Abstract
An 8-year-old domestic shorthair cat was evaluated for a several day history of anorexia and vomiting. Abdominal distention was noted on physical examination and diagnostics including abdominal radiographs and abdominal ultrasound demonstrated the presence of free fluid in the peritoneal cavity. Septic peritonitis was diagnosed on cytologic evaluation of the peritoneal fluid. The cat was taken to surgery and a foreign body composed of plant material was found embedded within the spleen. A splenectomy was performed and both open and active peritoneal drainages were used to successfully treat this case.
An 8-year-old, 9.4 kg male castrated domestic shorthair cat was admitted to our clinic for evaluation of a 5-day history of anorexia and lethargy. The cat lived strictly indoors, and there was one other healthy cat in the household. No previous pertinent medical history was noted.
The initial physical examination revealed that the cat had an increased temperature of 39.3°C (reference range, 37.9–39.2°C), and the heart rate (246 beats/min) and the respiration rate (78 breaths/min) were increased. His pulse quality was strong, and no arrhythmias were auscultated. The cat was alert and no abnormal mentation was noted. Other physical examination findings included a distended abdomen and increased bronchovesicular sounds.
Initial diagnostics included non-invasive blood pressure measurement (Doppler), pulse oximetry, complete blood count, biochemical profile, prothrombin time (PT), feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) testing (SNAP FIV/FeLV Test; Idexx Laboratories), urinalysis, abdominal and thoracic radiographs, and abdominal ultrasound. The systolic blood pressure was 130 mmHg, and the oxygen saturation as measured by pulse oximetry was 100%. Abnormalities found on complete blood count and biochemical profile included a neutrophilia (22,970/μl; reference range, 2300–14,000/μl) with a band neutrophilia of 490/μl, hyponatremia (142 mmol/l; reference range, 146–157 mmol/l), hypoproteinemia (5.5 g/dl; reference range, 6.0–8.6 g/dl), hypoalbuminemia (2.0 g/dl; reference range, 2.4–3.8 g/dl), and an increase in the aspartate transaminase (AST) activity (63 U/l; reference range, 1–37 U/l). The PT was not prolonged at 11 s (reference range, 9.6–13.2 s), and FeLV and FIV testing were negative. The urine specific gravity was determined to be 1.054.
Abdominal radiographs demonstrated poor serosal detail and abdominal distention. Ultrasonographic examination identified a large amount of echogenic free fluid throughout the peritoneal cavity, and the mesentery contained several hypoechoic regions. A sample of fluid was aspirated and submitted for cytologic evaluation. Peritoneal fluid analysis revealed many degenerate neutrophils and both intra- and extracellular bacterial rods were present. Thoracic radiographs were within normal limits.
Based on the diagnosis of septic peritonitis, an exploratory laparotomy was performed. Prior to anesthetic induction, intravenous crystalloid fluid therapy with Normosol-R (Abbott Laboratories) at a rate of 3 ml/kg/h and broad spectrum antibiotic therapy with ampicillin (Sandoz: 22 mg/kg, IV, q8 h) and enrofloxacin (Baytril; Baxter Healthcare: 5 mg/kg, IV, q24 h) were initiated. At surgery, an intraperitoneal foreign body resembling plant material was found embedded within the spleen and peri-splenic mesentery (Fig 1). Diffuse peritonitis and suppurative peritoneal effusion were also noted. Swabs of the peritoneal cavity and a splenic sample were submitted for both aerobic and anaerobic bacterial cultures.
Fig 1.
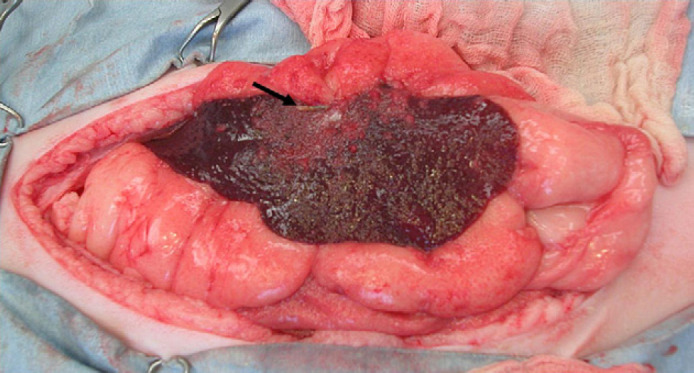
Intra-operative view of the spleen in this cat. Note the foreign body designated by the black arrow.
