Abstract
Nosebleed or epistaxis is one of the most common forms of presenting an emergency in the ENT field. Since March 11, 2020, the World Health Organisation has proclaimed COVID-19 a global pandemic, and the world has been closed down. The main objective of the study is to analyse and compare the dynamics of epistaxis aetiology among the cases that required hospitalisation in the pre-pandemic period and the period of the COVID 19 pandemic. The study is multicenter retrospective from October 2018 to May 2022, including 380 cases of hospitalised epistaxis, with the mention that March 2020 is considered the beginning of the pandemic period. 60.8% of the patients enrolled in the study in the pre-pandemic period (60.8%) and 39.2% in the pandemic period. Differences between groups were not statistically significant between study entries (pre-pandemic vs. pandemic) and age (p=0.331), gender (p=0.916) or existence of local causes for epistaxis (p=0.895). Patients with general causes for epistaxis were more frequently enrolled in the pandemic period, while patients without general causes for epistaxis were more frequently enrolled in the pre-pandemic period. Patients with a hospitalisation period of more than 5 days were more frequently enrolled in the pre-pandemic period while patients with a hospitalisation period of 3 to 5 days were more frequently enrolled in the pandemic period. Also, patients with idiopathic epistaxis were more frequently enrolled in the pre-pandemic period. Based on the results presented in our study, the period of the Covid 19 pandemic directly influenced both the number of patients and the period of hospitalisation.
Keywords: Epistaxis , nosebleed , hospitalization
Introduction
One of the most common types of presenting an emergency in the ENT profession is nosebleed or epistaxis.
Most of the cases are not life-threatening and are easy to solve.
Most of the time, the identification of the aetiology overlaps with the measures to stop the bleeding, or even follows it.
The aetiology of nosebleeds can be represented by local causes, general causes or can be idiopathic.
The most common local causes are represented by: trauma, anatomical deformations, inflammatory reactions, intranasal tumours or environmental factors [1].
General causes of epistaxis include hypertension, coagulation disorders, inherited haemorrhagic diatheses and vascular or cardiovascular diseases [2, 3].
Idiopathic epistaxis is a diagnosis of exclusion of all possible aetiologies previously presented [4].
In January 2020, a scientist discovered a pneumonia-like disease with interhuman transmission in Wuhan, China, and the region was placed under lockdown two days later.
Meanwhile, in the United States, the first case in point of a man returning to Washington from Wuhan surfaces, prompting the declaration of a public health emergency [5].
On February 26, the first case of COVID-19 was declared in Romania.
Since March 11, 2020, the World Health Organisation has proclaimed COVID-19 a global pandemic, and the world has been closed down.
COVID-19 is the disease caused by the SARS-CoV 2 virus which certainly represented one of the biggest global challenges in recent human history [6].
The Covid 19 pandemic produced important changes in all aspects of society, particularly and directly in medical practice.
Restrictive measures were imposed, and additional and specific medical practice measures were implemented, especially in surgical specialties.
Because ENT specialists were in a high-risk group due to performing aerosol-generating operations, only medical-surgical emergencies were provided for a long time.
Ever since the beginning of the pandemic, Real Time PCR testing has evolved rapidly and was the gold standard even after the start of the vaccination campaign.
Vaccination against COVID-19 in the European Union started on December 27 and by August 31, 2021, the goal of fully vaccinating 70% of the adult population was reached
In the EU, 7 vaccines are authorized by the European Medicines Agency (types of vaccine: mRNA, viral vector, based on viral proteins and vaccine with inactivated virus) [6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17].
The immediate availability of the anti-COVID-19 vaccination in Romania made possible a fairly quick return to current medical practice and the relaxation of measures.
The aim of our study was to evaluate the aetiological aspects of one of the important emergencies in the field of ENT, that of epistaxis that requires hospitalisation, comparing the pre-pandemic and the pandemic period.
Materials and Methods
From October 2018 to May 2022, 380 cases of hospitalised epistaxis were studied in the ENT Clinic of the Craiova County Emergency Hospital and the Institute of Speech-Language and Functional ENT Surgery "Prof. Dr. D. Hociotă" Bucharest, with the mention that March 2020 is considered the start of the pandemic period.
