Abstract
A young adult cat was evaluated for respiratory failure. Diagnostic testing, including computed tomography and culture, documented a primary Mycoplasma species pneumonia. The cat was treated aggressively with supportive care, including mechanical ventilation and appropriate antibiotics including doxycycline, and made a complete recovery. This is a unique case in respect of the severity of the Mycoplasma species pneumonia as well as the cat's recovery from severe lung injury.
A 3-year-old, 9.5 lb (4.3 kg), spayed female domestic shorthair cat was referred to Cummings School of Veterinary Medicine at Tufts University for evaluation of acute onset of respiratory distress, which was preceded by 2 days of coughing. The cat was an indoor-only cat, from a multi-cat (five) household, which also included a rabbit. Recurrent upper respiratory tract infections had occurred in the home, and a new kitten with an upper respiratory tract infection had recently been introduced. The cat in the case report had not had any prior signs of upper respiratory tract infection. Vaccination against feline rhinotracheitis, calici- and panleukopenia viruses had occurred within the previous year. A test for feline leukemia and immunodeficiency viruses was negative.
The referring veterinarian evaluated the cat for coughing 2 days prior to presentation. At that time, fenbendazole at an unknown dose was prescribed, reportedly due to concern for potential lung worm infections such Aelurostrongylus abstrusus. The cat was discharged home for supportive care. The following day, the cat represented to the referring veterinarian and was reported to have open-mouth breathing and hypothermia. Thoracic radiographs showed moderate, diffuse bronchial and hazy interstitial patterns. There was also a mild alveolar pattern in the ventral aspect of the left caudal lung lobe. No new therapy was prescribed and the cat was discharged for on-going supportive care at home (due to lack of overnight hospitalization at the primary care hospital).
The next day, progressive respiratory distress developed and the cat was represented to the primary care veterinarian. At that point, the cat was referred for 24 h care and on-going evaluation. On presentation to the Tufts emergency service, the cat was assessed as weak with respiratory distress and hypothermia (35.9°C [96.7°F]). The compressibility of the thoracic cavity was considered normal, percussion was not performed. Increased bronchovesicular sounds were present bilaterally, although no murmur, crackles or wheezes were ausculted. The cat was placed in an oxygen cage with the fraction inspired oxygen (FiO2) set at 60%. Venous blood gas analysis showed a combined respiratory and metabolic acidosis (acidemia) (7.231; reference range 7.337–7.467), hypercapnea (54.1 mmHg, reference range 40–44 mmHg), and normal bicarbonate (22.9 mmol/l, reference range 20–24 mmol/l). Further testing was not performed given the cat's fragile condition and treatment was initiated with intravenous fluids (lactated Ringer's solution at 3 ml/kg/h [1.4 ml/lb/h]), cefazolin (22 mg/kg [10 mg/lb] IV q 8 h) and supplemental heat.
The next morning (day 2) a complete blood count, serum chemistry profile, coagulation profile and heartworm antigen and antibody levels were obtained. Additionally, a transoral tracheal wash was performed under brief propofol sedation. A moderate neutrophilic leukocytosis with a left shift was present. The serum chemistry panel was unremarkable except for hyperglycemia (207 mg/dl, reference range 70–120 mg/dl). The coagulation profile was unremarkable and both the feline heartworm antibody and antigen tests were negative.
Cytological evaluation of the endotracheal aspirate documented suppurative inflammation with evidence of previous hemorrhage. The neutrophils were predominately degenerate and often contained cellular debris. Some macrophages exhibited erythrophagia and/or contained hemosiderin. Some coccobacilli-like structures were present within neutrophils, but they were not seen on the Gram-stained preparation.
Based upon the cytological evaluation and while awaiting culture results, antimicrobial therapy was changed to potentiated ticarcillin (50 mg/kg [22.8 mg/lb] IV q 8 h), and doxycycline (10 mg/kg [4.5 mg/lb] IV q 12 h). Fluid support and supplemental oxygen were continued. The cat's clinical condition continued to decline over the following 6 h.
