Abstract
To determine whether the ability of Staphylococcus aureus to bind collagen involves an adhesin other than the collagen adhesin encoded by cna, we examined the collagen binding capacity (CBC) of 32 strains of S. aureus. With only two exceptions, a high CBC corresponded with the presence of cna. Both exceptions involved cna-positive strains with a low CBC. The first was a single strain (ACH5) that encoded but did not express cna. The second were the mucoid strains Smith diffuse and M, both of which encoded and expressed cna but bound only minimal amounts of collagen. Analysis of capsule mutants suggests that the reduced CBC observed in the mucoid strains was due to masking of the collagen adhesin on the cell surface and that this masking effect is restricted to heavily encapsulated strains. Differences in the CBC of the remaining cna-positive strains were correlated to variations in the level of cna transcription and were independent of the number of B domain repeats in the cna gene. In all cna-positive strains other than ACH5, cna transcription was temporally regulated, with cna mRNA levels being highest in cells taken from exponentially growing cultures and falling to almost undetectable levels as cultures entered the post-exponential growth phase. The CBC was also highest with cells taken from exponentially growing cultures. Mutation of agr resulted in a slight increase in cna transcription and a corresponding increase in CBC during the exponential growth phase but did not affect the temporal pattern of cna transcription. Mutation of sar resulted in a more dramatic increase in CBC and a delay in the post-exponential-phase repression of cna transcription. Mutation of both sar and agr had an additive effect on both CBC and cna transcription. We conclude that (i) cna encodes the primary collagen-binding adhesin in S. aureus, (ii) sar is the primary regulatory element controlling expression of cna, and (iii) the regulatory effects of sar and agr on cna transcription are independent of the interaction between sar and agr.
Staphylococcus aureus binds a number of host proteins, including fibronectin, laminin, vitronectin, fibrinogen, collagen, thrombospondin, elastin, osteopontin and bone sialoprotein (26). The S. aureus adhesins responsible for this binding have been termed MSCRAMMs to denote their role as microbial surface components recognizing adhesive matrix molecules (26). Genes encoding MSCRAMMs that bind fibronectin (fnbA and fnbB), fibrinogen (fib, clfA, and fbpA), elastin (ebpS), and collagen (cna) have been identified (26). Each of these genes encodes an adhesin with apparent specificity for a single host protein. In contrast, map appears to encode a broad-specificity MSCRAMM that mediates the low-level binding of several host proteins, including bone sialoprotein, fibronectin, fibrinogen, vitronectin, thrombospondin, collagen and osteopontin (15, 21). We recently demonstrated that map is encoded by most and possibly all S. aureus strains (33). fib, clfA, ebpS, and at least one of the two fnb genes are also highly conserved. In contrast, the collagen adhesin gene (cna) is relatively rare, being present in only 10 of the 25 strains that we examined (33).
Because cna is the only recognized S. aureus gene that encodes an adhesin that specifically binds collagen (30), the observation that most strains do not encode cna (33) suggests that most strains do not bind collagen. That inference is consistent with earlier surveys of S. aureus isolates (36) and is supported by the observation that mutation of cna in S. aureus Phillips eliminated the ability to bind collagen (28). However, cna mutagenesis has not been done in any strain other than Phillips, and it remains possible that at least some cna-negative S. aureus strains bind collagen either by virtue of the enhanced expression of a broad-specificity adhesin like Map or the presence of a collagen-binding MSCRAMM other than cna. Indeed, Nilsson et al. (22) reported that mutation of the staphylococcal accessory regulator (sar) in S. aureus DB resulted in a 16-fold enhancement in the capacity to bind type II collagen despite the fact that DB does not encode cna.
