Abstract
Mast cell tumours (MCTs) are relatively common tumours of cats, and are the second most common cutaneous tumours in cats in the USA. While the primary splenic form of the disease is far less common, it is usually associated with more severe clinical signs. Signalment, clinical and survival characteristics of mast cell neoplasia were characterised in 41 cats. The most common tumour location was cutaneous/subcutaneous head and trunk. Stage 1a was the most common tumour stage at first diagnosis (n=20), followed by stage 4 (both stage 4a and stage 4b; n=10). Of 22 cats that underwent excisional biopsy, mast cell neoplasia recurred in four cats during the study period. Three of the 41 cats presented with simultaneous cutaneous and either splenic or lymph node tumours. A comparison between cats with only cutaneous tumours (n=30) and those with tumours involving the spleen or lymph nodes (n=11) showed longer survival times for the cutaneous-only group (P=0.031). Twelve of the 41 cats died of mast cell neoplasia during the study period. When a subgroup of cats with only cutaneous tumours (no lymph node or visceral involvement) were divided according to whether there were multiple (five or more) tumours (n=6) or a single tumour (n=19), cats with single tumours survived longer than those with multiple tumours (P=0.001). Solitary cutaneous feline MCTs without spread to the lymph nodes usually manifest as benign disease with a relatively protracted course. However, multiple cutaneous tumours, recurrent tumours and primary splenic disease should receive a guarded prognosis due to the relatively short median survival times associated with these forms of the disease.
While mast cell tumours (MCTs) are the second most common cutaneous tumours in cats (Thamm and Vail 2001), the primary splenic form of the disease is much less common. The two forms of the disease appear to arise independently and one early paper reports that they are only rarely present at the same time (Liska et al 1979). As in other species, feline mast cells contain prominent granules, which stain blue with Giemsa and purple with toluidine blue. These granules contain a number of vasoactive substances including mast cell tryptase, chymase, heparin and histamine (Beadleston et al 1997).
Splenomegaly is a characteristic feature of the primary splenic form of feline mast cell neoplasia. Mastocytaemia is sometimes also present (Liska et al 1979). Cats with primary splenic mastocytosis may develop single or multiple gastric or duodenal ulcers, presumably secondary to histamine production by the tumour, and presenting signs often include vomiting and anorexia (Liska et al 1979). A second distinct form of visceral MCT occurs in cats – intestinal or alimentary mast cell neoplasia. Intestinal mastocytoma is the third most common primary intestinal tumour in cats after lymphoma and adenocarcinoma (Carpenter et al 1987). Gastric or duodenal ulceration has not been reported in association with intestinal MCTs and it is thought this may be the result of a lack of granules in these neoplastic cells (Barker and Van Dreumel 1985). Intestinal mastocytomas may metastasise to mesenteric lymph nodes, liver, spleen and rarely, lungs (Molander-McCrary et al 1998).
Dermal tumours are observed most commonly on the head, neck and trunk (Molander-McCrary et al 1998). They develop in subcutaneous tissue or dermis, are papular or nodular and may be hairy, alopecic or have an ulcerated surface. There are two presentations of cutaneous mast cell neoplasia seen frequently in clinical practice:
Solitary, firm, round, well-circumscribed, variably-sized masses (0.5–3.0 cm in diameter), dermoepidermal or subcutaneous in location; and
Multiple, raised, firm, round, well-demarcated, white to yellow, small (0.2–1.0 cm in diameter) papules and nodules that are fixed to the skin (Scott 1980).
The form of cutaneous mast cell neoplasia described in 1 (above) has also been described as mast cell type. Affected cats are generally over 4 years old, there is no specific breed predisposition and the tumour is generally benign histologically (Wilcock et al 1986) and behaviourally (Johnson et al 2002). However, one study reported that about 15% of these tumours were histologically aggressive and showed a tendency to recur or spread to distant sites within 2–3 months of surgical excision (Wilcock et al 1986). More recent work has shown that while mitotic activity was a powerful indicator of the behaviour of feline cutaneous MCTs, a prognostic indication must be obtained from simultaneous evaluation of cell type, growth pattern, histological grading, mitotic activity and lymphocytic infiltrates (Lepri et al 2003).
Young (<4 years old), Siamese cats are predisposed to the development of a miliary histiocytic form of cutaneous mast cell disease, corresponding to 2 (above). This form presents clinically as multiple miliary subcutaneous masses with a benign biological behaviour (Wilcock et al 1986). Some histiocytic MCTs regress spontaneously within 2 years of detection (Scott 1980).
