Abstract
The purpose of this study was to clarify whether cats have a colostral and milk phase of lactation differentiated by concentrations of immunoglobulins, and whether colostrum ingestion by newborn kittens is essential for optimal transfer of passive immunity. Milk from specific pathogen-free queens was analyzed for IgG and IgA concentrations from parturition through 6 weeks of lactation. Serum IgG and IgA concentrations from birth through 8 weeks of age were determined for colostrum-fed kittens, colostrum-deprived kittens that were fed a milk replacer, and colostrum-deprived kittens that were fostered onto queens in the milk phase of lactation. The total IgG and IgA concentrations in milk were significantly higher on the day of parturition than on day 7 of lactation, indicating cats do have a colostral phase of lactation. The predominant immunoglobulin in both colostrum and milk was IgG. The serum IgG concentrations in colostrum-deprived kittens fostered on queens in the milk phase of lactation were similar to colostrum-deprived kittens fed a milk replacer, and the concentrations were significantly lower than in colostrum-fed kittens for the first 4 weeks of life. The serum IgA concentrations in both colostrum-deprived groups were significantly lower than colostrum-fed kittens on day 2 after parturition, but were similar thereafter. Colostrum-deprived kittens fostered onto queens in the milk phase of lactation had failure of passive transfer of maternal antibodies. Protective concentrations of immunoglobulins can be restored in kittens with failure of passive transfer of immunity by parenteral administration of adult cat serum, but not by fostering on queens in mid-lactation.
Passive transfer of maternal antibodies to neonates provides protection against infectious diseases before development of their own adaptive immunity (Mason et al 1930, Brambell 1966, Simpson-Morgan and Smeaton 1972). Many mammalian neonates rely on ingestion of antibody-rich colostrum for passive transfer of maternal antibodies (Smith and Little 1922, Bruner et al 1948, Comline et al 1951, Olsson 1959, Harding et al 1961, Hardy 1964, Gillette and Filkins 1966, Kruze 1970). Colostrum is a transient but concentrated source of maternal immunoglobulins, particularly IgG and IgA (Rouse and Ingram 1970, Bourne and Curtis 1973, McGuire and Crawford 1973, Porter 1973, Heddle and Rowley 1975, Kohn et al 1989, Le Jan 1993, Sheoran et al 2000).
Colostral immunoglobulins ingested by the nursing newborn are transferred intact from the intestinal lumen into the circulation by a non-selective transport mechanism (Brambell 1966, Jeffcott 1971, Stott et al 1979, Staley and Bush 1985). In addition to the non-selective transport, neonatal enterocytes have specific receptors (FcγRn) for the uptake of IgG (Kacskovics et al 2000, Ghetie and Ward 2002, Mayer et al 2002). The capacity for intestinal absorption of colostral immunoglobulins decreases rapidly and generally ceases by 24 h after parturition (Hardy 1964, Brambell 1966, Gillette and Filkins 1966, Klaus et al 1969, Jeffcott 1971, Heddle and Rowley 1975, Casal et al 1996). Concomitant with closure of intestinal absorption, the immunoglobulin concentration in mammary secretions markedly decreases as lactation switches from the colostral to milk phase by 3 days after parturition (Rouse and Ingram 1970, Curtis and Bourne 1971, Bourne and Curtis 1973, McGuire and Crawford 1973, Heddle and Rowley 1975, Norcross 1982, Le Jan 1993, Sheoran et al 2000).
The serum concentration of passively acquired immunoglobulins in neonates is ultimately determined by the quantity in the colostrum, the volume of colostrum ingested, and the time of ingestion. Neonates that fail to acquire adequate amounts of antibodies before cessation of intestinal absorption are at significant risk of infection. Failure of passive transfer of immunity (FPT) is a well-documented cause of infection-related illness and death in large animal neonates (McGuire et al 1977, Robinson et al 1993, Raidal 1996, Donovan et al 1998), and several colostrum replacements and immunoglobulin supplements are available for prevention and treatment of FPT.
Similar to other mammalian species, neonatal kittens rely on ingestion of colostrum for passive transfer of maternal antibodies (Harding et al 1961, Yamada et al 1991, Casal et al 1996, Pu and Yamamoto 1998, Levy et al 2001). Extrapolation from studies in large animal species would suggest that kittens with failure of passive transfer of immunity are at significant risk for infection. Kittens at risk for FPT include those that are orphaned or rejected before nursing, kittens from very large litters, small or weak kittens, kittens from queens that fail to lactate on the day of birth, and kittens that must be removed before nursing to avoid neonatal isoerythrolysis. A recent study (Levy et al 2001) demonstrated that adult cat serum administered parenterally is an effective immunoglobulin supplement for kittens with FPT due to colostrum deprivation. Another study (Casal et al 1996) reported that, in contrast to other mammalian species, immunoglobulin concentrations were similar in feline colostrum and milk, and proposed that newborn kittens at risk for FPT could be fostered on queens at any stage of lactation for acquisition of adequate passive immunity.