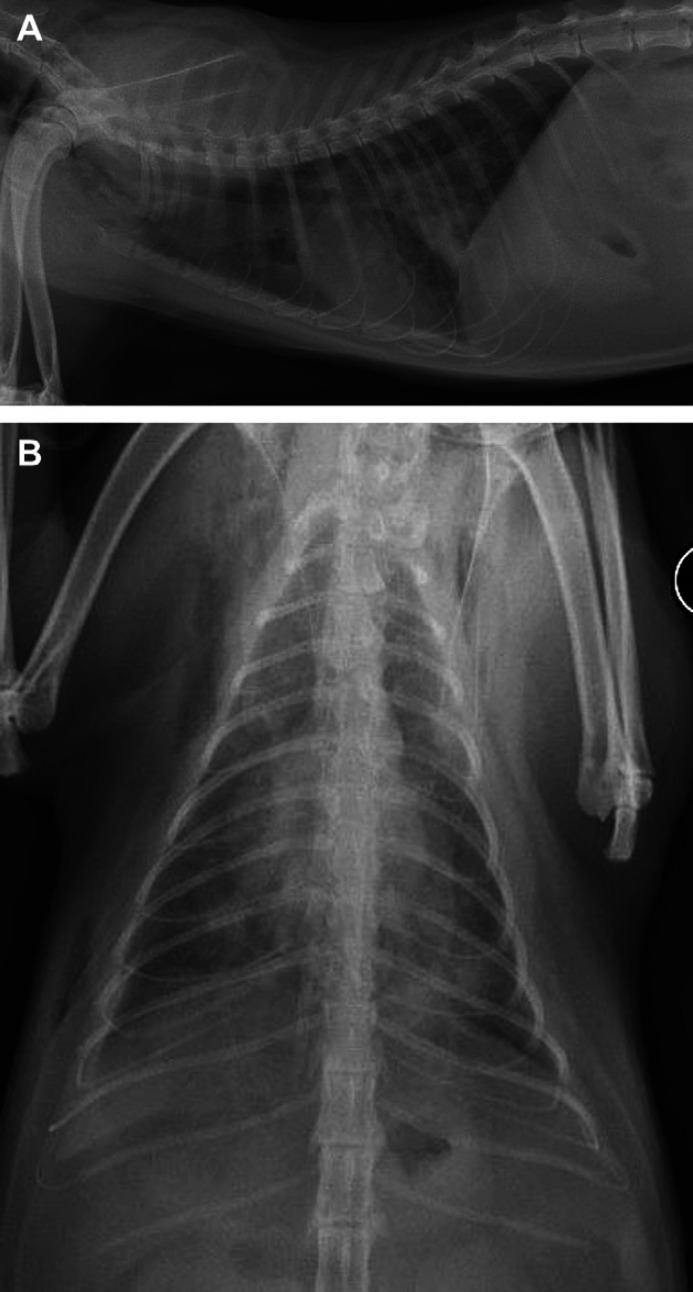
Thoracic radiographs (Fig 1) were repeated. Compared to referral radiographs, the diffuse, hazy interstitial pattern and lung consolidation were more extensive. Moderate pneumomediastinum and subcutaneous emphysema were also evident. An abbreviated echocardiogram was performed and excluded primary cardiac disease as a cause of the lung abnormalities.
Fig 1.
(A) Right lateral radiograph. (B) Ventrodorsal thoracic radiograph. There is a heavy interstitial and patchy alveolar lung pattern bilaterally. Pneumomediastinum, subcutaneous emphysema, and mild right-sided pleural effusion are also noted.
Due to concerns about the progression of respiratory distress and imminent respiratory failure, anesthesia and intermittent positive pressure ventilation (IPPV) were initiated. Computed tomography (CT) with angiography was performed to further evaluate the lung parenchyma and to assess for pulmonary thromboembolism. CT was performed with a Picker PQ 5000 single slice spiral scanner using the following scan and display parameters: 120 kV, 200 mA, 5 mm slice thickness, 3 mm reconstruction index, 1.5 pitch factor, window center of −600, and window width of 1600. The cat was positioned in ventral recumbency.
