Abstract
A retrospective study of 128 feline bone marrow reports identified 13 cases of aplastic anemia. Clinical diagnoses included chronic renal failure (n=5), feline leukemia virus infection (n=2), hyperthyroidism treated with methimazole (n=1) and idiopathic aplastic anemia (n=5). In some cats, starvation may play a role in the development of marrow aplasia. Some cats with aplastic anemia can have prolonged survival without resolution of the pancytopenia.
Aplastic anemia is characterized by failure of blood cell production resulting in a hypocellular bone marrow that is replaced by fat (Brunning 1989, Weiss 2000). This typically results in bicytopenia or pancytopenia in the blood. Aplastic anemia has been described in a variety of species including dogs, cats, and humans (Brunning 1989, Dornsife et al 1989, Weiss 2000). The cause of aplastic anemia is uncertain in all species but is thought to be multifactorial (Miura et al 1991, Nissen 1991). Previous studies implicated immune-mediated suppression of hematopoiesis (Miura et al 1991). More recently, overproduction of the myelosuppressive cytokines interferon-γ and tumor necrosis factor-α have been implicated in aplastic anemia in humans (Verma et al 2002).
Aplastic pancytopenia has also been associated with starvation (Lewis and Rebar 1979, Smith and Spivak 1985). In humans with anorexia nervosa or starvations caused by neglect, bone marrow is hypocellular and fat undergoes serous atrophy (Pearson 1967, Smith and Spivak 1985). One case of aplastic anemia associated with serous atrophy of fat was described in an emaciated goat (Lewis and Rebar 1979).
Little is known about the incidence of aplastic anemia in cats and few associated disease conditions have been identified. Aplastic anemia has been associated with feline leukemia virus infection, parvovirus infection, and adverse drug reactions including chemotherapeutic agents and griseofulvin toxicity (Peterson et al 1988, Dornsife et al 1989, Rottmann et al 1991, Weiss and Evanson 2000).
A retrospective study of feline bone marrow specimens submitted between July 1, 1996 and June 30, 2004 was conducted. To assure a diagnosis of aplastic anemia, only cats with complete blood counts within 24 h of bone marrow collection, aspiration smears and core biopsy specimens were included. One hundred twenty-eight specimens were identified. Aplastic anemia was defined as over 95% of the marrow space occupied by adipose tissue in unit particles or core biopsy specimens and by concurrent bicytopenia or pancytopenia in the blood. Anemia was defined as a hematocrit of less than 30%, leukopenia was as total leukocyte count of less than 3000/μl and thrombocytopenia as platelet count of under 200,000/μl.
All bone marrow specimens were reviewed by the author. Sixteen met the criteria for the study and 13 (10.2%) of these had bicytopenia or pancytopenia. These cases were further evaluated by reviewing case records, clinicopathological data and necropsy data. The clinical/pathological diagnoses for these cases included chronic renal failure (n=5), feline leukemia virus infection (n=2), hyperthyroidism/methimazole toxicity (n=1), and idiopathic aplastic anemia (n=5).
Five cats had a diagnosis of chronic renal failure (Table 1). The diagnosis was based on the presence of persistent azotemia and isosthenuria. All cats had a history of anorexia and weight loss and serological tests for feline leukemia virus and feline immunodeficiency virus were negative. Four cats also had negative direct immunofluorescent tests for feline leukemia virus infection performed on bone marrow. Cat 1 was pancytopenic when initially evaluated. A bone marrow evaluated at that time indicated severe panhypoplasia. The cat was treated with erythropoietin and the hematocrit returned to the low reference interval. The cat survived for 6 years with mild pancytopenia. Cat 2 had mild leukopenia and thrombocytopenia when renal failure was initially diagnosed. The leukopenia and thrombocytopenia persisted over the subsequent 16 months and the cat became progressively anemic. The bone marrow was virtually acellular. Adipose tissue within the marrow had a pink granular appearance consistent with serous atrophy of fat (Fig. 1; Brunning 1989). This cat was euthanased the next day because of a splenic sarcoma. Cat 3 initially had a moderate non-regenerative anemia that worsened despite erythropoietin therapy. Three months later the cat was pancytopenic and the bone marrow was aplastic. After 5 months of severe pancytopenia, the cat was euthanased. Cat 4 had moderate pancytopenia and bone marrow aplasia at the time of initial detection of renal failure. Despite this, the cytopenias in the blood did not worsen and the cat survived for more than 3 years. Cat 5 was referred for evaluation of chronic renal failure. The cat had been treated with erythropoietin before referral. The cat had a severe non-regenerative anemia and moderate thrombocytopenia. A bone marrow evaluation revealed severe panhypoplasia. The cat died 2 days later.
Table 1.