The purpose of this study was to clarify whether cats have similar immunoglobulin concentrations in colostrum and milk, or if the concentrations in colostrum are significantly higher as in other species. The concentrations of IgG and IgA were measured in colostrum and milk samples from queens from parturition through 6 weeks of lactation. To determine whether the stage of lactation was important to optimal transfer of passive immunity, serum IgG and IgA concentrations were determined for neonatal kittens that ingested colostrum, for kittens deprived of colostrum and fostered on queens in early to mid-lactation, and for kittens deprived of colostrum and fed an artificial milk replacer.
Materials and methods
Animals
Sixty-five blood type A specific pathogen-free queens and their 182 kittens were included in the study. Queens were under constant observation during the final days of gestation, and all deliveries were attended. The research protocol was approved by the Institutional Animal Care and Use Committee and was conducted in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Treatment groups
There were three treatment groups in the study—colostrum-fed kittens, colostrum-deprived kittens fed an artificial milk replacer, and colostrum-deprived kittens fostered onto queens in early to mid-lactation. All kittens were removed from the queens immediately after birth to prevent ingestion of colostrum, and blood samples were collected for determination of baseline serum IgG and IgA concentrations. Kittens in each litter were randomly assigned to either the colostrum-fed or colostrum-deprived groups. Kittens in the colostrum-fed group (n=125) were immediately returned to their queens to nurse for the duration of the study. One group of colostrum-deprived kittens (n=39) was fed an artificial milk replacer (Eukanuba Milk Replacer Formula for Kittens; Iams) for the first 48 h postpartum, and then returned to their queens for the duration of the study. Another group of colostrum-deprived kittens (n=18) was fostered onto surrogate queens (n=5) that were 1–3 weeks in lactation. Nine kittens were fostered onto two surrogate queens that were on day 7 of lactation, five kittens were fostered onto two surrogate queens on day 14 of lactation, and four kittens were fostered onto a queen on day 21 of lactation. The fostered kittens nursed for 48 h on the surrogate queens, and then were returned to their maternal queen for the duration of the study.
Serum and milk samples
Blood samples (1 ml) were collected via jugular venepuncture into serum separator tubes from all queens on the day of parturition. Blood samples (0.25 ml) were collected from kittens via jugular venepuncture into serum separator microtubes at parturition, on days 2 and 7, then weekly until 8 weeks after parturition. Serum was harvested from the blood samples by centrifugation. Milk (approximately 250–500 μl) was collected daily from each queen from parturition to day 7, then at 2, 4, and 6 weeks of lactation. The samples were collected by gentle milking of the secretions from several mammary glands directly into cryovials and were not further processed. Serum and milk samples were stored at −80°C pending immunoglobulin analyses.
Measurement of IgG
Total IgG concentrations in serum and milk samples were determined by a commercial radial immunodiffusion kit (Feline IgG RID kit; VMRD, Inc) according to the manufacturer's instructions. Standards with IgG concentrations of 32–2000 mg/dl included with the kits were analyzed with each batch of samples. The diameter of the precipitin ring for each standard was measured to generate a semilogarithmic standard curve of IgG concentration versus diameter. The standards and samples were tested in duplicate. Samples with IgG concentrations more than 2000 mg/dl were diluted into the range of the standards. Samples with IgG concentrations less than 32 mg/dl were recorded as 0 for purposes of statistical analysis.
Measurement of IgA
Total IgA concentrations in serum and milk samples were determined by a commercial ELISA assay (Feline IgA ELISA kit; Bethyl Laboratories) according to the manufacturer's instructions. Standards with IgA concentrations of 0.001–0.1 mg/dl provided with the kits were included on each microtiter plate containing test samples. The IgA standards, serum sample dilutions (1:250–1:8000), and milk sample dilutions (1:250–1:2000) were tested in duplicate. A quadratic standard curve was generated from the absorbance values of the IgA standards using a software program (Microplate Manager; Bio-Rad Laboratories). The IgA concentrations in the test samples were calculated from the standard curve by the software program.
Statistical analysis
Data were analyzed using a software program (SigmaStat 3.0 for Windows, SPSS, Inc). Normally distributed data with equal variance were analyzed by the Student's t-test. Data that were not normally distributed or had unequal variance were analyzed by the Mann–Whitney rank sum test. The Spearman rank order correlation test was used to calculate correlation coefficients (r). For all analyses, P<0.05 was considered significant. The data are reported as mean±SD (range).
Results
Serum and milk IgG and IgA concentrations in queens
The total IgG concentration in serum of queens at parturition was 1502±544 mg/dl (range, 500–2800 mg/dl). The total IgG concentration in milk at parturition from the same queens was 6205±2385 mg/dl (range, 1860–13,600 mg/dl). The milk IgG concentration was 4.5±1.9-fold greater (P<0.001) than the serum IgG concentration at parturition. The milk IgG concentrations at parturition were positively correlated with the serum IgG concentrations in the respective queens (r=0.54, P<0.001).