Identification and characterization of the S. aureus adhesins that contribute to collagen binding is important in light of the fact that S. aureus strains that cause musculoskeletal infections (e.g., septic arthritis and osteomyelitis) almost invariably bind collagen (2, 13, 26). This correlation suggests that therapeutic strategies aimed at the inhibition of collagen binding might be useful for the prevention and treatment of musculoskeletal infection. However, the development of such strategies will require a clear understanding of the bacterial factors that contribute to collagen binding. To that end, we characterized 32 strains of S. aureus with respect to their collagen binding capacity (CBC) and the presence and expression of cna. Our results indicate that (i) cna encodes the only S. aureus MSCRAMM that mediates the high-level binding of collagen, (ii) variations in CBC among cna-positive strains are due to capsule production and/or variations in the level of cna transcription and are independent of B-domain variations within the collagen adhesin itself, (iii) cna transcription is temporally regulated in a manner that is at least partially dependent on the sar regulatory locus, and (iv) the regulatory effects of sar on cna transcription are independent of the interaction between sar and agr.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The S. aureus strains used in this study are summarized in Table 1. Because the prototypical 8325-4 strain of S. aureus does not encode cna (33), we introduced cna into a representative 8325-4 strain (RN6390) and a sar mutant derived from RN6390 (SarR) (5). The cna gene was introduced both by transformation with a plasmid containing the FDA574 cna gene cloned into pLI50 (pLI50::cna) and by Φ11-mediated transduction from CYL574 (28). The latter results in the introduction of cna into the chromosomal lipase (geh) gene (geh::cna). Transformants were selected by plating on tryptic soy agar (TSA) containing 10 μg of chloramphenicol per ml and confirmed by plasmid profile analysis (data not shown). Transductants were selected on TSA containing 5 μg of tetracycline per ml and confirmed by Southern blot using cna and geh probes (data not shown). The RN6390 (geh::cna) and SarR (geh::cna) derivatives were designated UAMS-174 and UAMS-175, respectively (Table 1). We also generated agr-null mutants of UAMS-174 and UAMS-175 by Φ11-mediated transduction using RN6911 (23) as a donor strain. Transductants were selected on TSA containing 2 μg of minocycline per ml and confirmed by Southern blot using cna, agr, and sar probes (data not shown). The agr-null mutants of UAMS-174 and UAMS-175 were designated UAMS-171 and UAMS-172, respectively (Table 1).
TABLE 1.
Strains used
| Strain | cna (no. of B domains) | Reference | Description or comments |
|---|---|---|---|
| UAMS-1 | Yes (1) | 7 | Clinical isolate (osteomyelitis) |
| U-6911 | Yes (1) | 8 | agr-null mutant derived from UAMS-1 |
| Phillips | Yes (2) | 28 | Clinical isolate (osteomyelitis) |
| FDA574 | Yes (3) | 30 | Source of cna for cloning experiments |
| UAMS-639 | Yes (4) | 3 | Clinical isolate (tracheal culture) |
| Becker | Yes (1) | 16 | Microencapsulated (type 8) |
| UAMS-604 | Yes (3) | 34 | Clinical isolate (blood) |
| ACH2 | Yes (2) | This study | Clinical isolate (septic arthritis) |
| ACH4 | Yes (3) | This study | Clinical isolate (septic arthritis) |
| ACH5 | Yes (3) | This study | Clinical isolate (septic arthritis) |
| SD | Yes (1) | 6 | Heavily encapsulated (type 2) |
| SC | Yes (1) | 6 | Capsule-deficient derivative of SD |
| M | Yes (1) | 6 | Heavily encapsulated (type 1) |
| MV | Yes (1) | 18 | Capsule-deficient derivative of M |
| CYL5556 | Yes (1) | 24 | cap1 mutant of M |
| CYL6194 | Yes (1) | This study | cap8 mutant of UAMS-1 |
| CYL5972 | Yes (1) | This study | cap8 mutant of Becker |
| UAMS-2 | No | This study | Clinical isolate (draining sinus tract) |
| UAMS-603 | No | 34 | Clinical isolate (wound) |
| S6C | No | 9 | Hyperproducer of staphylococcal enterotoxin B |
| DB | No | 4 | Clinical isolate (blood) |
| 11D2 | No | 4 | sarA mutant derived from DB |
| ISP479C | No | 7 | Wild-type 8325-4 strain |
| ISP546 | No | 7 | agrA mutant derived from ISP479C |
| RN6390 | No | 23 | Wild-type 8325-4 strain |
| SarR | No | 5 | sarA mutant derived from RN6390 |
| RN6911 | No | 23 | agr-null mutant derived from RN6390 |
| UAMS-173 | No | This study | sar agr double mutant derived from RN6390 |
| UAMS-174 | Yes (3) | This study | RN6390 (geh::cna) |
| UAMS-175 | Yes (3) | This study | SarR (geh::cna) |
| UAMS-171 | Yes (3) | This study | agr-null mutant derived from UAMS-174 |
| UAMS-172 | Yes (3) | This study | agr-null mutant derived from UAMS-175 |
| UAMS-176 | Yes (3) | This study | RN6390 (pLI50::cna) |
| UAMS-177 | Yes (3) | This study | SarR (pLI50::cna) |
| UAMS-88 | No | This study | S. epidermidis control strain |
Capsule-deficient mutants of the cna-positive, serotype 8 strains Becker and UAMS-1 were generated by allele replacement mutagenesis. The DNA region encoding the cap8 gene cluster required for capsule biosynthesis has been cloned and sequenced (31). A derivative of Becker (CYL5972) with most of the cap8 gene cluster deleted was constructed by using the temperature-sensitive delivery vector pCL52.1 (19). Briefly, a 3.8-kb SalI fragment containing cap8A, cap8B′, and approximately 2.6 kb of the DNA upstream of the cap8A gene was ligated to a 4.4-kb SalI fragment containing cap8O, cap8P, and approximately 2.0 kb downstream of the cap8P gene. The ligated fragment was cloned into the multiple cloning site of pCL52.1 to form pCL7842. The two fragments were shown by restriction enzyme digestion to be oriented in the same manner as found in the wild-type chromosome (data not shown). pCL7842 was used to transform S. aureus RN4220 and was then transduced from RN4220 into Becker by using phage 52A (19). RN4220 transformants and Becker transductants were isolated at the permissive temperature of 30°C. After a shift to the nonpermissive temperature (43°C) and selection for episomal integrants, a mutant (CYL5972) in which the plasmid had excised from the chromosome, leaving the deleted version of the cap8 gene cluster, was isolated and verified as previously described (19).