Clinicopathological features of cats with aplastic anemia
| Cats 1–5 | Cats 6–7 | Cat 8 | Cats 9–13 | |
|---|---|---|---|---|
| Age (years) | 16±3 | 1±1 | 16 | 6±3 |
| Clinical diagnosis | Chronic renal failure | Feline leukemia virus infection | Methimazole toxicity | Idiopathic |
| Hematocrit (%) | 17±3 | 11±2 | 19 | 18±3 |
| Total leukocytes (/μl) | 2200±820 | 1200±980 | 4500 | 2320±770 |
| Neutrophils (/μl) | 1900±430 | 1100±850 | 2300 | 890±130 |
| Platelets (/μl) | 49,500±18,000 | 52,600±20,200 | <1000 | 23,000±14,000 |
Values represent mean±SD.
Fig 1.
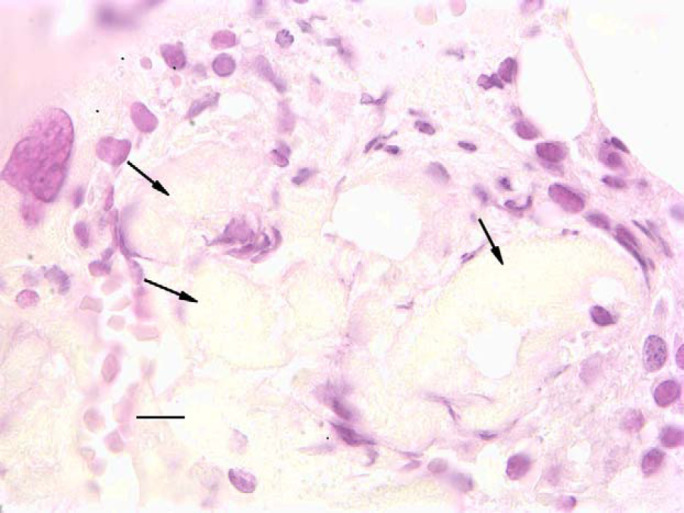
Bone marrow core biopsy from a cat (case 2) with chronic renal failure. Notice that the marrow is poorly cellular and that the adipose tissue is replaced by an amorphous granular material (arrows) consistent serous degeneration of fat. Cells present are small lymphocytes. Bar=20 μm.
Cats 6 and 7 tested positive for feline leukemia virus infection (Table 1). Both cats were less than 2 years old. Evaluation of the blood indicated that both cats were pancytopenic. Examination of bone marrow indicated severe panhypoplasia in both cats. Both cats were euthanased within 2 weeks.
Cat 8 had a history of hyperthyroidism (Table 1). It had been treated with methimazole for 3 years; however, the dosage had been doubled (2.5 mg BID) 1 week before hematological evaluation. Tests for feline leukemia virus (by bone marrow direct immunofluorescence assay) and feline immunodeficiency virus were negative. The cat had a mild normocytic normochromic non-regenerative anemia, leukopenia and severe thrombocytopenia. The bone marrow was aplastic with most cells present consisting of small lymphocytes and plasma cells. Methimazole toxicity was suspected as a cause of the hematological dyscrasia. During evaluation an intra-abdominal mast cell tumor was identified and the cat was euthanased.
Cats 9–13 were classified as idiopathic aplastic anemia (Table 1). Four cats had a history of chronic illness characterized by partial to complete anorexia and weight loss. Two cats had oral ulcers and one had gastric ulcer. These cats had been anorectic for 2, 5 and 9 months, respectively, and had lost over a third of their body weight. All cats tested negative for feline leukemia virus (four cats were tested by bone marrow direct immunofluorescence assay) and feline immunodeficiency virus infection. None of the cats were tested for monocytic ehrlichiosis, however, ehrlichiosis was considered unlikely because it is not endemic in the upper Midwest area of the USA. Three cats had severe pancytopenia and one cat had mild pancytopenia. One cat was not anemic but had severe leukopenia and thrombocytopenia. All cats had acellular bone marrow. Adipose tissue in one of the emaciated cats was replaced by a pink granular material consistent with serous atrophy of fat. All five cats were euthanased within 2 weeks of initial evaluation.
Results of this study indicate that several disease conditions are associated with aplastic anemia in cats. Aplastic anemia has been previously described with feline leukemia virus subgroup C infection and with treatment with methimazole (Peterson et al 1988, Dornsife et al 1989). Feline leukemia virus appears to destroy hematopoietic precursor cells in bone marrow. Treatment with methimazole or propylthiouracil results in a relatively high incidence of hematological dyscrasias. In a study of 262 cats treated with methimazole, leukopenia developed in 4.7% and thrombocytopenia developed in 2.7% (Peterson et al 1988).