The total IgA concentration in serum of queens at parturition was 190±137 mg/dl (range, 17–677 mg/dl). The total IgA concentration in milk at parturition from the same queens was 143±116 mg/dl (range, 10–577 mg/dl). The milk IgA concentration was not significantly different (P=0.05) from the serum IgA concentration. There was no correlation between the serum and milk IgA concentrations in the respective queens (r=0.24, P=0.08).
The total IgG concentration in milk from the queens decreased significantly (P<0.001) during the first 7 days of lactation from the peak at parturition of 6205±2385 to 533±729 mg/dl on day 7 (Fig 1). The IgG concentration further declined to 205±128 mg/dl by day 42 (6 weeks) of lactation. Similarly, the total IgA concentration in milk from the queens decreased significantly (P<0.001) during the first 7 days of lactation from the peak at parturition of 143±116 to 29±23 mg/dl on day 7 (Fig 2). The milk IgA concentration did not change (P>0.05) further from 1 to 6 weeks of lactation.
Fig 1.
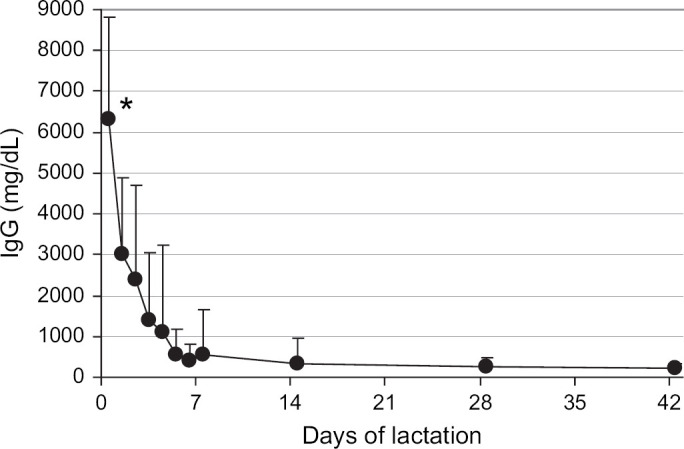
Mean IgG concentrations in the milk of queens from parturition through 6 weeks of lactation. The mean for parturition (day 0) represents 65 queens, the means from days 1 to 6 represent 24 to 29 queens, and the means for days 7 to 42 represent 45 to 49 queens. Error bars indicate one SD of the mean. *The mean IgG in milk at parturition (day 0) was significantly different from mean IgG in milk on days 1–42.
Fig 2.
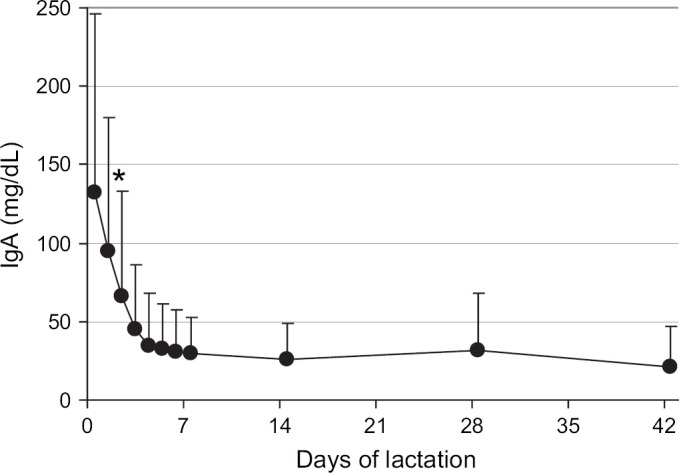
Mean IgA concentrations in the milk of queens from parturition through 6 weeks of lactation. The mean for parturition (day 0) represents 57 queens, the means from days 1 to 6 represent 24 to 28 queens, and the means for days 7 to 42 represent 36 to 41 queens. Error bars indicate one SD of the mean. *The mean IgA in milk at parturition (day 0) was significantly different from mean IgA in milk on days 2–42.
Serum IgG and IgA concentrations in kittens
None of the kittens had detectable IgG or IgA in the serum at parturition. For kittens that ingested colostrum, the peak serum total IgG concentration on day 2 after parturition was 1915±851 mg/dl (range, 350–6000 mg/dl). This peak concentration was positively correlated with the serum IgG (r=0.44, P<0.001) and milk IgG (r=0.33, P<0.001) of their dams at parturition. Similarly, the peak serum total IgA concentration in kittens on day 2 was 32±31 mg/dl (range, 6–176 mg/dl), and this peak concentration was positively correlated with the serum IgA (r=0.19, P=0.03) and milk IgA (r=0.65, P<0.001) of their dams at parturition.