The main objective of the study is to analyse and compare the dynamics of epistaxis aetiology among the cases that required hospitalisation in the pre-pandemic period and the period of the COVID 19 pandemic.
The data was collected from the observation sheets of hospitalised patients and from the electronic database of the Hippocrates medical program: the number of hospitalisations, the gender and age of the patients, the cause of epistaxis and the period of hospitalisation.
All patients in the batch during the pandemic period were tested for SARS-CoV-19 infection at admission, through the Real Time PCR COVID test, then only the rapid COVID-19 test was used.
Where applicable, the steps in place for diagnosis and isolation were followed, as were the measures for medical and auxiliary employees to restrict the degree of infection transmission.
This study has the approval of the ethics committee of the University of Medicine and Pharmacy of Craiova and the patients included in the batch have given their written consent to the use of medical data for educational and scientific purposes.
Data Analysis
All the data from the study was analysed using IBM SPSS Statistics 25 and illustrated using Microsoft Office Excel/Word 2016.
Qualitative variables were written as counts or percentages and were tested between groups using Fisher’s Exact Test.
Results
Data from Table 1 shows the descriptive characteristics of the entire study group patients
Table 1.
Descriptive characteristics of the studied patients
|
Parameter (Nr., %) |
Value |
|
Study entry |
|
|
Pre-pandemic |
231 (60.8%) |
|
Pandemic |
149 (39.2%) |
|
Age |
|
|
Young adults |
97 (25.5%) |
|
Middle aged |
125 (32.9%) |
|
Old adults |
158 (41.6%) |
|
Gender |
|
|
Female |
173 (45.5%) |
|
Male |
207 (54.5%) |
|
Epistaxis-Local cause |
73 (19.2%) |
|
Epistaxis-General cause |
233 (61.3%) |
|
Idiopathic epistaxis |
91 (23.9%) |
|
Hospitalisation period |
|
|
3-5 days |
274 (72.1%) |
|
>5 days |
106 (27.9%) |
60.8% of the patients enrolled in the study in the pre-pandemic period (60.8%) and 39.2% in the pandemic period. 25.5% of the patients were young adults, 32.9% were middle-aged and 41.6% were old adults. 45.5% of the patients were women, 54.5% were men. 19.2% of the patients had local causes for epistaxis (mostly after surgery or because of neoplasia), 61.3% of the patients had general causes for epistaxis (mostly because of arterial hypertension), 23.9% of the patients had idiopathic epistaxis. 72.1% of the patients were hospitalised for about 3 to 5 days and 27.9% for more than 5 days.
During the pandemic period of the study, 3 patients with known COVID-19 infection and 3 others with post-COVID-19 status were hospitalised, all 6 patients being hypertensive and on anticoagulant treatment.
Data from Table 2 shows the distribution of the patients according to characteristics and study entry.
Table 2.
Distribution of the patients according to characteristics and study entry
|
Study entry / Age |
Pre-pandemic |
Pandemic |
p* |
|
Young adults |
65 (28.1%) |
32 (21.5%) |
0.331 |
|
Middle aged |
72 (31.2%) |
53 (35.6%) |
|
|
Old adults |
94 (40.7%) |
64 (43%) |
|
|
Study entry / Gender |
Pre-pandemic |
Pandemic |
p* |
|
Female |
106 (45.9%) |
67 (45%) |
0.916 |
|
Male |
125 (54.1%) |
82 (55%) |
|
|
Study entry / Local cause - Epistaxis |
Pre-pandemic |
Pandemic |
p* |
|
Absent |
186 (80.5%) |
121 (81.2%) |
0.895 |
|
Present |
45 (19.5%) |
28 (18.8%) |
|
|
Study entry / General cause - Epistaxis |
Pre-pandemic |
Pandemic |
p* |
|
Absent |
102 (44.2%) |
45 (30.2%) |
0.007 |
|
Present |
129 (55.8%) |
104 (69.8%) |
|
|
Study entry / Idiopathic - Epistaxis |
Pre-pandemic |
Pandemic |
p* |
|
Absent |
165 (71.4%) |
124 (83.2%) |
0.010 |
|
Present |
66 (28.6%) |
25 (16.8%) |
|
|
Study entry / Hospitalisation period |
Pre-pandemic |
Pandemic |
p* |
|
3-5 days |
181 (78.4%) |
93 (62.4%) |
0.001 |
|
>5 days |
50 (21.6%) |
56 (37.6%) |
Fisher’s Exact Test
According to the Fisher’s Exact Tests, differences between groups were not statistically significant between study entries (pre-pandemic vs. pandemic) and age (p=0.331), gender (p=0.916) or existence of local causes for epistaxis (p=0.895).
