 | Normal | Reasonable distinct corticomedullary definition. Cortical thickness in right cranial renal pole varied. Mild hyperechoic patchy appearance in the right cranial pole. | No abnormality in biopsy of left kidney 7 months after ultrasonography |
| 7 | Left kidney within normal range in size. Right kidney was smaller than normal. |
|
ND |
| 8 | Normal | Normal | ND |
| 9 | Normal | Distinct corticomedullary definition. Mild hyperechoic patchy areas in the renal poles. | ND |
| 10 | Normal | Normal | ND |
| 11 | Small |
|
Patchy collagen infiltration in cortex and medulla, aggregate of mononuclear cells, crystals and crystal fragments |
| 13 | ND | ND | ND |
| 14 | Small |
|
Severe interstitial fibrosis, scattered infiltration of mononuclear cells, atrophic epithelium, crystals |
| 15 | ND | ND | Atrophic epithelium in dilated tubuli, mild interstitial fibrosis, crystals |
| 16 | ND | ND | Normal kidney tissue |
ND=No data.
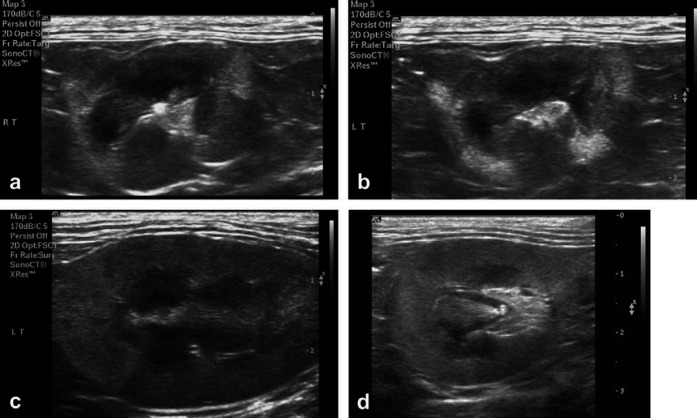
Fig 2.
Characteristic ultrasound findings in affected Ragdolls with CKD: patchy hyperechoic areas and variable findings. Cat 11: small kidneys, long axis (a) and short axis view (b). The corticomedullary junction is different in different parts of the kidney, and there is variable cortical thickness. In both kidneys there were few hyperechoic foci located mainly in the renal pelvis. (c) Cat 6: hyperechoic cranial pole of the kidneys. (d) Cat 4: left kidney, short axis view: hyperechoic regions, mainly in the renal poles, and a hyperechoic small structure in the pelvic region.
On histological analysis, cat 14 showed large areas of interstitial fibrosis and scattered occurrence of lymphocytic cells (Fig 3a). A few dilated tubules with degenerated epithelial cells were also seen in these areas together with a moderate periglomerular fibrosis. In the other parts of the cortex the tubules were dilated with atrophic or degenerated epithelium. Crystals with radiating striations and others with an ovoid shape were seen in large numbers in polarised light in all parts of the cortex, consistent with oxalate crystals (Fig 3b).
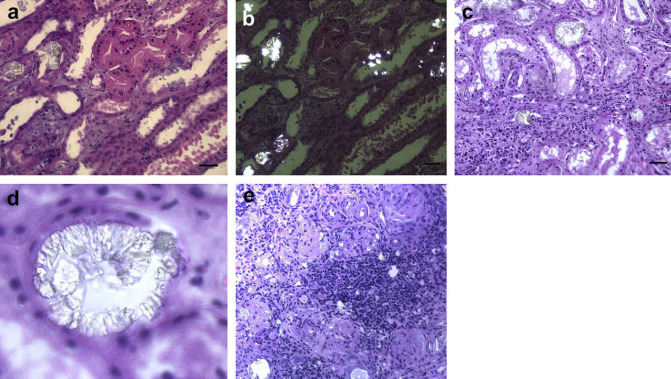
Fig 3.
Histopathological changes in three affected Ragdolls (numbers as in Table 1). (a) Cat 14, infiltration of mononuclear cells and fibrous tissue in the interstitium; (b) cat 14: crystals in polarised light; (c) cat 15: crystals in tubules and moderately dilated tubules; (d) cat 15: crystal structure from a tubule; (e) cat 11: collagen infiltration, aggregate of mononuclear cells, crystals and crystal fragments.
In cat 15 both the convoluted loops and the loops of Henle were in most areas dilated to a moderate degree (Fig 3c). The epithelium in these tubules had an atrophic appearance. In few places infiltrates of a small number of mononuclear cells and a moderate infiltration of fibrous tissue could be seen. Several uroliths were seen and polarised light revealed similar crystals to cat 14 (Fig 3d). These findings led to a clinical suspicion of PH and other family members were reviewed.
The ultrasound and histopathological characteristics of the cats are shown in Table 2. Several had regions of hyperechogenicity on ultrasound (cats 4, 6, 9 and 11) (Fig 2). In cat 11 the lesions were severe and extensive, and the same oxalate-like crystals were abundant in kidney tissue (Fig 3e).