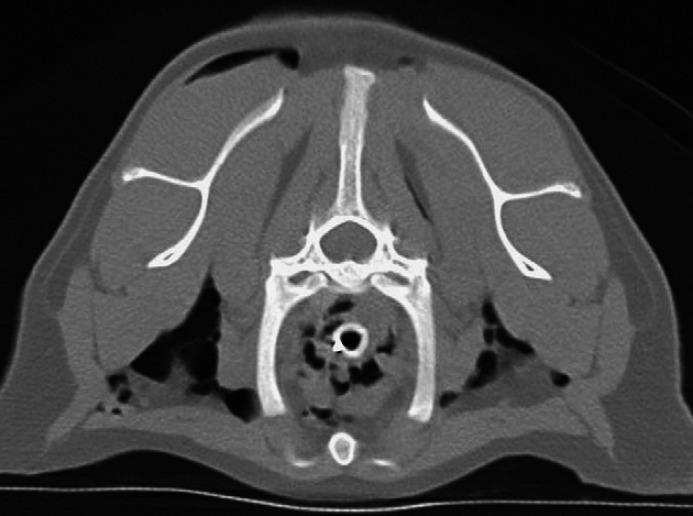
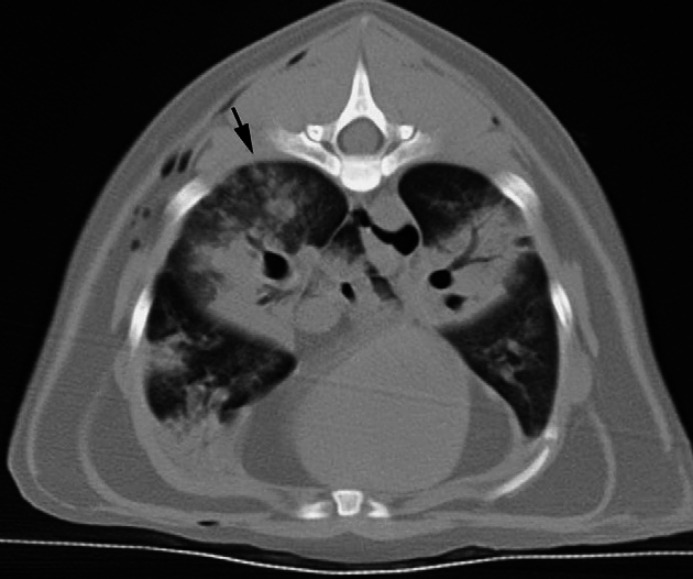
On the CT images there was mild pleural effusion, moderate bilateral subcutaneous emphysema and numerous gas bubbles within the mediastinum (Fig 2). Diffuse, bilateral, ground glass attenuation, as well as bilateral patchy reticular and ill-defined nodular opacities, and severe, patchy (lobular) regions of air space consolidation were evident in all lobes (Fig 3). Consolidation was most severe in the cranial aspect of the right caudal lobe and the majority of the left caudal lobe; margination between the consolidated and non-consolidated portions within the lobes was irregular, but sharply defined (Fig 3). Bronchial/bronchiolar wall thickening was noted in some lobes. A repeat spiral scan was performed immediately following intravenous injection of iohexol (Omipaque 300, Amersham Health). A 3 mm filling defect was suspected in the left caudal pulmonary artery just cranial to the left caudal lobe consolidation; however, interpretation was hampered by artifactual streaks and overall poor filling of the arteries bilaterally. Compared with the non-contrast examination, consolidation was more extensive, now involving the right cranial lobe and more of the right caudal lobe. In summary, CT findings were compatible with diffuse bilateral interstitial and air space disease, with some small airway and pleural involvement. Differential diagnoses included acute interstitial pneumonia, acute lung injury, diffuse hemorrhage, and pulmonary thromboembolism with superimposed lung infarction or hemorrhage. The CT findings were interpreted to be atypical for bacterial pneumonia or neoplasia; nevertheless, diffuse lung infection including the atypical pneumonias, notably Mycoplasma species were certainly compatible.
Fig 2.
Transverse thoracic CT image at the level of the first ribs. Note the pneumomediastinum and the bilateral subcutaneous emphysema.
Fig 3.