A splenectomy was performed, and the spleen was submitted for histopathologic evaluation. A thorough abdominal exploratory surgery revealed no fenestrations in the gastrointestinal tract and no abnormalities in any other abdominal organs. Because of the severity of the peritonitis and the continued gross contamination that was refractory to lavage, open peritoneal drainage was elected. Additionally, open peritoneal drainage was utilized because foreign material is often associated with anaerobic infections.
To perform open peritoneal drainage, sutures of 3–0 nylon (Ethicon) were placed in the linea alba 1 cm apart along the length on the abdominal incision. The sutures were tightened to allow for approximately 1 cm of peritoneal opening. Sterile sponges were placed along the incision, and the abdomen was bandaged.
Crystalloid fluid therapy and antibiotics were continued postoperatively. Colloid fluid therapy (Hetastarch; Hospira) at a rate of 1.5 ml/kg/h, metronidazole (Flagyl; Plivia: 10 mg/kg, IV, q12 h), and famotidine (Pepcid; Baxter Healthcare: 0.5 mg/kg, IV, q12 h) were also added to the treatment regimen after the completion of surgery. Buprenorphine (Buprenex; Bedford Labs: 0.01 mg/kg, IV, q6 h) was given for pain control.
Physical examination findings 1 day postoperatively included slightly tacky mucous membranes, and slightly increased bronchovesicular sounds bilaterally. The bandage placed over the open abdominal incision was still intact and strike-through had not occurred. The patient was normothermic (37.9°C). No episodes of hypotension or poor oxygen saturation were noted.
Biochemical panel abnormalities noted 1 day postoperatively included hypocalcemia (7.1 mg/dl; reference range, 9.1–11.2 mg/dl), hyponatremia (142 mmol/l), hypokalemia (3.4 mmol/l; reference range, 3.5–4.8 mmol/l), hypermagnesemia (3.0 mg/dl; reference range, 1.9–2.6 mg/dl), hypoalbuminemia (1.3 g/dl), hypoglobulinemia (2.9 g/dl; reference range, 3.1–5 g/dl), hypocholesterolemia (83 mg/dl; reference range, 96–248 mg/dl), hyperbilirubinemia (2.7 mg/dl; reference range, 0.1–0.8 mg/dl) and an AST of 239 U/l. Venous blood gas analysis demonstrated a pH of 7.348, a PCO2 of 32.5 mmHg and a base excess of −8 mmol/l. The platelet count was 202,000/μl, and there was a prolongation of the PT at 14.2 s and partial thromboplastin time (PTT) at 46.6 s (reference range, 12.6–15.7 s). Because of the prolongation of the clotting times, fresh frozen plasma was administered at a rate of 10 ml/kg every 8 h.
A second abdominal exploratory surgery was performed approximately 12 h after the first surgery. The peritoneal cavity appeared healthier at the second surgery and only a minimal amount of effusion was noted. Based on the findings of the second surgery, the abdomen was closed and a closed-suction drain (Jackson-Pratt Drainage System; Cardinal Health) was placed. An esophagostomy tube was also placed at the time of the second surgery to facilitate nutritional support.
Fluid production from the closed-suction drain ranged from 1 to 2 ml/kg/h, and unfortunately, the patient removed the drain 22 h following placement.
Three days following surgery, the cat was much more alert and comfortable. The previously described fluid therapy and drug regimen were continued. A prolongation of the clotting times was still present (PT=14.3 s and PTT=22.9 s) and administration of fresh frozen plasma was continued (10 ml/kg, q8 h).