Patients with general causes for epistaxis were more frequently enrolled in the pandemic period (69.8% vs. 55.8%), while patients without general causes for epistaxis were more frequently enrolled in the pre-pandemic period (44.2% vs. 30.2%) (p=0.007) and patients with a hospitalisation period of more than 5 days were more frequently enrolled in the pre-pandemic period (78.4% vs. 62.4%) while patients with a hospitalisation period of 3 to 5 days were more frequently enrolled in the pandemic period (37.6% vs. 21.6%) (p=0.001).
According to causes of epistaxis and age categories Figure 1, middle aged adults were not significantly associated with any type of epistaxis (general/local/idiopathic) while old adults were more associated with general causes of epistaxis (125 cases (+)-53.6% vs. 33 cases (-)-22.4%) while young adults were less associated with general causes of epistaxis (28 cases (+)-12% vs. 69 cases (-)-46.9%) (p<0.001).
Figure 1.

Distribution of the patients according to age category and causes of epistaxis
The situation for local causes of epistaxis was opposite, young adults being more associated with local causes of epistaxis (33 cases (+)-45.2% vs. 64 cases (-)-20.8%) while old adults were less associated with local causes of epistaxis (16 cases (+)-21.9% vs. 142 cases (-)-46.3%) (p<0.001).
For the idiopathic cause of epistaxis, the situation is similar, young adults being more associated with idiopathic epistaxis (38 cases (+)-41.8% vs. 59 cases (-)-20.4%) while old adults being less associated with idiopathic epistaxis (23 cases (+)-25.3% vs. 135 cases (-)-46.7%) (p<0.001).
According to local causes of epistaxis and study entry Figure 2, none of the local causes of epistaxis were significantly more observed in the pandemic or pre-pandemic period (neoplastic-p=0.271, postoperative-p=0.428, traumatic-p=0.709 or inflammatory-p=0.385).
Figure 2.

Distribution of the patients according to study entry and local causes of epistaxis
According to general causes of epistaxis and study entry Figure 3, epistaxis cases determined by arterial hypertension (p=0.250), antiplatelet therapy (p=0.641), hepatic/renal causes (p=0.848) or endocrine causes (p=0.392) were not significantly more observed in the pandemic or pre-pandemic period.
Figure 3.

Distribution of the patients according to study entry and general causes of epistaxis
However, in the pandemic period there were significantly more cases of epistaxis determined by atrial fibrillation (26 cases-17.4% vs. 18 cases-7.8%, p=0.005), oral anticoagulation (28 cases-18.8% vs. 9 cases-3.8%, p<0.001) and haematological causes (32 cases-21.5% vs. 13 cases-5.6%, p<0.001).
Also, patients with idiopathic epistaxis were more frequently enrolled in the pre-pandemic period (28.6% vs. 16.8%) (p=0.010) (Figure 4).
Figure 4.

Distribution of the patients according to study entry and existence of idiopathic epistaxis
When analysing patients enrolled in the pre-pandemic period according to causes of epistaxis and hospitalisation period Figure 5, none of the causes of epistaxis were significantly more associated with a higher period of hospitalisation (local causes-p=0.106, general causes-p=0.421 or idiopathic-p=0.726).
Figure 5.

Distribution of the patients observed in the pre-pandemic period according to hospitalisation period and causes of epistaxis
When analysing patients enrolled in the pandemic period according to causes of epistaxis and hospitalisation period Figure 6, none of the causes of epistaxis were significantly more associated with a higher period of hospitalisation (local causes-p=1.000, general causes-p=0.197 or idiopathic-p=0.174).
Figure 6.