Anemia is a frequent finding in chronic renal failure (DiBartola et al 1987, Cowgill 1992). Aplastic anemia has not been previously documented, but some cats develop pancytopenia (Weiss and Evanson 2000). The advanced age of these cats and the negative feline leukemia virus and feline immunodeficiency virus test results suggests that the aplasia was unlikely to be caused by an infection with these viruses. Interestingly, some cats have prolonged survival in spite of persistent pancytopenia and aplastic bone. Therefore, the author would suggest that the prognosis for cats with aplastic anemia should be considered guarded rather than grave.
A common feature of some cats with chronic renal failure and idiopathic aplastic anemia was prolonged anorexia and emaciation. In three cats with idiopathic aplastic anemia, anorexia was prolonged and severe. One cat with renal failure and one emaciated cat with idiopathic aplastic anemia had serous atrophy of fat. This rare lesion has only been described in bone marrow of humans and a goat in association with emaciation or starvation (Lewis and Rebar 1979, Smith and Spivak 1985). In these cases, bone marrow has been hypocellular or aplastic (Pearson 1967). Therefore, the presence of prolonged anorexia, emaciation, or serous atrophy of fat in some cats with aplastic anemia suggests that the aplasia may be the result of starvation.
In conclusion, in this study of 13 cases, aplastic anemia was seen most commonly in cats with chronic renal failure and as an idiopathic condition. In both conditions, starvation may play a role in the development of the marrow aplasia. Some cats with aplastic anemia can have prolonged survival without resolution of the pancytopenia.
References
- Brunning R.B. Bone marrow. Rosai A. Ackerman's Surgical Pathology, 1989, C.V. Mosby: St. Louis, 1251–1303. [Google Scholar]
- Cowgill L. Pathophysiology and management of anemia in chronic progressive renal failure, Seminars in Veterinary Medicine and Surgery 7, 1992, 175–189. [PubMed] [Google Scholar]
- DiBartola S.P., Rutgers H.C., Zack P.M., Tarr M.J. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984), Journal of the American Veterinary Medical Association 190, 1987, 1196–1202. [PubMed] [Google Scholar]
- Dornsife R.E., Gasper P.E., Mullins J.I., Hoover E.A. In vitro erythrocytopathic activity of an aplastic anemia-inducing feline retrovirus, Experimental Hematology 17, 1989, 138–144. [PubMed] [Google Scholar]
- Lewis H.B., Rebar A.H. Bone Marrow Evaluation in Veterinary Practice, 1979, Ralston Purina Co.: St. Louis, MO. [Google Scholar]
- Miura A., Endo K., Sugawara T., Kameoka J., Watanabe N., Meguro K., Fukuhara O., Stao I., Suzuki C., Yoshinaga K. T cell-mediated inhibition of erythropoiesis in aplastic anemia: the possible role of IFN-γ and TNF-α, British Journal of Haematology 78, 1991, 442–449. [DOI] [PubMed] [Google Scholar]
- Nissen C. The pathophysiology of aplastic anemia, Seminars in Hematology 28, 1991, 313–318. [PubMed] [Google Scholar]
- Pearson H.A. Marrow hypoplasia in anorexia nervosa, Journal of Pediatrics 71, 1967, 211–215. [DOI] [PubMed] [Google Scholar]
- Peterson M.E., Kintzer P.P., Hurvitz A.I. Methimazole treatment of 262 cats with hyperthyroidism, Journal of Veterinary Internal Medicine 2, 1988, 150–157. [DOI] [PubMed] [Google Scholar]
- Rottmann J.B., English R.V., Breitschwerdt E.B., Duncan D.E. Bone marrow hypoplasia in a cat treated with griseofulvin, Journal of the American Veterinary Medical Association 198, 1991, 429–431. [PubMed] [Google Scholar]
- Smith R.L., Spivak J.L. Marrow cell necrosis in anorexia nervosa and involuntary starvation, British Journal of Haematology 60, 1985, 525–530. [DOI] [PubMed] [Google Scholar]
- Verma A., Deb D.K., Sassano A., Kambhampati S., Wickrema A., Uddin S., Mohindru M., Van Besien K., Platanias L.C. Activation of the p38 mitogen-activated protein linase signaling pathway mediates cytokine-induced hematopoietic suppression in aplastic anemia, Journal of Immunology 168, 2002, 5984–5988. [DOI] [PubMed] [Google Scholar]
- Weiss D.J. Aplastic anemia. Feldman B.F., Zinkl J.G., Jain N.C. Schalm's Veterinary Hematology, 5th edn, 2000, Town Lippincott Williams & Wilkins, 212–215. [Google Scholar]
- Weiss D.J., Evanson O.A. A retrospective study of feline pancytopenia, Comparative Haematology International 10, 2000, 50–55. [Google Scholar]