The patient remained in the hospital for 4 more days after the second surgery and progressively improved. Culture results from the abdominal cavity demonstrated the presence of β-Streptococcus, Clostridium perfringens and Prevotella bivia. All bacteria were susceptible to the current antibiotic therapy. Histopathology revealed the presence of acute fibrinous splenitis as well as pyogranulomatous peritonitis. Intralesional bacterial colonies were present within the peri-splenic mesentery.
The patient began to eat 2 days prior to discharge, and the esophagostomy tube was removed. The patient was discharged with amoxicillin/clavulanic acid (Clavamox; Pfizer) (14 mg/kg, PO, q12 h) and metronidazole (10 mg/kg, PO, q12 h). These antibiotics were continued for 4 weeks.
The patient was seen 14 days after discharge for suture removal and re-evaluation. The owner noted no abnormalities during that time. The cat is clinically normal 14 months after discharge.
Feline splenic disease is uncommonly diagnosed (Ishmael and Howell 1968, Spangler and Culbertson 1992). One study, found that feline splenic samples comprised only 0.3% of all submitted post mortem samples during the evaluated time period (Spangler and Culbertson 1992). The cat in this current report was diagnosed with splenitis.
Splenitis is a rare diagnosis in cats, reported to account for approximately 2% of all feline splenic diseases (Spangler and Culbertson 1992). Furthermore, splenitis in cats has been sub-categorized into suppurative, granulomatous, pyogranulomatous, necrotizing, eosinophilic, and lymphoplasmacytic variations based on the predominate cell type found (Couto 1990, Neer 1996). To the authors' knowledge, splenitis secondary to a splenic foreign body has not been previously described in the cat, and this diagnosis appears to be extremely rare in all species. Human reports of splenic foreign bodies include migration of biliary stents into the pulp of the spleen with the development of a splenic abscess, the migration of a Kirschner wire that had been utilized for shoulder dislocation fixation, and migration of an ingested sewing needle from the gastrointestinal tract into the spleen (Gongaware et al 1972, Potter et al 1988, Baccarani et al 2003). Splenic hematoma development from an intraperitoneal sewing needle in a dog has been reported, but the needle was not located within the splenic parenchyma at surgery (Ramani et al 2001).
The treatment of splenic abscessation in humans is described more extensively than companion animals and ranges from conservative management to aggressive surgical exploration. Some reports describe treatment with antimicrobial therapy alone and outcomes have varied (Fernandes et al 1992, Lucien and Leong 1997, Sangchan et al 2003). Other treatment options include ultrasound or computed tomography (CT)-guided percutaneous abscess drainage or immediate splenectomy at the time of diagnosis (Lucien and Leong 1997, Chou et al 1998, Sangchan et al 2003, Tung et al 2006). Several studies have reported success rates of 100% with splenectomy combined with antimicrobial therapy, and this management option is generally considered as the definitive treatment for splenic abscessation (Lucien and Leong 1997, Green 2001, Tung et al 2006).
The most common cause of septic peritonitis in cats is gastrointestinal leakage (47%) with most cases occurring secondary to gastrointestinal neoplasia (Costello et al 2004). Other reported causes of septic peritonitis in cats include trauma (bite wounds, motor vehicular accidents, and gunshot wounds), leakage from the urogenital system, hepatic abscessation, pancreatic abscessation, liver lobe necrosis and severe omphalitis (Woolfson and Dulisch 1986, Greenfield and Walshaw 1987, Hardie 1989, Aumann et al 1998, Staatz et al 2002, Bonczynski et al 2003, Costello et al 2004, Levin et al 2004, Sergeeff et al 2004). Septic peritonitis secondary to a splenic foreign body has not been previously reported.