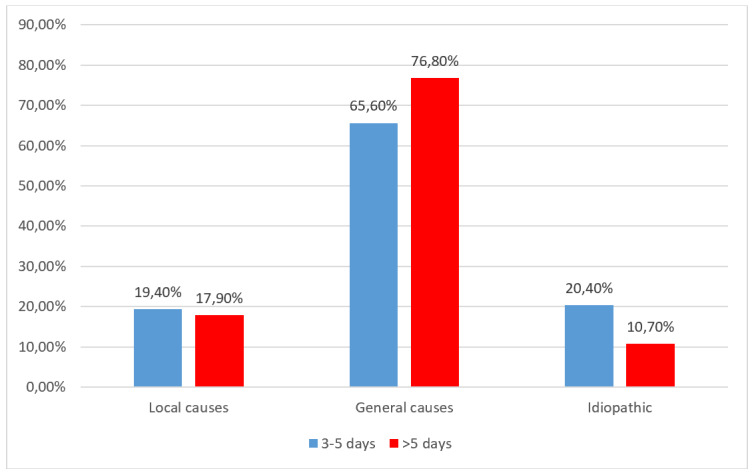
Distribution of the patients observed in the pandemic period according to hospitalisation period and causes of epistaxis
Discussions
The COVID-19 pandemic has greatly complicated the practice in the ENT specialty, because it is of particular interest to the upper airways, requiring additional protective measures.
With the increase in the number of cases, the hospitals become overwhelmed.
The overloading of the medical system and the personal protective equipment (PPE) crisis, led to a reorganisation of medical systems all over the world [18, 19, 20, 21, 22].
Between groups (pre-pandemic vs. pandemic) and age, gender, or existence of local causes for epistaxis, we observed no differences statistically significant.
Epistaxis was found to be more common in elderly adults above 65 years of age, both in pre-pandemic (94, 40.7%) and pandemic period (64, 43%) without having statistic signification, which is similar to the results of Pallin et al. [4].
Our obtained data coincided with that published regarding gender distribution, with most writers reporting a male prevalence of nosebleed [23, 24, 25].
We found that patients with general causes for epistaxis were more frequently enrolled in the pandemic period, while patients without general causes for epistaxis were more frequently enrolled in the pre-pandemic period.
According to causes of epistaxis and age categories, our data shows that middle aged adults were not significantly associated with any type of epistaxis (general/local/idiopathic) while old adults were more associated with general causes of epistaxis, while young adults were less associated with general causes of epistaxis.
The situation for local causes of epistaxis was opposite, young adults being more associated with local causes of epistaxis, while old adults were less associated with local causes of epistaxis.
For the idiopathic cause of epistaxis, the situation is similar, young adults being more associated with idiopathic epistaxis while old adults being less associated with idiopathic epistaxis.
There is a paucity of data regarding this within the literature, and further studies needed.
Our study did not lead to statistically relevant differences between pandemic or pre-pandemic period regarding the local aetiology of the specific cause (neoplastic, postoperative, traumatic, or inflammatory).
Tumour and post-surgical pathology as local causes of epistaxis diverge from the literature and are overrepresented in this study group.
Among the general causes of epistaxis identified in the studied group, there are listed those determined by arterial hypertension, antiplatelet therapy, hepatic/renal causes, or endocrine causes were not significantly more observed in the pandemic or pre-pandemic period.
However, in the pandemic period there were significantly more cases of epistaxis determined by atrial fibrillation, oral anticoagulation, and haematological causes.
The prevalence of hypertension in Romania is 46% according to the national survey SEPHAR IV (Study for Evaluation of Prevalence of Hypertension and Cardiovascular Risk in an Adult Population in Romania) [26].
The most common general cause of epistaxis in our study, not influenced by the pandemic, is hypertension.
Old adults have comorbidities such as arterial hypertension, vascular or cardiovascular diseases and are also the ones who most frequently follow anticoagulant or antiplatelet treatment.
Over time, many researchers have tried to prove the influence of arterial hypertension as the aetiology of epistaxis, but there is not enough evidence to support this fact, most being case series, without a control group.
The lack of documentation of blood pressure values before epistaxis as well as the inability to demonstrate causality or effect or coincidence in patients with epistaxis is an important variable [27, 28].
Even the studies that investigated a series of cases using a control group failed to define the association between epistaxis and hypertension, the nosebleed not being triggered by hypertension, but still managed to demonstrate the difficulty in controlling hypertensive epistaxis [29].