Transverse thoracic CT image at the level of the ninth rib. Note the severe, patchy air space consolidation in the caudal lobes, and in the right middle and accessory lobes. Note the irregular but sharply defined margins between the consolidated and non-consolidated portions of the lobes. There is a diffuse, hazy ground glass opacity and reticular markings in most of the remaining lungs and ill-defined nodular opacities in the dorsal aspect of the right caudal lobe (arrow).
An arterial blood gas was performed shortly after induction on 100% FiO2, which documented a PaO2 of 118 mmHg and a PCO2 of 74.8 mmHg, with a PaO2:FiO2 ratio of 118 (reference range>500, Haskins 2004). Ventilation was performed with synchronous intermittent mandatory ventilation (SIMV) mode with a set tidal volume (VT) of 42 ml (10 ml/kg [4.5 ml/lb]), respiratory rate of 20 bpm and positive end-expiratory pressure (PEEP) of 5 cmH2O. The sedation protocol included intermittent diazepam (0.01 mg/kg [0.005 mg/lb] IV boluses as needed), hydromorphone and ketamine continuous rate infusion (CRI) (hydromorphone 0.004 mg/kg/h [0.002 mg/lb/h], ketamine 0.3 mg/kg/h [0.14 mg/lb/h]). Following 6 h of IPPV, the pulmonary function had improved, with oxygen saturation (SpO2) of 98% with a FiO2 of 0.5 and an end tidal CO2 (ETCO2) of 25 mmHg. Due to the cat's size, no further arterial blood gases were obtained that day.
The following morning (day 3), the ketamine CRI was discontinued to lighten the plane of sedation and to better assess the viability of the cat's respiratory efforts. An arterial line was successfully placed for direct blood pressure and blood gas measurements. The IPPV was continued. In the afternoon, desaturation associated with a large (300 ml) pneumothorax was identified on the right side, so a thoracostomy tube was placed and intermittent hand suction used to control signs of pleural space disease. In addition, the endotracheal tube was changed twice due to obstruction with mucoid secretions and a small amount of blood. However, the cat's pulmonary status improved, and the sedation was discontinued. The cat was weaned from the ventilator after 31 h of ventilation. The cat was then placed in oxygen cage with a FiO2 of 0.5. An arterial blood gas, while breathing spontaneously, documented a PaO2 of 105 mmHg and PaCO2 of 37 mmHg with a PaO2:FiO2 ratio of 210. The cat continued to slowly convalesce, returning to normal within 2 days. On day 5, the chest tubes were removed and aerosolized therapy with albuterol was added with one puff given twice daily using a feline specific-spacer (Aerokat, Trudell Medical, Ontario, Canada). The cat's therapy continued with potentiated ticarcillin and doxycycline. On day 5, the cat began eating and grooming, and the supplemental oxygen was discontinued. Final bacterial culture and sensitivity data, available on day 6, documented a pure heavy growth of Mycoplasma species. Culture was performed as per protocol at the laboratory [Idexx] with the sample being plated out on blood and MacConkey's agar (Fischer Scientific, Lowell, MA), and checked for growth every 24 h. This should have detected most potentially significant aerobic bacteria, including Bordetella bronchiseptica.
Based on these results, the potentiated ticarcillin and intravenous fluids were discontinued. On day 7, the cat was discharged with doxycycline (5.8 mg/kg [2.6 mg/lb] PO q 12 h for 1 month). At time of manuscript submission, 16 months later, the cat is doing well, with no cough or other signs of respiratory disease.
This case is unique in the severity of the interstitial pneumonia and respiratory failure associated with Mycoplasma species infection. IPPV was required to improve oxygenation and limit carbon dioxide retention. IPPV is used rarely in cats, with one report describing a dismal 14% survival rate for cats with respiratory failure, and another, more recent review reporting a 21% survival to discharge (Lee et al 2005, Hopper et al 2007). Successful outcome following respiratory failure in this cat was attributed to supportive care and appropriate antimicrobial therapy.