Surgical intervention and the administration of antibiotics are essential to the treatment of septic peritonitis. In this patient, broad spectrum antibiotic therapy was initiated with the use of ampicillin, enrofloxacin, and metronidazole in an attempt to eliminate infectious organisms within the peritoneal cavity. Enrofloxacin and other fluoroquinolones should be used with caution in cats as dose-dependent toxicity resulting in irreversible retinal degeneration can occur (Ford et al 2007). Current recommendations to reduce the risk of retinal toxicity include using split dosing and avoidance of rapid intravenous infusions (Wiebe and Hamilton 2002). Because of the diffuse peritoneal contamination noted at surgery, open peritoneal drainage was elected. In this particular case, open peritoneal drainage was useful in that anaerobic bacteria were isolated. Anaerobes are less likely to be isolated in environments with prolonged air exposure (Stone et al 1975, Hosgood 1993, Lanz et al 2001).
Management of septic peritonitis by the use of open peritoneal drainage has been detailed in feline patients. One study reported a survival rate of 50% (3/6) for cats managed with open peritoneal drainage (Costello et al 2004). Other studies have found better success reporting survival rates of 67–89%; however, these studies included both dogs and cats in their findings (Greenfield and Walshaw 1987, Staatz et al 2002). The surgeon electing open peritoneal drainage should be prepared for multiple anesthetic episodes as well as potential complications and disadvantages such as prolonged hospital stays, abdominal organ evisceration, anemia, hypoproteinemia, fluid loss and nosocomial infection (Greenfield and Walshaw 1987, Lanz et al 2001, Staatz et al 2002). Surgical exploration and splenectomy followed by open and active peritoneal drainage were successful in the cat described in this report.
References
- Aumann M., Worth L.T., Drobatz K.J. Uroperitoneum in cats: 26 cases (1986–1995), Journal of the American Animal Hospital Association 34, 1998, 315–324. [DOI] [PubMed] [Google Scholar]
- Baccarani U., Risaliti A., Sainz-Barriga M. Ileosplenic fistula and splenic abscesses caused by migration of biliary stents in a liver transplant recipient, Gastrointestinal Endoscopy 58, 2003, 811–813. [DOI] [PubMed] [Google Scholar]
- Bonczynski J.J., Ludwig L.L., Barton L.J., Loar A., Peterson M.E. Comparison of peritoneal fluid and peripheral blood pH, bicarbonate, glucose, and lactate concentration as a diagnostic tool for septic peritonitis in dogs and cats, Veterinary Surgery 32, 2003, 161–166. [DOI] [PubMed] [Google Scholar]
- Chou Y., Tiu C., Chiou H., Hsu C.C., Chiang J.H., Yu C. Ultrasound-guided interventional procedures in splenic abscesses, European Journal of Radiology 28, 1998, 167–170. [DOI] [PubMed] [Google Scholar]
- Costello M.F., Drobatz K.J., Aronson L.R., King L.G. Underlying cause, pathophysiologic abnormalities, and response to treatment in cats with septic peritonitis: 51 cases (1990–2001), Journal of the American Veterinary Medical Association 225, 2004, 897–902. [DOI] [PubMed] [Google Scholar]
- Couto C.G. A diagnostic approach to splenomegaly in cats and dogs, Veterinary Medicine 85, 1990, 220–238. [Google Scholar]
- Fernandes E.T., Tavares P.B., Garcette C.B.M. Conservative management of splenic abscesses in children, Journal of Pediatric Surgery 27, 1992, 1578–1579. [DOI] [PubMed] [Google Scholar]
- Ford M.M., Dubielzig R.R., Giuliano E.A., Moore C.P., Narfström K.L. Ocular and systemic manifestations after oral administration of a high dose of enrofloxacin in cats, American Journal of Veterinary Research 68, 2007, 190–202. [DOI] [PubMed] [Google Scholar]