Cat 4 had developed temporary but severe azotaemia at 1 year of age after a jaw luxation. With 4 weeks of treatment he recovered and had been clinically healthy since then. Cat 6 had been very thin during the previous lactation, but otherwise clinically healthy. The other cats among the 11 Ragdolls tested for PH had never shown signs of disease.
Results of Testing for PH
Individual urine oxalate results plus AGT, GR and HPR activities are shown in Table 3.
Table 3.
Clinicopathological findings in 11 Ragdoll cats and one DSH control screened for PH
| Analysis (reference range) | UOx/UCreat (<84 umol/mmol creatinine) | AGT (human liver: 19.1–47.9 umol/h/mg protein) | GR (human liver: 23–207 nmol/min/mg protein) | HPR (human liver: 92–876 nmol/min/mg protein) |
|---|---|---|---|---|
| Cat | ||||
| 1 | 3 | – | – | – |
| 2 | <1 | 36.5 | 91 | 519 |
| 3 | 5 | – | – | – |
| 4∗ | 8 | 38.1 | 94 | 700 |
| 5 | 1 | 35.0 | 152 | 1622 |
| 6∗ | 10 | – | – | – |
| 7∗ | 10 | 23.8 | 58 | 537 |
| 8 | 6 | 33.9 | 63 | 454 |
| 9 | 9 | – | – | – |
| 10 | 13 | – | – | – |
| 11∗ | 25 | – | – | – |
| 12 (control) | 8 | 43.6 | 131 | 799 |
Numbers are the same as in Table 1.
UOx/UCreat = urine oxalate to creatinine ratio;
– = No biopsy or insufficient biopsy material for analysis.
Cats with abnormal findings on ultrasound.
All Ragdolls had urine oxalate/creatinine ratios within the reference range. Liver enzyme activity in cats 4 and 7, both of which had ultrasound abnormalities, was similar to that seen in cats without ultrasound abnormalities (cats 2, 5, 8) and the control cat (cat 12). Thus there was no evidence to support a diagnosis of PH1 or PH2 in these animals.
Discussion
The examination with polarised light revealed an unusually large number of crystals in two of the animals raising the suspicion of oxalosis. While occasional oxalate crystals may sometimes be observed in end stage renal disease of unspecified aetiology, the large amount of crystals or stones reported here is uncommon. The nature of these crystals and the other findings recorded were in accordance with those found in cases of inherited hyperoxaluria 7 and ethylene glycol poisoning. 13 However, urine oxalate/creatinine ratios were similar to control animals and to those previously reported in cats. 10 Catalytic activities for AGT, GR and HPR were similar to those described by Danpure 6 and values found were similar in one control and unaffected family members, effectively excluding PH type 1 and type 2.
The ultrasound findings tended to exclude acute ethylene glycol toxicity where the renal cortex typically becomes uniformly hyperechoic within a few hours. 14 The medulla then becomes hyperechoic subsequent to the cortical changes. These changes produce an overall uniformly hyperechoic kidney – not patchy hyperechoic areas as observed in the affected Ragdolls.
A recent report on renal failure associated with melamine and cyanuric acid in cats also revealed crystals but in this case with concentric and radiating striations in tubules of the kidneys. 15 The crystals in the examined Ragdoll kidneys have features more consistent with oxalate crystals; some are present in proximal tubules and they lack concentric rings (CA Brown, personal communication 2007). However, there was no evidence of either hyperoxaluria in the animals tested nor was there any abnormality in AGT and GR activity in those animals on whom tissue was available for analysis arguing against a specific inherited disease of oxalate metabolism.
It is not quite clear whether or not the ultrasound findings are different from what is observed in non-specific CKD or end stage renal disease in cats as there is very little is published in the scientific literature on this topic. Whether or not oxalate deposition is part of the histological picture, non-specific CKD can also be familial. Kidneys in cats with CKD can present with more variable ultrasound findings than dogs. 14 The nature of mild renal disease observed by ultrasound in the cats who are still alive cannot be determined without a kidney biopsy.
The pattern of development of renal disease in the Ragdolls where azotaemia occurs at 2–3 years of age, is different to that described for PH2 in DSH cats 5,7 where affected animals developed azotaemia and neurological disease between 5 and 9 months of age. This finding may suggest chronic changes in the kidneys of Ragdolls, predisposing for an acute-on-chronic episode of kidney disease once a second trigger is present.
The suspicion of an inherited renal disease in Ragdolls is high and a screening regime for animals for breeding has been implemented in Sweden and Finland through first-line practitioners. A Dutch board-certified ultrasonographer has also been screening Ragdolls with familial kidney disease in the Netherlands (M Schmidt, personal communication 2007). The characteristic ultrasound findings are of small, irregular shaped kidneys, sometimes different sizes of the right and left kidneys, hyperechogenic wedge-shaped areas in the cortex, reduced corticomedullary junction, in the patchy/lobular pattern commonly observed in cats. The findings are not necessarily distinguishable from non-specific CKD in cats (M Schmidt, personal communication 2007).