Mycoplasma species are a unique genus of bacteria that lack cell walls. The role of Mycoplasma species infection in atypical pneumonia in people has been recognized for decades. Lack of Gram-staining of organisms visualized in a cytological evaluation emphasizes the importance of bacterial culture, although isolation of these organisms usually requires special culture media (Rosendal 1990, Johnson et al 2004). In recent years, the development of polymerase chain reaction (PCR) analysis has proven very useful in supporting infection. Unfortunately, PCR was not readily available at the time of the cat in this report's illness.
While Mycoplasma species are well recognized as etiologic agents in upper respiratory tract and ocular disease in cats (Johnson et al 2005, Bannasch and Foley 2005), as well as in other species, their role as lower respiratory tract pathogens is less clear. Some investigators believe Mycoplasma species only colonizes inflamed or infected lower airways, while others believe Mycoplasma species capable of being a primary cause of pneumonia (Kirschner et al 1990, Chandler and Lappin 2002).
Interestingly, Mycoplasma species have not been cultured from the airways of normal cats, despite their common residence in the upper airway (Randolph et al 1993, Chandler and Lappin 2002). Prior published reports of Mycoplasma species as the primary agent in cats with infectious lower respiratory tract disease are limited to a case series of three cats with pneumonia, 11 of 21 cases in a retrospective on lower respiratory tract infections in cats and one retrospective study in both cats and dogs (Foster et al 1998, Chandler and Lappin 2002, Foster et al 2004). Other reports document the presence of Mycoplasma species in cats with upper respiratory tract infections (Johnson et al 2005, Bannasch and Foley 2005).
Appropriate antibiotic treatment for cats with suspected pneumonia should include antibiotics for Mycoplasma species. Macrolides, azalides, lincosamides, tetracyclines, chloramphenicol and fluoroquinolones are considered appropriate for use in empiric treatment of suspected Mycoplasma species infection.
This patient's updated vaccination status and successful recovery makes it unlikely that respiratory viruses contributed to the development of respiratory failure. However, feline calicivirus vaccines do not always prevent infection and primary viral infection with secondary Mycoplasma species remains a possibility in this cat.
CT is a widely established technique to evaluate diffuse lung disease in people (Webb et al 2001a). Reports of its use for such in animals have increased in recent years. On CT images in people, Mycoplasma species pneumonia is considered a diffuse process characterized primarily by parenchymal opacification (Webb et al 2001b). CT findings consist mainly of unilateral or bilateral consolidation, often with a patchy, lobular distribution, patchy ground glass attenuation, centrilobular nodules indicating bronchiolitis, and thickening of the peribronchovascular and interlobular septal interstitium (Reittner et al 2000). Bronchial wall thickening, small pleural effusions and air trapping have also been described (Webb et al 2001b). Most of these abnormalities, and the additional findings of pneumomediastinum and subcutaneous emphysema, were evident in the cat of this report. Pneumomediastinum and subcutaneous emphysema were believed to be due to ruptured alveoli or bronchioles with subsequent dissection of air into the mediastinum and along fascial planes. Although transtracheal washes or traumatic jugular venepuncture may also result in pneumomediastinum; although neither appeared to be responsible in this case.
The pneumothorax was clearly iatrogenic in nature. The cat in this report had significant pulmonary disease, with hypoxemia and hypercarbia. Positive pressure ventilation, particularly with high peak airway pressures is associated with a risk of pneumothorax (Haskins and King 2004). The use of low tidal volumes has been used in people with lung injury, this strategy may also be useful in cats (ARDSNET 2000).
This case highlights Mycoplasma species as a probably sole pathogen in pneumonia and airway disease in the cat and the potential for complete resolution of parenchymal and airway clinical signs with aggressive treatment, including mechanical ventilation and appropriate antibiotics.
Acknowledgments
The authors thank Linda Kinney BS, CVT, ALAT for her assistance with the CT scan described in this report.