The aim of this study was to characterise the signalment, clinical and survival characteristics of a relatively large group of cats with histologically-confirmed mast cell neoplasia using a multi-centre retrospective and prospective study design.
Materials and methods
In the retrospective arm of the study, records from the Department of Veterinary Pathology, University of Queensland were searched for all histologically- or cytologically-confirmed cases of feline mast cell neoplasia diagnosed from January 1995 until December 2002 (n=11). In the prospective arm of the study, records for all cats positive for mast cell neoplasia by histological or cytological diagnosis were requested from two Australian pathology laboratories (IDEXX-VPS, Woollongabba, Queensland and Department of Veterinary Pathology, University of Queensland, St Lucia, Queensland) from June 2001 until December 2003 (n=18). Records were collected from all cats referred to the Oncology Service at the Veterinary Hospital, University of Pennsylvania, Philadelphia, PA, from January 2002 until December 2003 for management of histologically-diagnosed mast cell neoplasia (n=12).
Clinical details collected included age at diagnosis; gender; breed; results of haematology, performed at the time of diagnosis; details of any concurrent diseases; live/dead status; cause of death, if appropriate; number of days from first diagnosis of mast cell neoplasia (by cytology or histopathology) to either date last known or date of death; necropsy results; and presence or absence of excisional biopsy. Tumour details collected included location: head (cutaneous tumours on the head and neck); visceral (in the abdominal cavity); trunk (cutaneous tumours on the shoulders, chest abdomen or tail); multiple skin (more than five cutaneous tumours at presentation) and limbs (cutaneous tumours on the limbs); size; and tumour stage (Table 1). Cats with primary splenic or intestinal MCTs (without cutaneous involvement) were classified as stage 4. Initial staging protocol, where used, consisted of serum biochemistry; haematology; a buffy coat analysis; and abdominal ultrasound examination and/or bone marrow aspirate cytology as required, depending on the tumour size, clinical signs and clinical judgment of the attending clinician.
Table 1.
Tumour staging criteria
| Tumour stage | Criteria for tumour stage |
|---|---|
| 1 | One dermal tumour with no spread to regional lymph nodes (RLN) |
| 2 | One dermal tumour with spread to RLN |
| 3 | Multiple dermal tumours; large infiltrating tumours±RLN involvement |
| 4 | Any tumour with distant metastasis or recurrence with metastasis |
| Stage+a | Stage+no evidence of systemic disease |
| Stage+b | Stage+evidence of systemic disease |
In cats that underwent excisional biopsy, margin status (dirty – containing neoplastic mast cells, or clean – without any evidence of neoplastic mast cells, on histopathological examination) was recorded, as were details of any recurrence of mast cell neoplasia after surgery. More specifically, the location of the recurrence in relation to the first instance of mast cell neoplasia was recorded (whether the recurrence was at or close to the site where the primary tumour was removed; if any de novo primary tumours occurred distant to the previous tumour; or if there was metastasis or visceral involvement subsequent to the first diagnosis of mast cell neoplasia), as was the number of days from excisional biopsy to the following diagnosis of mast cell neoplasia.
Survival time was assessed as time from first diagnosis until death from mast cell neoplasia, or euthanasia on human grounds because of complications resulting from mast cell neoplasia. Cats that died of causes not attributable to their mast cell neoplasia were censored on the date of their death. Cats that were lost to follow-up were censored at the end of the study (the end of February 2003), as were cats that were alive at the end of the study.
Survival analysis was performed with Kaplan–Meier survival curves using GraphPad Prism 4.02 (GraphPad Software Inc, San Diego). Significances of differences between Kaplan–Meier survival curves were attested using pairwise Logrank tests.
Results
Signalment
Of the 41 cats, 20 were female spayed and 21 were male neutered. Although most cats were Domestic Shorthairs (n=27), there were five Siamese, four Domestic Longhairs, two Burmese and one cat from each of the following breeds – Abyssinian, Persian and Maine Coon. Mean age was 10.5 years (±0.57 SEM) and the age range was 4–17 years. There were seven cats aged less than 7 years at diagnosis and the age range for those cats was 4–6 years. Only one of these seven cats was Siamese.