The specialised literature reports a prevalence of arterial hypertension associated with epistaxis between 24% and 64% [30, 31, 32, 33, 34, 35].
The relationship between oral anticoagulants and epistaxis is poorly documented in the international literature.
The prescription of oral anticoagulants is increasing [36].
The results of the study regarding the ingestion of anticoagulant or antiplatelet drugs (15%) are inferior compared to the studies by Kumar S and Saraceni Neto P, who report that between 24% and 33% of patients hospitalised with epistaxis are under the administration of anticoagulant drugs and/or antiplatelet agents. [37, 38].
The cause of epistaxis is not always identifiable.
The percentage of idiopathic epistaxis is higher (23.90%) in this study compared to the data reported by Qureishi in 2012, but lower compared to the results of Iseh's study (29.2%) in 2008 [39].
Patients with idiopathic epistaxis were more frequently enrolled in the pre-pandemic period, result compared to the study by Devabalan et al. [40].
Patients who were hospitalised for more than 5 days were more likely to be registered in the pre-pandemic era, whereas patients who were hospitalised for 3 to 5 days were more likely to be enrolled in the pandemic period.
Conclusions
The Covid 19 pandemic period directly influenced the number of hospitalisations of patients with nosebleeds as well as the length of hospitalisation in both ENT departments from Craiova and Bucharest.
Based on the results presented in our study, the most common general cause of epistaxis, not influenced by the pandemic, is hypertension.
Also, there were significantly more cases of epistaxis determined by atrial fibrillation, oral anticoagulation, and haematological causes in the pandemic period.
Our study did not lead to statistically relevant differences between pandemic or pre-pandemic period regarding the local aetiology of the specific cause.
Conflict of interests
None to declare.
References
- 1.Purkey MR, Seeskin Z, Chandra R. Seasonal variation and predictors of epistaxis. Laryngoscope. 2014;124(9):2028–2033. doi: 10.1002/lary.24679. [DOI] [PubMed] [Google Scholar]
- 2.INTEGRATE (UK, Stevens K, Smith ME, Williams RJ, Ellis M, Hardman JC, Hopkins C. National prospective observational study of inpatient management of adults with epistaxis-a National Trainee Research Collaborative delivered investigation. Rhinology. 2019;57(3):180–189. doi: 10.4193/Rhin18.239. [DOI] [PubMed] [Google Scholar]
- 3.Clark M, Berry P, Martin S, Harris N, Sprecher D, Olitsky S, Hoag JB. Nosebleeds in hereditary hemorrhagic telangiectasia: Development of a patient-completed daily eDiary. Laryngoscope Investig Otolaryngol. 2018;3(6):439–445. doi: 10.1002/lio2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pallin D. J., Chng Y. M., McKay M. P., Emond J. A., Pelletier A. J., Camargo C. A., Jr. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Annals of Emergency Medicine. 2005;46(1):77–81. doi: 10.1016/j.annemergmed.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Assaf AM, Haddadin RN, Akour AA. COVID-19: Between Past and Present. Viral Immunol. 2021;34(3):145–157. doi: 10.1089/vim.2020.0102. [DOI] [PubMed] [Google Scholar]
- 6.James JJ. COVID-19: From Epidemic to Pandemic. Disaster Med Public Health Prep. 2020;14(6):e3–e5. doi: 10.1017/dmp.2020.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yüce M, Filiztekin E, Özkaya KG. COVID-19 diagnosis-A review of current methods. Biosens Bioelectron. 2021;172:112752–112752. doi: 10.1016/j.bios.2020.112752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortner A, Schumacher D. First COVID-19 Vaccines Receiving the US FDA and EMA Emergency Use Authorization. Discoveries (Craiova) 2021;9(1):e122–e122. doi: 10.15190/d.2021.