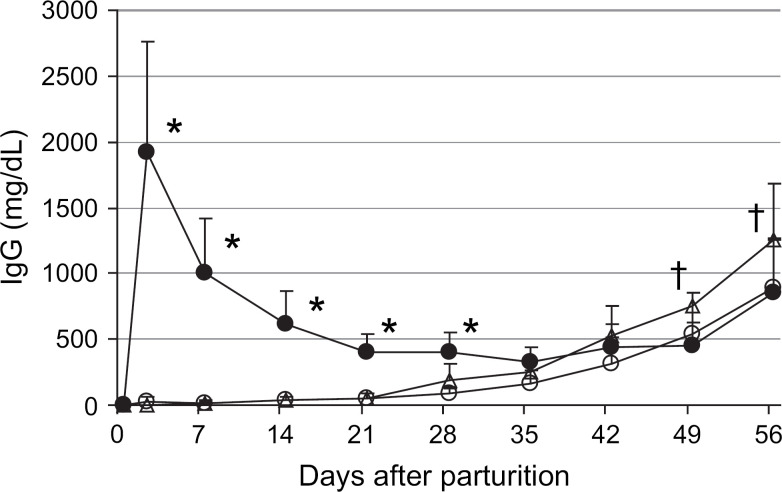
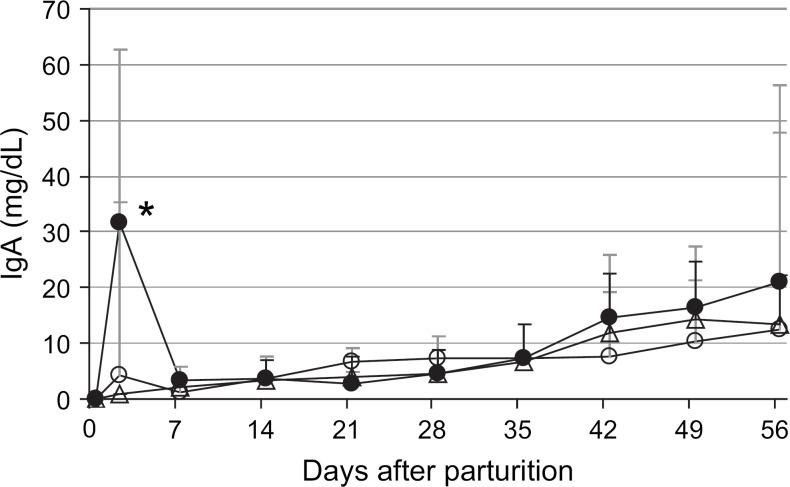
Following the peak on day 2, the maternally derived serum IgG concentrations in the colostrum-fed kittens steadily declined to a nadir of 329±107 mg/dl (range, 200–600 mg/dl) at 5 weeks of age, followed by an increase to 848±425 mg/dl (range, 250–2650 mg/dl) at 8 weeks of age (Fig 3). From the peak on day 2, serum IgA concentrations decreased to 3±2 mg/dl on day 7, after which the concentration remained steady at 3–5 mg/dl (range, 1–22mg/dl) for the first 4 weeks of age, followed by an increase to 21±35 mg/dl (range, 5–208 mg/dl) by 8 weeks of age (Fig 4).
Fig 3.
Mean serum IgG concentrations from parturition through 8 weeks of age in kittens that ingested colostrum (n=55–125, closed circles), colostrum-deprived kittens that ingested milk from surrogate queens in mid-lactation (n=18, open circles), and colostrum-deprived kittens fed a milk replacer (n=39, open triangles). Error bars indicate one SD of the mean. *Colostrum-fed kittens were significantly different from colostrum-deprived kittens. †Colostrum-deprived kittens fed milk replacer were significantly different from colostrum-deprived kittens fostered on surrogate queens and colostrum-fed kittens.
Fig 4.
Mean serum IgA concentrations from parturition through 8 weeks of age in kittens that ingested colostrum (n=55–125, closed circles), colostrum-deprived kittens that ingested milk from surrogate queens in mid-lactation (n=18, open circles), and colostrum-deprived kittens fed a milk replacer (n=39, open triangles). Error bars indicate one SD of the mean. *Colostrum-fed kittens were significantly different from colostrum-deprived kittens on day 2.
There was no detectable serum IgG and IgA on day 2 in colostrum-deprived kittens fed a milk replacer for 48 h. For colostrum-deprived kittens fostered on surrogate queens at 1–3 weeks of lactation, the serum IgG concentration on day 2 was 31±30 mg/dl (range, 0–84 mg/dl), and the serum IgA concentration was 4±2 mg/dl (range, 1–9 mg/dl). The IgG concentration in the milk of the surrogate queens ranged from 125 to 390 mg/dl, while the IgA concentration ranged from 10 to 80 mg/dl. There was no correlation of foster kitten serum IgG and IgA concentrations on day 2 with the IgG (r=0.074, P=0.8) or IgA (r=0.14, P=0.6) concentrations in the milk of their surrogate queens. The serum IgG and IgA concentrations on day 2 for both groups of colostrum-deprived kittens were significantly (P<0.001) lower than for kittens that ingested colostrum at birth.
The serum IgG concentrations in both groups of colostrum-deprived kittens were significantly (P<0.001) less than colostrum-fed kittens for the first 4 weeks after parturition (Fig 3). Starting at 2 weeks of age, the serum IgG concentrations in the colostrum-deprived kittens fostered on surrogate queens or fed a milk replacer steadily increased to 8 weeks of age due to endogenous synthesis of antibodies (Fig 3). At 7 and 8 weeks, the serum IgG concentrations in the colostrum-deprived kittens fed a milk replacer were significantly (P<0.001) higher than all other kittens.