Clinical details
The WHO tumour stage guidelines were used for this study, and the criteria for each stage are explained in Table 1. Stage 1a was the most common tumour stage at first diagnosis (n=20), followed by stage 4 (n=10). Five of 19 cats with either stage 3 or 4 disease at presentation had a history of chronic vomiting. The cat with an intestinal MCT (stage 4) had no history of vomiting.
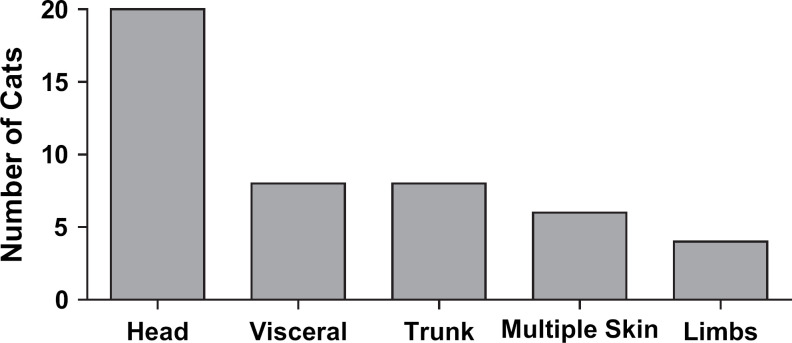
Tumour location is shown in Fig 1. The most common tumour location was the head (cutaneous), followed by the trunk (cutaneous) and then visceral (primary splenic, primary intestinal or primary lymph node). Of 30 cats with cutaneous/subcutaneous MCTs confined to the head and trunk, 27 of them had cutaneous tumours only, while in the remaining three cats the spleen (n=1) or distant lymph nodes (n=2; mesenteric lymph nodes) were also involved. Of the 41 cats in the study, 23 had one or two cutaneous tumours with no regional lymph node (RLN) or visceral involvement; four cats had three to five cutaneous tumours with no RLN or visceral involvement; in four cats there were more than five cutaneous tumours with or without RLN involvement; in two cats there were more than five cutaneous tumours with visceral involvement; five cats had the primary splenic form of the disease; two cats had MCTs involving lymph nodes but no cutaneous involvement (right prescapular lymph node and right submandibular lymph node); and one cat had an intestinal MCT.
Fig 1.
Tumour location at diagnosis. Of the 41 cats in the study, five had tumours in more than one location at the time of diagnosis. Cats with more than five cutaneous tumours at presentation were counted in the multiple skin group.
Twenty-five of the 41 cats had the full initial staging protocol (as described in Materials and methods) performed at the time of first tumour diagnosis. The remaining 16 cats were staged by collecting the following information – tumour size; tumour location; number of tumours; and the extent of any lymph node involvement was determined by local and distant lymph node palpation followed by biopsy if lymph node enlargement was detected. Results revealed mast cells in the buffy coat smear in two of the 25 cats. One of these two cats had presented with multiple (>5) cutaneous tumours and the other with mast cell neoplasia of the spleen and one jejunal lymph node.
Twenty-two cats in the study underwent excisional biopsy, and accompanying histopathology reported that 13 had clean margins and nine had dirty margins. The remaining 19 cats did not undergo excisional biopsy. Mast cell neoplasia was diagnosed in these 19 cats by either incisional biopsy (n=10), or by cytology (n=9). In all but four of the 19 cats, excisional biopsy was not attempted either because the mast cell neoplasia affected the liver, or because there were multiple (>5) cutaneous tumours.
There was no further diagnosis of mast cell neoplasia during the study period in seven of the nine cats with dirty margins and 11 of the 13 cats with clean margins. Of the two cats with dirty margins that had a further diagnosis of mast cell neoplasia during the study period, one had a local recurrence of the tumour, while the other developed multiple cutaneous tumours and a splenic tumour within 6 months of diagnosis of the original tumour. The two cats that had clean margins on the original excisional biopsy, but were diagnosed again with mast cell neoplasia during the study period, were originally diagnosed with solitary cutaneous tumours. One of these two cats developed a single cutaneous tumour at the same site at 205 days after the original excision, and the other cat developed single cutaneous tumours at different locations on the head (original tumour – left side of neck; second tumour – right preauricular area; third tumour – dorsum of nose) on two separate occasions approximately 8 months and 13 months after the original tumour was diagnosed.