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mojsoska B, Larsen S, Olsen DA, Madsen JS, Brandslund I, Alatraktchi FA. Rapid SARS-CoV-2 Detection Using Electrochemical Immunosensor. Sensors (Basel) 2021;21(2):390–390. doi: 10.3390/s21020390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eichler HG, Cavaleri M, Enzmann H, Scotti F, Sepodes B, Sweeney F, Vamvakas S, Rasi G. Clinical Trials for COVID-19: Can we Better Use the Short Window of Opportunity. Clin Pharmacol Ther. 2020;108(4):730–733. doi: 10.1002/cpt.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baden LR, El Sahly, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ElBagoury M, Tolba MM, Nasser HA, Jabbar A, Elagouz AM, Aktham Y, Hutchinson A. The find of COVID-19 vaccine: Challenges and opportunities. J Infect Public Health. 2021;14(3):389–416. doi: 10.1016/j.jiph.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durand J, Dogné JM, Cohet C, Browne K, Gordillo-Marañón M, Piccolo L, Zaccaria C, Genov G. Safety Monitoring of COVID-19 Vaccines: Perspective from the European Medicines Agency. Clin Pharmacol Ther. 2023;113(6):1223–1234. doi: 10.1002/cpt.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavaleri M, Enzmann H, Straus S, Cooke E. The European Medicines Agency's EU conditional marketing authorisations for COVID-19 vaccines. Lancet. 2021;397(10272):355–357. doi: 10.1016/S0140-6736(21)00085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellino S. COVID-19 vaccines approved in the European Union: current evidence and perspectives. Expert Rev Vaccines. 2021;20(10):1195–1199. doi: 10.1080/14760584.2021.1962304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotez PJ, Nuzhath T, Callaghan T, Colwell B. COVID-19 vaccine decisions: considering the choices and opportunities. Microbes Infect. 2021;23(4-5):104811–104811. doi: 10.1016/j.micinf.2021.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Aguanno V, Ralli M, Greco A, de Vincentiis. Clinical Recommendations for Epistaxis Management During the COVID-19 Pandemic. Otolaryngol Head Neck Surg. 2020;163(1):75–77. doi: 10.1177/0194599820926497. [DOI] [PubMed] [Google Scholar]
- 19.Cui C, Yao Q, Zhang D, Zhao Y, Zhang K, Nisenbaum E, Cao P, Zhao K, Huang X, Leng D, Liu C, Li N, Luo Y, Chen B, Casiano R, Weed D, Sargi Z, Telischi F, Lu H, Denneny JC, Shu Y, Liu X. Approaching Otolaryngology Patients During the COVID-19 Pandemic. Otolaryngol Head Neck Surg. 2020;163(1):121–131. doi: 10.1177/0194599820926144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sampathkumar P, Beam E, Breeher LE, O'Horo JC. Precautions, Utilization of Personal Protective Equipment, and Conservation Strategies During the COVID-19 Pandemic. Mayo Clin Proc. 2020;95(9S):S11–S13. doi: 10.1016/j.mayocp.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chhibber A, Kharat A, Kneale D, Welch V, Bangpan M, Chaiyakunapruk N. Assessment of health equity consideration in masking/PPE policies to contain COVID-19 using PROGRESS-plus framework: a systematic review. BMC Public Health. 2021;16;21(1):1682–1682. doi: 10.1186/s12889-021-11688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dharmarajan H, Belsky MA, Anderson JL, Sridharan S. Otolaryngology Consult Protocols in the Setting of COVID-19: The University of Pittsburgh Approach. Ann Otol Rhinol Laryngol. 2022;131(1):12–26. doi: 10.1177/00034894211005937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eziyi JAE, Akinpelu OV, Amusa YB, Eziyi AK. Epistaxis in Nigerians: A 3-year Experience. East Cent Afr J Surg. 2009;14(2):93–98. [Google Scholar]
- 24.Mgbor NC. Epistaxis in Enugu: A 9 year Review. Nig J of otolaryngology. 2004;1(2):11–14. [Google Scholar]
- 25.Kaygusuz I, Karlidag T, Keles E, Yalcin S, Alpay HC, Sakallioglu O. Retrospective Analysis of 68 Hospitalized Patients with Epistaxis. Firat Tip Dergisi. 2004;9(3):82–85. [Google Scholar]