In contrast to IgG, the serum IgA concentrations in the colostrum-deprived kittens fostered on surrogate queens or fed a milk replacer were similar to the concentrations in colostrum-fed kittens from 1 to 8 weeks of age (Fig 4).
Discussion
The results of this study demonstrate that cats, like most other mammalian species, have both colostral and milk phases of lactation distinguished by the concentrations of IgG and IgA. Similar to horses, pigs, and dogs (Rouse and Ingram 1970, Bourne and Curtis 1973, Heddle and Rowley 1975, Norcross 1982, Sheoran et al 2000), the IgG concentration was nearly five times higher in colostrum than in serum of the queens. This is also consistent with previous studies in cats (Gorman and Halliwell 1989, Pu and Yamamoto 1998, Levy et al 2001, Crawford et al 2003, MacDonald et al 2004). In contrast to previous studies (Casal et al 1996, Levy et al 2001, Crawford et al 2003, MacDonald et al 2004), the colostral IgG concentrations in the current study were weakly correlated with the serum IgG concentrations of the queen. This discrepancy may be explained by the analysis of a larger number of cats in the current study.
The predominant immunoglobulin in feline colostrum was IgG, consistent with previous studies in cats, dogs, horses, and pigs (Rouse and Ingram 1970, Bourne and Curtis 1973, Heddle and Rowley 1975, Norcross 1982, Gorman and Halliwell 1989, Casal et al 1996, Pu and Yamamoto 1998, Sheoran et al 2000). As in previous reports for cats (Gorman and Halliwell 1989, Casal et al 1996, Pu and Yamamoto 1998), the IgA concentration in the colostrum was similar to that in serum of the queens. Therefore, cats are different from horses, pigs, and dogs in which IgA is more concentrated in colostrum than in serum (Rouse and Ingram 1970, Bourne and Curtis 1973, Heddle and Rowley 1975, Norcross 1982, Le Jan 1993, Sheoran et al 2000).
During the first 7 days of lactation, there was a five-fold decrease in IgG concentration in milk and a 10-fold decrease in IgA. The milk IgG concentration steadily decreased throughout lactation while the IgA concentration remained at a constant low level for 6 weeks. These findings are similar to previous studies in cats (Pedersen 1987, Gorman and Halliwell 1989, Pu and Yamamoto 1998) as well as horses, pigs, and dogs (Rouse and Ingram 1970, Bourne and Curtis 1973, McGuire and Crawford 1973, Heddle and Rowley 1975, Norcross 1982, Sheoran et al 2000). However, the results are contradictory to those of another study in cats (Casal et al 1996) in which IgG and IgA concentrations in whole and de-fatted colostrum and milk were similar. In other studies, including the current one, the immunoglobulins were measured in unprocessed whole milk samples. In pigs and cows, immunoglobulins in colostrum and milk are associated with fat globules in the cream layer (Le Jan 1993), and the association of immunoglobulins with milk fat is the basis of the milk ring test for Brucella species antibodies (Patterson et al 1974, Sutra et al 1986). Like other species, feline colostrum and milk contain substantial amounts of lipids (Keen et al 1982, Adkins et al 1997).
In the cats in this study as well as in previous studies (Pedersen 1987, Gorman and Halliwell 1989, Pu and Yamamoto 1998), IgG was not only the predominant immunoglobulin in colostrum, but also in milk. This is similar to ruminants, but different from other mammals such as dogs, horses, and pigs in which IgA is the predominant immunoglobulin in milk (Rouse and Ingram 1970, Bourne and Curtis 1973, McGuire and Crawford 1973, Heddle and Rowley 1975, Le Jan 1993, Sheoran et al 2000).
Similar to previous studies in cats (Yamada et al 1991, Casal et al 1996, Pu and Yamamoto 1998, Levy et al 2001, Crawford et al 2003, MacDonald et al 2004), the serum IgG concentrations in kittens that ingested colostrum gradually declined from the peak on day 2 to a nadir at 4–5 weeks of age due to catabolism of maternal IgG. The serum IgG concentrations in colostrum-deprived kittens fed a milk replacer or fostered onto surrogate queens in the milk phase of lactation were significantly lower for the first 4 weeks of life compared to kittens that ingested colostrum. Numerous studies in large animal species have shown that serum IgG concentration is the best predictor of whether neonates will be protected against infection, and that the concentration should be >400 mg/dl for adequate protection (LeBlanc et al 1992, Rea et al 1996, Franz et al 1998, Virtala et al 1999). Large animal neonates with serum IgG concentrations of less than 400 mg/dl are diagnosed with failure of passive transfer of immunity. Although the minimum IgG concentration required for adequate passive transfer of immunity in kittens is unknown, all of the colostrum-deprived kittens that ingested milk from surrogate queens or were fed a milk replacer had serum IgG concentrations less than 400 mg/dl for the first 4 weeks of life, and thus had FPT based on the definition for large animal neonates.