Of the five Siamese cats included in the study, four underwent excisional biopsy and histopathology. In none of these was the histiocytic variant, previously reported in Siamese cats, identified on histopathology.
Survival
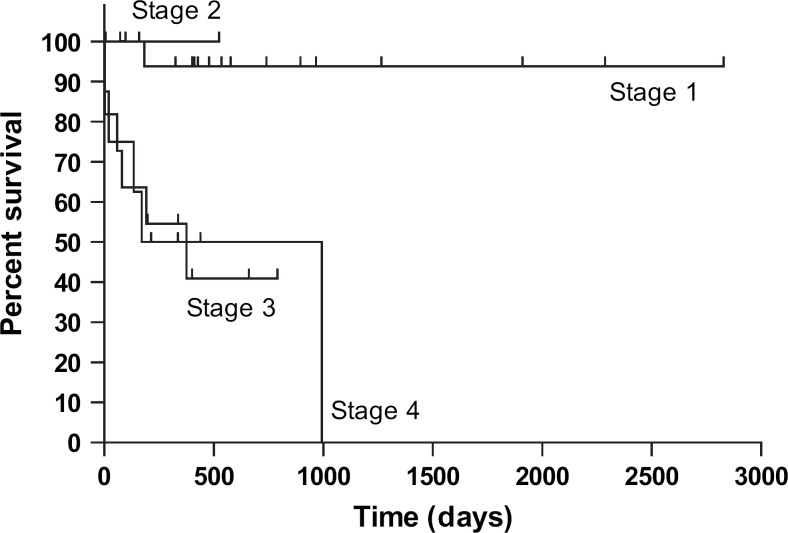
Figure 2 shows the Kaplan–Meier survival curves for the group by tumour stage. Cats with stage 1 disease at diagnosis survived longer than those with stage 3 or stage 4 disease at first diagnosis (stage 3: P<0.001; stage 4: P=0.016; Logrank test). There were no statistically significant differences between survival times when other combinations of stages at first diagnosis were compared. Tumour stage at first presentation is presented in Table 2, together with the number of cats that died because of mast cell disease in the study period, and survival times. Twelve of the 41 cats died of mast cell neoplasia during the study period. Median survival times were available from all cats first presented with stage 3 and stage 4 disease (stage 3: 582 days, n=8; stage 4: 283.5 days, n=10). As over half of cats with stage 1 (n=21) and stage 2 (n=2) disease at first diagnosis were still alive at the end of the study period, median survival time could not be calculated for these groups.
Fig 2.
Kaplan–Meier survival curves – tumour stage at diagnosis (n=41). Stage 1 vs stage 3 P<0.001; stage 1 vs stage 4 P=0.016; Logrank test.
Table 2.
Disease outcome by tumour stage at first presentation (n=41)
| Tumour stage | Number of cats | Number of cats dead due to mast cell disease in the study period | Survival times* |
|---|---|---|---|
| 1a | 20 | 1 | 183 |
| 2a | 2 | 0 | 59 |
| 3a | 6 | 2 | 3, 21 |
| 3b | 3 | 3 | 134, 170, 994 |
| 4 | 10 | 6 | 1, 3, 59, 80, 192, 375 |
Survival time=days between tumour diagnosis and death due to mast cell neoplasia, in the study period.
The range of survival times in cats that died of mast cell neoplasia varied widely when grouped by stage at first diagnosis (stage 1: 183 days, n=1; stage 2: n=0; stage 3: 3–994 days, n=5; stage 4: 1–375 days, n=6).
When cats were divided according to whether only cutaneous tumours (n=30) or visceral/lymph node neoplasia was present (n=11), there was a statistically significant difference in survival (P=0.031). Median survival times were not available for the two groups as more than half of each group were still alive at the end of the study period, but the ranges of survival times in cats that died of mast cell neoplasia from each group were 3–994 days for the cutaneous group (n=6) and 1–80 days for the visceral/lymph node group (n=5).
When a subgroup of cats with only cutaneous tumours (no lymph node or visceral involvement; n=25) were divided according to whether there were multiple (five or more) tumours (n=6) or a single tumour (n=19 – one stage 1a cat was lost to follow-up and unable to be included in the survival analysis), cats with single tumours survived longer than those with multiple tumours (P=0.001). Median survival times were not available for the single tumour group as more than half of those cats were still alive, but median survival time for the multiple tumour group was 375 days. One of the 19 cats with single cutaneous tumours went on to die of mast cell neoplasia during the study period (at 183 days after initial diagnosis), while four of the six cats with multiple tumours succumbed to the disease in the same period (range 3–994 days).