- 26.Dorobantu M, Cojocaru C, Stanciulescu L, Pop C, Bala C, Benedek T, Parepa I, Lighezan D, Darabont R, Darabantiu D, Mitu F, Gheorghita V, Scafa-Udriste A, Gheorghe-Fronea OF. Ups and downs of conducting a national representative survey on hypertension during pandemic time: main results of SEPHAR IV. J Hypertens. 2023;41(8):1271–1280. doi: 10.1097/HJH.0000000000003461. [DOI] [PubMed] [Google Scholar]
- 27.Knopfholz J, Lima-Junior E, Précoma-Neto D, Faria-Neto JR. Association between epistaxis and hypertension: a one year follow-up after an index episode of nose bleeding in hypertensive patients. Int J Cardiol. 2009;134(3):e107–9. doi: 10.1016/j.ijcard.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Lubianca Net, Fuchs FD, Facco SR, Gus M, Fasolo L, Mafessoni R, Gleissner AL. Is epistaxis evidence of end-organ damage in patients with hypertension. Laryngoscope. 1999;109(7 Pt 1):1111–5. doi: 10.1097/00005537-199907000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Sarhan NA, Algamal AM. Relationship between epistaxis and hypertension: A cause and effect or coincidence. J Saudi Heart Assoc. 2015;27(2):79–84. doi: 10.1016/j.jsha.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herkner H. Havel C., Müllner M., Gamper G., Bur A., Temmel A.F. Active epistaxis at ED presentation is associated with arterial hypertension. Am J Emerg Med. 2002;20(2):92–95. doi: 10.1053/ajem.2002.31577. [DOI] [PubMed] [Google Scholar]
- 31.Theodosis P. Mouktaroudi M., Papadogiannis D., Ladas S., Papaspyrou S. Epistaxis of patients admitted in the emergency department is not indicative of underlying arterial hypertension. Rhinology. 2009;47(3):260–263. doi: 10.4193/Rhin08.157. [DOI] [PubMed] [Google Scholar]
- 32.Nash C. M., Field S.M.B. Epidemiology of epistaxis in a canadian emergency department. Israeli J Emerg Med. 2008;8(3):24–28. [Google Scholar]
- 33.Page C. Biet A., Liabeuf S., Strunski V., Fournier A. Serious spontaneous epistaxis and hypertension in hospitalized patients. Eur Arch Otorhinolaryngol. 2011;268(12):1749–1753. doi: 10.1007/s00405-011-1659-y. [DOI] [PubMed] [Google Scholar]
- 34.Viducich R. A., Blanda M.P., Gerson L.W. Posterior epistaxis: clinical features and acute complications. Ann Emerg Med. 1995;25(5):592–596. doi: 10.1016/s0196-0644(95)70169-9. [DOI] [PubMed] [Google Scholar]
- 35.Pollice P. A., Yoder M.G. Epistaxis: a retrospective review of hospitalized patients. Otolaryngol Head Neck Surg. 1997;117(1):49–53. doi: 10.1016/S0194-59989770205-5. [DOI] [PubMed] [Google Scholar]
- 36.Desai NR, Krumme AA, Schneeweiss S, Shrank WH, Brill G, Pezalla EJ, Spettell CM, Brennan TA, Matlin OS, Avorn J, Choudhry NK. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation-quality and cost implications. Am J Med. 2014;127(11):1075–1082. doi: 10.1016/j.amjmed.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 37.Kumar S, Shetty A, Rockey J, Nilssen E. Contemporary surgical treatment of epistaxis. What is the evidence for sphenopalatine artery ligation. Clin Otolaryngol Allied Sci. 2003;28(4):360–3. doi: 10.1046/j.1365-2273.2003.00724.x. [DOI] [PubMed] [Google Scholar]
- 38.Saraceni Neto, Nunes LM, Gregório LC, Santos Rde, Kosugi EM. Surgical treatment of severe epistaxis: an eleven-year experience. Braz J Otorhinolaryngol. 2013;79(1):59–64. doi: 10.5935/1808-8694.20130011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iseh KR, Muhammad Z. Pattern of epistaxis in Sokoto, Nigeria: a review of 72 cases. Ann Afr Med. 2008;7(3):107–11. doi: 10.4103/1596-3519.55668. [DOI] [PubMed] [Google Scholar]
- 40.Devabalan Y, Cereceda-Monteoliva N, Lorenz H, Magill JC, Unadkat S, Ferguson M, Rennie C. Coronavirus disease 2019: changing the future of emergency epistaxis management. J Laryngol Otol. 2021;135(8):675–679. doi: 10.1017/S0022215121001456. [DOI] [PMC free article] [PubMed] [Google Scholar]