References
- Acute Respiratory Distress Syndrome Network (ARDSNET). Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome, New England Journal of Medicine 342, 2000, 1301–1308. [DOI] [PubMed] [Google Scholar]
- Bannasch M.J., Foley J.E. Epidemiological evaluation of multiple respiratory pathogens in cats in animal shelters, Journal of Feline Medicine and Surgery 7, 2005, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J.C., Lappin M.R. Mycoplasmal respiratory infections in small animals: 17 cases (1988–1999), Journal of the American Animal Hospital Association 38, 2002, 111–119. [DOI] [PubMed] [Google Scholar]
- Foster S.F., Barrs V.R., Martin P., Malik R. Pneumonia associated with Mycoplasma spp in three cats, Australian Veterinary Journal 76, 1998, 460–464. [DOI] [PubMed] [Google Scholar]
- Foster S.F., Martin P., Allan G.S., Barrs V.R., Malik R. Lower respiratory infection in cats: 21 cases (1995–2000), Journal of Feline Medicine and Surgery 6, 2004, 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper K., Haskins S.C., Kass P.H., Rezende M.L., Aldrich J. Indications, management and outcome of long-term positive pressure ventilation in dogs and cats: 148 cases (1990–2001), Journal of the American Veterinary Medical Association 230, 2007, 64–75. [DOI] [PubMed] [Google Scholar]
- Haskins S.C. Interpretation of blood gas measurements. King L.G. Textbook of Respiratory Disease in Dogs and Cats, 2004, Saunders: St Louis, Missouri. [Google Scholar]
- Haskins S.C., King L.G. Positive pressure ventilation. King L.G. Textbook of Respiratory Disease in Dogs and Cats, 2004, Saunders: St Louis, Missouri. [Google Scholar]
- Johnson L.R., Drazenovich N.L., Foley J.E. A comparison of routine culture with polymerase chain reaction technology for the detection of Mycoplasma species in feline nasal samples, Journal of Veterinary Diagnostic Investigation 16, 2004, 347–351. [DOI] [PubMed] [Google Scholar]
- Johnson L.R., Foley J.E., DeCock H.E.V., Clarke H.E., Maggs D.J. Assessment of infectious organisms associated with chronic rhinosinusitis in cats, Journal of the American Veterinary Medical Association 227, 2005, 579–585. [DOI] [PubMed] [Google Scholar]
- Kirschner B.K., Port C.D., Magoc T.J., Sidor T.J., Ruben Z. Spontaneous bronchopneumonia in laboratory dogs infected with untyped Mycoplasma spp, Laboratory Animal Science 40, 1990, 625–628. [PubMed] [Google Scholar]
- Lee J.A., Drobatz K.J., Koch M.W., King L.G. Indications for and outcome of positive pressure ventilation in cats: 53 cases (1993–2002), Journal of the American Veterinary Medical Association 226, 2005, 924–931. [DOI] [PubMed] [Google Scholar]
- Randolph J.F., Moise N.S., Scarlett J.M., Shin S.J., Blue J.T., Corbett J.R. Prevalence of mycoplasmal and ureaplasmal recovery from traceobronchial lavages and of mycoplasmal recovery from pharyngeal swab specimens in cats with or without pulmonary disease, American Journal of Veterinary Research 54, 1993, 897–900. [PubMed] [Google Scholar]
- Reittner P., Muller N.L., Heyneman L., Johkoh T., Park J.S., Lee K.S., Honda O., Tomiyama N. Mycoplasma pneumoniae pneumonia: radiographic and high-resolution CT features in 28 patients, American Journal of Roentgenology 174, 2000, 37–41. [DOI] [PubMed] [Google Scholar]
- Rosendal S. Mycoplasmal infection. Greene C.E. Infectious Diseases of the Dog and Cat, 1990, Saunders: Philadelphia, 446–449. [Google Scholar]
- Webb W.R., Muller N.L., Naidich D.P. Quick reference to high resolution computed tomography diagnosis of diffuse lung disease. Webb W.R., Muller N.L., Naidich D.P. High Resolution CT of the Lung, 3rd edn, 2001a, Lippincott, Williams and Wilkins: Philadelphia, pp. xv–liv. [Google Scholar]
- Webb W.R., Muller N.L., Naidich D.P. Diseases characterized primarily by parenchymal opacification. Webb W.R., Muller N.L., Naidich D.P. High Resolution CT of the Lung, 3rd edn, 2001b, Lippincott, Williams and Wilkins: Philadelphia, 355–420. [Google Scholar]