A range of chemotherapeutic agents was used to treat the cats in this study. Of the 22 cats for which a full drug history was available, 12 received chemotherapy for mast cell neoplasia. Of those 12 cats, six cats received prednisolone alone; one cat received prednisolone in combination with cyclophosphamide; three cats received vinblastine in combination with either prednisolone and deionised water (n=1), prednisolone and cyclophosphamide (n=1), or cyclophosphamide alone (n=1); and the remaining two cats received 1-(2-chloroethyl)-3-cyclohexyl-l-nitrosourea (CCNU), one in combination with prednisolone and the other with dexamethasone. For survival analysis, the 22 cats were stratified according to whether or not they underwent excisional biopsy (with clean margins) and whether or not they received chemotherapy, with resultant combinations creating four groups. There was not a statistically significant difference in survival between any pair combinations of the four groups.
Discussion
To the authors' knowledge, this is the largest published study reporting the full spectrum of feline mast cell neoplasia. As the non-cutaneous forms of the disease in cats are relatively uncommon, analytical studies of this kind are difficult to perform – the low case numbers reduce statistical power. While cutaneous MCTs are seen occasionally in feline practice, the primary splenic form of the disease is much less frequent. This study confirms the findings of other reports in that feline mast cell neoplasia was found mainly in older cats, with no sex predisposition, and the head and trunk were the most common anatomical sites for cutaneous tumours (Thamm and Vail 2001). In contrast to some other reports, Siamese did not appear to be overrepresented in this study, and a notable proportion of cats (three of 41) had simultaneous cutaneous and either splenic or lymph node disease (Scott 1980, Elmslie and Ogilvie 1994). However, to draw accurate conclusions about breed representation, knowledge of the proportions of different breeds presented at each of the different sites is required, which was outside the scope of this study.
Just under 20% of the tumours in this report were of the primary splenic form, which agrees with previous studies reporting the relatively high incidence of this form of mast cell disease in cats when compared to dogs (Carpenter et al 1987). While accompanying clinical signs with more advanced disease were generally non-specific, such as anorexia and weight loss, about 25% of cats with stage 3 or 4 disease at presentation had a history of chronic vomiting. This is presumably caused by the release of histamine from the tumour, resulting in hypergastrinaemia and vomiting (Liska et al 1979). When survival of the cats with only cutaneous MCTs was compared with those with visceral or lymph node tumours on initial presentation, those with cutaneous tumours survived longer (P=0.031), confirming the clinical logic that a more favourable prognosis should be given to tumours confined to the dermis.
While staging of MCTs in cats and dogs is similar, most cutaneous feline disease is behaviourally benign, and complete surgical excision without adjuvant chemotherapy is indicated in most cases (Thamm and Vail 2001). During the study period in this report, there was a recurrence of mast cell neoplasia in about a third of cats with complete excisional biopsies and about the same proportion of cats with incomplete excisional biopsies. This would appear to support previous work which reports that incomplete excision was not associated with a higher rate of tumour recurrence (Molander-McCrary et al 1998). Only two of the 22 cats with excisional biopsies had a recurrence of mast cell disease in a more aggressive (higher stage) form and the excision of the original tumour was incomplete in both cats. A variety of chemotherapeutic agents, including prednisolone, dexamethasone, vinblastine, CCNU, cyclophosphamide and deionised water were used to treat the cats in this study. For meaningful comparisons of survival to be made, it was essential that cats were stratified on both excisional biopsy status and chemotherapy status. When combinations of cats with or without full excisional biopsy of their tumours and with or without chemotherapy were compared, survival analysis did not reveal that any of the four groups survived longer than the others. If the cohort of cats studied was larger, perhaps a combination of chemotherapy and excisional biopsy may have shown more promise, and in that case would have warranted recommendation in a clinical setting. However, as mentioned earlier, reduced statistical power is one of the challenges provided by the study of a relatively rare disease. It could also be argued that as most cats with stage 1, 2 or 3a disease had not died by the end of the study, adjuvant chemotherapy is not indicated in these cases, as surgery alone is enough to ensure a long survival time.