The serum IgG concentrations steadily increased in all kittens from 4 to 8 weeks of age due to endogenous IgG synthesis. The lower serum IgG concentrations at 7 and 8 weeks of age in kittens that ingested milk in the first 48 h postpartum most likely represented suppression of endogenous IgG synthesis by passively acquired maternal antibodies as previously demonstrated in pigs, calves, and foals (Jeffcott 1974, LaMotte 1977, Klobasa et al 1981, 1990).
Similar to IgG, peak serum IgA concentrations were significantly higher in kittens that ingested colostrum compared to colostrum-deprived kittens that were fed milk replacer or fostered onto surrogate queens. In contrast to IgG, the serum IgA concentrations in all of the kittens, regardless of whether they ingested colostrum or not, were similar by 1 week of age and increased at a similar rate thereafter. Previous studies in cats and dogs reported that serum IgA concentrations quickly plummeted from the peak following colostrum ingestion to a nadir at 1–2 weeks after parturition, followed by a slow but steady increase with age due to endogenous synthesis (Poffenbarger et al 1991, Yamada et al 1991, Casal et al 1996, Pu and Yamamoto 1998). The reported half-life of maternally derived IgA in kittens is approximately 2 days, which is much shorter than that reported for maternally derived IgG of 4–12 days (Yamada et al 1991, Casal et al 1996, Crawford et al 2003, MacDonald 2004).
In conclusion, the colostrum of cats contained IgG concentrations greater than those in serum while the IgA concentrations were similar to serum. During the first 7 days of lactation, the IgG and IgA concentrations in milk declined from a peak on the day of parturition to low levels that persisted for the duration of lactation. Therefore, cats, similar to other mammalian species, have both colostral and milk phases of lactation defined by different concentrations of IgG and IgA. Unlike other non-ruminant mammals, the predominant immunoglobulin in both colostrum and milk was IgG. Similar to colostrum-deprived kittens fed a milk replacer, colostrum-deprived kittens fostered onto surrogate queens in the milk phase of lactation had failure of passive transfer of immunity that persisted for 4 weeks. Kittens that are not protected by passively acquired immunity are expected to be at greatest risk of infection during the first 4 weeks after parturition, prior to the development of adaptive immunity. Protective concentrations of immunoglobulins can be restored in kittens with FPT by parenteral administration of adult cat serum (Levy et al 2001), but not by fostering on queens in mid-lactation.
Acknowledgements
The authors acknowledge Megan Ross and Alex Trapp for technical assistance. This study was funded in part by a grant from the Harold H. Morris Trust Fund for Research in Diseases of Small Animals and the Merck-Merial Veterinary Scholar Program.
References
- Adkins Y., Zicker S.C., Lepine A., Lonnerdal B. Changes in nutrient and protein composition of cat milk during lactation, American Journal of Veterinary Research 58, 1997, 370–375. [PubMed] [Google Scholar]
- Bourne F.L., Curtis J. The transfer of immunoglobulins IgG, IgA, and IgM from serum to colostrum and milk in the sow, Immunology 24, 1973, 157–162. [PMC free article] [PubMed] [Google Scholar]
- Brambell F.W.R. The transmission of immunity from mother to young and the catabolism of immunoglobulins, Lancet ii, 1966, 1087–1093. [DOI] [PubMed] [Google Scholar]
- Bruner D.W., Edwards P.R., Doll E.R. Passive immunity in the newborn foal, Cornell Veterinarian 38, 1948, 363–366. [Google Scholar]
- Casal M.L., Jezyk P.F., Giger U. Transfer of colostral antibodies from queens to their kittens, American Journal of Veterinary Research 57, 1996, 1653–1658. [PubMed] [Google Scholar]
- Comline R.S., Roberts H.E., Titchen D.A. Route of absorption of colostral globulin in the newborn animal, Nature 167, 1951, 561–562. [DOI] [PubMed] [Google Scholar]
- Crawford P.C., Hanel R.M., Levy J.K. Evaluation of treatment of colostrum-deprived kittens with equine IgG, American Journal of Veterinary Research 64, 2003, 969–975. [DOI] [PubMed] [Google Scholar]
- Curtis J., Bourne F.J. Immunoglobulin quantitation in sow serum, colostrum and milk and the serum of young pigs, Biochimica Biophysica Acta 236, 1971, 319–339. [DOI] [PubMed] [Google Scholar]
- Donovan G.A., Dohoo I.R., Montgomery D.M., Bennett F.L. Associations between passive immunity and morbidity and mortality in dairy heifers in Florida, USA, Previews in Veterinary Medicine 34, 1998, 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz L.C., Landon J.C., Lopes L.A., Marinho L.A., Sarma C., Bruemmer J., et al. Oral and intravenous immunoglobulin therapy in neonatal foals, Journal of Equine Veterinary Science 18, 1998, 742–748. [Google Scholar]