It is not clear from the pedigree presented (Fig 1) whether the kidney disease is inherited or acquired. An autosomal recessive inheritance is excluded because all offspring would be expected to be affected if both cats 6 and 9 are affected (Fig 1). Similarly X-linked recessive inheritance does not fit with the observed picture unless cat 6 has two defective X chromosomes. Autosomal dominant inheritance cannot be excluded. However, the disease pattern might also be consistent with a primary renal disease with secondary processes of renal tubular crystal retention or stone formation, as described in recent reviews. 16,17
Future studies should aim at including a high enough number of cats to evaluate the hypothesis of inherited disease and further characterise the nature of the disease and its aetiology.
Acknowledgements
The authors are grateful to Dr JH Jansen, Norwegian School of Veterinary Science, for help with contacts and discussions during the study.
References
- 1.Danpure C.J., Rumsby G. Molecular aetiology of primary hyperoxaluria and its implications for clinical management, Expert Rev Mol Med 6, 2004, 1–16. [DOI] [PubMed] [Google Scholar]
- 2.Lorenzo V., Alvarez A., Torres A., Torregrosa V., Hernandez D., Salido E. Presentation and role of transplantation in adult patients with type 1 primary hyperoxaluria and the I244T AGXT mutation: Single-center experience, Kidney Int 70, 2006, 1115–1119. [DOI] [PubMed] [Google Scholar]
- 3.Danpure C.J., Jennings P.R., Jansen J.H. Enzymological characterization of a putative canine analogue of primary hyperoxaluria type 1, Biochim Biophys Acta 1096, 1991, 134–138. [DOI] [PubMed] [Google Scholar]
- 4.Jansen J.H., Arnesen K. Oxalate nephropathy in a Tibetan spaniel litter. A probable case of primary hyperoxaluria, J Comp Pathol 103, 1990, 79–84. [DOI] [PubMed] [Google Scholar]
- 5.Blakemore W.F., Heath M.F., Bennett M.J., Cromby C.H., Pollitt R.J. Primary hyperoxaluria and l-glyceric aciduria in the cat, J Inherit Metab Dis 11 (suppl 2), 1988, 215–217. [DOI] [PubMed] [Google Scholar]
- 6.Danpure C.J., Jennings P.R., Mistry J., et al. Enzymological characterization of a feline analogue of primary hyperoxaluria type 2: A model for the human disease, J Inherit Metab Dis 12, 1989, 403–414. [DOI] [PubMed] [Google Scholar]
- 7.McKerrell R.E., Blakemore W.F., Heath M.F., et al. Primary hyperoxaluria (l-glyceric aciduria) in the cat: A newly recognised inherited disease, Vet Rec 125, 1989, 31–34. [DOI] [PubMed] [Google Scholar]
- 8.deLorenzi D., Bernardini M., Pumarola M. Primary hyperoxaluria (l-glyceric aciduria) in a cat, J Feline Med Surg 7, 2005, 357–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sewell A.C., Moritz A., Duran M. D-(+)-glyceric aciduria in an Afghan hound, J Inherit Metab Dis 20, 1997, 395–396. [DOI] [PubMed] [Google Scholar]
- 10.Sewell A.C. Feline primary hyperoxaluria, J Feline Med Surg 8, 2006, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giafi C.F., Rumsby G. Kinetic analysis and tissue distribution of human d-glycerate dehydrogenase/glyoxylate reductase and its relevance to the diagnosis of primary hyperoxaluria type 2, Ann Clin Biochem 35 (Pt 1), 1998, 104–109. [DOI] [PubMed] [Google Scholar]
- 12.Rumsby G., Weir T., Samuell C.T. A semiautomated alanine: Glyoxylate aminotransferase assay for the tissue diagnosis of primary hyperoxaluria type 1, Ann Clin Biochem 34 (Pt 4), 1997, 400–404. [DOI] [PubMed] [Google Scholar]
- 13.Maxie G.M. Jubb, Kennedy, and Palmer's pathology of domestic animals, 5th edn., 2007, Saunders: Edinburgh. [Google Scholar]
- 14.Nyland T.G., Mattoon J.F. The urinary tract, Small animal diagnostic ultrasound, 2nd edn, 2002, Saunders: Amsterdam, 158–195. [Google Scholar]
- 15.Brown C.A., Jeong K.S., Poppenga R.H., et al. Outbreaks of renal failure associated with melamine and cyanuric acid in dogs and cats in 2004 and 2007, J Vet Diagn Invest 19, 2007, 525–531. [DOI] [PubMed] [Google Scholar]
- 16.Verkoelen C.F. Crystal retention in renal stone disease: A crucial role for the glycosaminoglycan hyaluronan?, J Am Soc Nephrol 17, 2006, 1673–1687. [DOI] [PubMed] [Google Scholar]
- 17.Verkoelen C.F., Verhulst A. Proposed mechanisms in renal tubular crystal retention, Kidney Int 72, 2007, 13–18. [DOI] [PubMed] [Google Scholar]