Mast cell neoplasia was the cause of death in at least some cats in all tumour stages except stage 2, and there were shorter survival times in cats with more advanced stage of disease at initial presentation. Survival times reported in this study varied widely when grouped by stage at diagnosis. Examination of survival ranges by stage at initial diagnosis yielded some interesting information. As expected, more advanced disease was associated with shorter minimum survival times, but very long survival times were also seen in some cats with relatively advanced disease at initial presentation. When subjected to statistical comparison, cats with stage 1 disease at presentation survived longer than any other stage. Perhaps if larger numbers of cats were available, increased statistical power may have enabled the demonstration of a statistically significant difference in survival between other combinations of stages at presentation. When survival of a subgroup of cats with cutaneous MCTs was analysed by dividing them into cats with single tumours and those with multiple (five or more) tumours on initial presentation, those with single tumours survived longer (P=0.001), although the range of survival times until death from mast cell neoplasia was very wide for the multiple tumour group. This result has clinical significance with regard to the formulation of a prognosis in cats with cutaneous mast cell neoplasia.
In conclusion, solitary cutaneous feline MCTs without spread to the RLNs usually manifest as benign disease with a relatively protracted course. However, multiple cutaneous tumours, recurrent tumours and primary splenic or lymph node disease should receive a guarded prognosis due to the relatively short median survival times associated with these forms of the disease.
Acknowledgements
The authors would like to thank the following veterinarians for the provision of clinical and histopathological information from cats with mast cell neoplasia – Dr Mary Atkinson, Dr Vanessa Barrs, Dr Melissa Carlisle, Dr Elizabeth Dill-Macky, Dr Anthony Gough, Dr Linda Hayes, Dr Veronika Langova, Dr Richard Ploeg, Dr Teena Price, Dr Ian Russ, Dr Rod Straw, Dr Doug Thamm, Dr Shirley Turner and Dr Hugh Wackett.
References
- Barker I., Van Dreumel A. The alimentary system. Jubb K.V.F., Kennedy P.C., Palmer N. 3rd edn, Pathology of Domestic Animals Vol. 2, 1985, Academic Press: Orlando, 1–237. [Google Scholar]
- Beadleston D., Roosje P., Goldschmidt M. Chymase and tryptase staining of normal feline skin and of feline cutaneous mast cell tumours, Veterinary Allergy and Clinical Immunology 5, 1997, 54–58. [Google Scholar]
- Carpenter J., Andrews L., Holzworth J. Tumours and tumour-like lesions. Holzworth J. Diseases of the Cat Vol. 1, 1987, WB Saunders: Philadelphia, 406–596. [Google Scholar]
- Elmslie R., Ogilvie G. Variables in behaviour and management of mast cell tumours. August J. Consultations in Feline Internal Medicine, 1994, WB Saunders: Philadelphia, 567–572. [Google Scholar]
- Johnson T.O., Schulman F.Y., Lipscomb T.P., Yantis L.D. Histopathology and biologic behavior of pleomorphic cutaneous mast cell tumors in fifteen cats, Veterinary Pathology 39, 2002, 452–457. [DOI] [PubMed] [Google Scholar]
- Lepri E., Ricci G., Leonardi L., Sforna M., Mechelli L. Diagnostic and prognostic features of feline cutaneous mast cell tumours: a retrospective analysis of 40 cases, Veterinary Research Communications 27 (Suppl. 1), 2003, 707–709. [DOI] [PubMed] [Google Scholar]
- Liska W., MacEwan E., Zaki F., Garvey M. Feline systemic mastocytosis: a review and results of splenectomy in seven cases, Journal of the American Animal Hospital Association 15, 1979, 589–597. [Google Scholar]
- Molander-McCrary H., Henry C., Potter K., Tyler J., Buss M. Cutaneous mast cell tumours in cats: 32 cases (1991–1994), Journal of the American Animal Hospital Association 34, 1998, 281–284. [DOI] [PubMed] [Google Scholar]
- Scott D. Feline dermatology 1900–1978: a monograph, Journal of the American Animal Hospital Association 16, 1980, 421–423. [Google Scholar]
- Thamm D., Vail D. Mast cell tumours. Withrow S., McEwen E.G. Small Animal Clinical Oncology, 2001, WB Saunders Co.: Philadelphia, 261–282. [Google Scholar]
- Wilcock B., Yager J., Zinc M. The morphology and behaviour of feline cutaneous mastocytomas, Veterinary Pathology 23, 1986, 320–324. [DOI] [PubMed] [Google Scholar]