- Gongaware R.D., Rose J.S., Berdon W.E., Weil R. Intrasplenic foreign body, American Journal of Surgery 124, 1972, 694–696. [DOI] [PubMed] [Google Scholar]
- Green B.T. Splenic abscess: report of six cases and review of the literature, American Surgeon 67, 2001, 80–85. [PubMed] [Google Scholar]
- Greenfield C.L., Walshaw R. Open peritoneal drainage for treatment of contaminated peritoneal cavity and septic peritonitis in dogs and cats: 24 cases (1980–1986), Journal of the American Veterinary Medical Association 191, 1987, 100–105. [PubMed] [Google Scholar]
- Hardie E.M. Peritonitis from urogenital conditions, Problems in Veterinary Medicine 1, 1989, 36–49. [PubMed] [Google Scholar]
- Hosgood G.L. Drainage of the peritoneal cavity, Compendium on Continuing Education for the Practicing Veterinarian 15, 1993, 1605–1616. [Google Scholar]
- Ishmael J., Howell J.M. Observations on the pathology of the spleen of the cat, Journal of Small Animal Practice 9, 1968, 7–13. [DOI] [PubMed] [Google Scholar]
- Lanz O.I., Ellison G.W., Bellah J.R., Weichman G., VanGilder J. Surgical treatment of septic peritonitis without abdominal drainage in 28 dogs, Journal of the American Animal Hospital Association 37, 2001, 87–92. [DOI] [PubMed] [Google Scholar]
- Levin G.M., Bonczynski J.J., Ludwig L.L., Barton L.J., Loar A.S. Lactate as a diagnostic test for septic peritoneal effusions in dogs and cats, Journal of the American Animal Hospital Association 40, 2004, 364–371. [DOI] [PubMed] [Google Scholar]
- Lucien L., Leong S.S. Splenic abscesses from 1987–1995, American Journal of Surgery 174, 1997, 87–93. [DOI] [PubMed] [Google Scholar]
- Neer T.M. Clinical approach to splenomegaly in dogs and cats, Compendium on Continuing Education for the Practicing Veterinarian 18, 1996, 35–48. [Google Scholar]
- Potter F.A., Fiorini A.J., Knox J., Rajesh P.B. The migration of a Kirschner wire from shoulder to spleen: brief report, Journal of Bone Joint Surgery (Br) 70, 1988, 326–327. [DOI] [PubMed] [Google Scholar]
- Ramani C., Jayaprakash R., Nagarajan L., David W.P.A., Balasubramanian N.N. Surgical management of subscapular splenic haematoma due to penetrating foreign body in a dog, Indian Veterinary Journal 78, 2001, 929–930. [Google Scholar]
- Sangchan A., Mootsikapun P., Mairiang P. Splenic abscess: clinical features, microbiologic finding, treatment and outcome, Journal of the Medical Association of Thailand 86, 2003, 436–441. [PubMed] [Google Scholar]
- Sergeeff J.S., Armstrong P.J., Bunch S.E. Hepatic abscesses in cats: 14 cases (1985–2002), Journal of Veterinary Internal Medicine 18, 2004, 295–300. [DOI] [PubMed] [Google Scholar]
- Spangler W.L., Culbertson M.R. Prevalence and type of splenic diseases in cats: 455 cases (1985–1991), Journal of the American Veterinary Medical Association 201, 1992, 773–776. [PubMed] [Google Scholar]
- Staatz A.J., Monnet E., Seim H.B. Open peritoneal drainage versus primary closure for the treatment of septic peritonitis in dogs and cats: 42 cases (1993–1999), Veterinary Surgery 31, 2002, 174–180. [DOI] [PubMed] [Google Scholar]
- Stone H.H., Kolb L.D., Geheber C.E. Incidence and significance of intraperitoneal anaerobic bacteria, Annals of Surgery 181, 1975, 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung C., Chen F., Lo C. Splenic abscess: an easily overlooked disease?, American Surgeon 4, 2006, 322–325. [PubMed] [Google Scholar]
- Wiebe V., Hamilton P. Fluoroquinolone-induced retinal degeneration in cats, Journal of the American Veterinary Medical Association 221, 2002, 1568–1571. [DOI] [PubMed] [Google Scholar]
- Woolfson J.M., Dulisch M.L. Open abdominal drainage in the treatment of generalized peritonitis in 25 dogs and cats, Veterinary Surgery 15, 1986, 27–32. [Google Scholar]