- Ghetie V., Ward E.S. Transcytosis and catabolism of antibody, Immunology Research 25, 2002, 97–113. [DOI] [PubMed] [Google Scholar]
- Gillette D.D., Filkins M. Factors affecting antibody transfer in the newborn puppy, American Journal of Physiology 210, 1966, 419–422. [DOI] [PubMed] [Google Scholar]
- Gorman N.T., Halliwell R.E. Immunoglobulin quantitation and clinical interpretation. Gorman N.T., Halliwell R.E. Veterinary Clinical Immunology, 1989, W B Saunders Co: Philadelphia, 55–73. [Google Scholar]
- Harding S.K., Bruner D.W., Bryant I.W. The transfer of antibodies from the mother cat to her newborn kittens, Cornell Veterinarian 51, 1961, 535–539. [PubMed] [Google Scholar]
- Hardy R.N. Intestinal absorption of macromolecules in the newborn pig, Proceedings in Physiology 25–26, 1964, 19P–20P. [Google Scholar]
- Heddle R.J., Rowley D. Dog immunoglobulins. I. Immunochemical characterization of dog serum, parotid saliva, colostrum, milk and small bowel fluid, Immunology 29, 1975, 185–195. [PMC free article] [PubMed] [Google Scholar]
- Jeffcott L.B. Duration of permeability of the intestine to macromolecules in the newly born foal, Veterinary Record 32, 1971, 340–341. [DOI] [PubMed] [Google Scholar]
- Jeffcott L.B. Studies on passive immunity in the foal. I. γ-Globulin and antibody variations associated with the maternal transfer of immunity and the onset of active immunity, Journal of Comparative Pathology 84, 1974, 93–101. [DOI] [PubMed] [Google Scholar]
- Kacskovics I., Wu Z., Simister N.E., Frenyo L.V., Hammarstrom L. Cloning and characterization of the bovine MHC class I-like Fc receptor, Journal of Immunology 164, 2000, 1889–1897. [DOI] [PubMed] [Google Scholar]
- Keen C.L., Lonnerdal B., Clegg M.S., Hurley L.S., Morris J.G., Rogers Q.R., et al. Developmental changes in composition of cats milk: trace elements, minerals, protein, carbohydrate and fat, Journal of Nutrition 112, 1982, 1763–1769. [DOI] [PubMed] [Google Scholar]
- Klaus G.G.B., Bennett A., Jones E.W. A quantitative study of the transfer of colostral immunoglobulins to the newborn calf, Immunology 16, 1969, 235–247. [PMC free article] [PubMed] [Google Scholar]
- Klobasa F., Werhahn E., Butler J.E. Regulation of humoral immunity in the piglet by immunoglobulins of maternal origin, Research in Veterinary Science 31, 1981, 195–206. [PubMed] [Google Scholar]
- Klobasa F., Butler J.E., Habe F. Maternal–neonatal immunoregulation: suppression of de novo synthesis of IgG and IgA, but not IgM, in neonatal pigs by bovine colostrum, is lost upon storage, American Journal of Veterinary Research 51, 1990, 1407–1412. [PubMed] [Google Scholar]
- Kohn C.W., Knight D., Hueston W., Jacobs R., Reed S.M. Colostral and serum IgG, IgA, and IgM concentrations in Standardbred mares and their foals at parturition, Journal of the American Veterinary Medical Association 195, 1989, 64–68. [PubMed] [Google Scholar]
- Kruze V. Absorption of immunoglobulin from colostrum in newborn calves, Animal Production 12, 1970, 627–638. [Google Scholar]
- LaMotte G.B. Total serum protein, serum protein fractions and serum immunoglobulins in colostrum-fed and colostrum-deprived calves, American Journal of Veterinary Research 38, 1977, 263–268. [PubMed] [Google Scholar]
- Le Jan C. Secretory component and IgA expression by epithelial cells in sow mammary gland and mammary secretions, Research in Veterinary Science 55, 1993, 265–270. [DOI] [PubMed] [Google Scholar]
- LeBlanc M.M., Tran T., Baldwin J.L., Pritchard E.L. Factors that influence passive transfer of immunoglobulins in foals, Journal of the American Veterinary Medical Association 200, 1992, 179–183. [PubMed] [Google Scholar]
- Levy J.K., Crawford P.C., Collante W.R., Papich M.G. Use of adult cat serum to correct failure of passive transfer in kittens, Journal of the American Veterinary Medical Association 219, 2001, 1401–1405. [DOI] [PubMed] [Google Scholar]
- MacDonald K., Levy J.K., Tucker S.J., Crawford P.C. Effects of passive transfer of immunity on results of diagnostic tests for antibodies against feline immunodeficiency virus in kittens born to vaccinated queens, Journal of the American Veterinary Medical Association 225, 2004, 1554–1557. [DOI] [PubMed] [Google Scholar]
- Mason J.H., Dalling T., Gordon W.S. Transmission of maternal immunity, Journal of Pathology 33, 1930, 783–797. [Google Scholar]
- Mayer B., Zolnai A., Frenyo L.V., Jancsik V., Szentirmay Z., Hammarstrom L., et al. Redistribution of the sheep neonatal Fc receptor in the mammary gland around the time of parturition in ewes and its localization in the small intestine of neonatal lambs, Immunology 107, 2002, 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire T.C., Crawford T.B. Passive immunity in the foal: measurement of immunoglobulin classes and specific antibody, American Journal of Veterinary Research 34, 1973, 1299–1303. [PubMed] [Google Scholar]
- McGuire T.C., Crawford T.B., Hallowell A.L., Macomber L.E. Failure of colostral immunoglobulin transfer as an explanation for most infections and deaths of neonatal foals, Journal of the American Veterinary Medical Association 170, 1977, 1302–1304. [PubMed] [Google Scholar]
- Norcross N.L. Secretion and composition of colostrum and milk, Journal of the American Veterinary Medical Association 181, 1982, 1057–1060. [PubMed] [Google Scholar]
- Olsson B. Studies on the formation and absorption of antibodies and immune globulins in piglets, Nordic Veterinary Medicine 11, 1959, 375–390. [Google Scholar]
- Patterson J.M., Roepke M.H., Deyoe B.L. Standardization of test-negative cream for Brucella milk ring test, American Journal of Veterinary Research 35, 1974, 119–120. [PubMed] [Google Scholar]
- Pedersen N.C. Basic and clinical immunology, Diseases of the Cat: Medicine and Surgery Vol. I, 1987, W B Saunders Co: Philadelphia, pp. 146–181 [Google Scholar]
- Poffenbarger E.M., Olson P.N., Chandler M.S., Seim H.B., Varman M. Use of adult dog serum as a substitute for colostrum in the neonatal dog, American Journal of Veterinary Research 52, 1991, 1221–1224. [PubMed] [Google Scholar]
- Porter P. Studies on porcine secretory IgA and its component chains in relation to intestinal absorption of colostral immunoglobulins by the neonatal pig, Immunology 24, 1973, 163–176. [PMC free article] [PubMed] [Google Scholar]
- Pu R., Yamamoto J.K. Immunology of the cat: passive transfer of maternal immunity. Pastoret P.P., Griebel P., Bazin H. Handbook of Vertebrate Immunology, 1998, Academic Press: San Diego, 305–308. [Google Scholar]
- Raidal S.L. The incidence and consequences of failure of passive transfer of immunity on a Thoroughbred breeding farm, Australian Veterinary Journal 73, 1996, 201–206. [DOI] [PubMed] [Google Scholar]
- Rea D.E., Tyler J.W., Hancock D.D., Besser T.E., Wilson L., Krytenberg D.S., et al. Prediction of calf mortality by use of tests for passive transfer of colostral immunoglobulin, Journal of the American Veterinary Medical Association 208, 1996, 2047–2049. [PubMed] [Google Scholar]
- Robinson J.A., Allen G.K., Green E.M., Fales W.H., Loch W.E., Wilkerson C.G. A prospective study of septicaemia in colostrum-deprived foals, Equine Veterinary Journal 25, 1993, 214–219. [DOI] [PubMed] [Google Scholar]
- Rouse B.T., Ingram D.G. The total protein and immunoglobulin profile of equine colostrum and milk, Immunology 19, 1970, 901–907. [PMC free article] [PubMed] [Google Scholar]
- Sheoran A.S., Timoney J.E., Holmes M.A., Karzenski S.S., Crisman M.V. Immunoglobulin isotypes in sera and nasal mucosal secretions and their neonatal transfer and distribution in horses, American Journal of Veterinary Research 61, 2000, 1099–1105. [DOI] [PubMed] [Google Scholar]
- Simpson-Morgan M.W., Smeaton T.C. The transfer of antibodies by neonates and adults, Advances in Veterinary Science Compendium of Medicine 16, 1972, 355–386. [PubMed] [Google Scholar]
- Smith T., Little R.B. The significance of colostrum to the newborn calf, Journal of Experimental Medicine 36, 1922, 181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley T.E., Bush L.J. Receptor mechanisms of the neonatal intestine and their relationship to immunoglobulin absorption and disease, Journal of Dairy Science 68, 1985, 184–194. [DOI] [PubMed] [Google Scholar]
- Stott G.H., Marx D.B., Menefee B.E., Nightengale G.T. Colostral immunoglobulin transfer in calves. I. Period of absorption, Journal of Dairy Science 62, 1979, 1632–1637. [DOI] [PubMed] [Google Scholar]
- Sutra L., Caffin J.P., Dubray G. Role of milk immunoglobulins in the Brucella milk ring test, Veterinary Microbiology 12, 1986, 359–366. [DOI] [PubMed] [Google Scholar]
- Virtala A.M., Grohn Y.T., Mechor G.D. The effect of maternally derived immunoglobulin G on the risk of respiratory disease in heifers during their first 3 months of life, Previews in Veterinary Medicine 39, 1999, 25–37. [DOI] [PubMed] [Google Scholar]
- Yamada T., Nagai Y., Matsuda M. Changes in serum immunoglobulin values in kittens after ingestion of colostrum, American Journal of Veterinary Research 52, 1991, 393–396. [PubMed] [Google Scholar]