To construct the UAMS-1 cap8 deletion mutant (CYL6194), plasmid pCL7960 was constructed by cloning three DNA fragments in tandem into the multiple cloning site of pCL52.1. The three fragments consisted of (i) a 3.2-kb EcoRI-SalI fragment containing cap8B, cap8C, cap8D, and cap8E′, (ii) a 1.7-kb Sau3A fragment containing the chloramphenicol resistance gene (cat) from pC194 (14), and (iii) a 4.4-kb SalI fragment containing cap8O, cap8P, and approximately 2.0 kb of DNA downstream from the cap8P gene. The three fragments were ligated in such a way that the cat gene was flanked by the two cap8 gene fragments in the same orientation found in the chromosome. The resulting plasmid (pCL7960) was transformed into RN4220 and then transduced into UAMS-1 as described above. After a shift to the nonpermissive temperature, derivatives of UAMS-1 containing cat were identified by plating on medium containing chloramphenicol. The fact that the intact cap8 gene cluster in one of these (CYL6194) was replaced by the cap8-cat construct was confirmed by Southern blotting (data not shown). The capsule-deficient phenotypes of CYL5972 and CYL6194 were confirmed by rocket immunoelectrophoresis using a type-specific, rabbit anti-type 8 antiserum (31).
For the isolation of RNA for Northern blot analysis and for the collagen binding assays (see below), all strains were grown without antibiotic selection. Growth rate was not affected by any of the mutations under these conditions. In all cases, the stability of each mutation in the absence of selection was confirmed by plating on both selective and nonselective media (data not shown). The only exceptions were the RN6390 and SarR strains carrying the pLI50::cna construct, both of which were grown in tryptic soy broth containing 5 μg of chloramphenicol per ml.
Northern blot analysis.
Total cellular RNA was isolated as previously described (8). Because all mutations are defined by stable, chromosomal insertions, and because antibiotic selection affects growth rate, all strains were grown in tryptic soy broth without antibiotic selection. The similarity in optical density of each culture at the time points when RNA samples were collected was confirmed by measuring the optical density (A560). Additionally, the stability of each mutation was confirmed by plating on selective and nonselective media at the conclusion of each experiment. RNA samples were denatured by using glyoxal and analyzed either by conventional Northern blotting (9) or by slot blotting. All gels were stained with ethidium bromide prior to transfer in order to confirm equal loads of RNA in each well. For slot blot analysis, RNA samples were vacuum drawn onto positively charged nylon membranes (Boehringer Mannheim, Indianapolis, Ind.), using a Bio-Dot SF slot blot apparatus (Bio-Rad Laboratories, Hercules, Calif.). After rinsing of each slot with Tris-EDTA buffer (0.1 M Tris-HCl [pH 8.0], 1 mM EDTA), the membrane was removed from the apparatus and the RNA was fixed to the membrane by using UV light (UV Crosslinker; Fisher Scientific, St. Louis, Mo.). The membrane was then processed according to our standard Northern blot protocol (8). Unless otherwise noted, 10 μg of total cellular RNA (based on A260) was loaded in each lane or slot. Northern blotting was done with a digoxigenin-dUTP-labeled cna probe as previously described (8).
Collagen binding assays.
Collagen binding assays were done with 125I-labeled collagen (types I and II; Sigma Chemical Co., St. Louis, Mo.) as previously described (8). Briefly, cells were harvested by centrifugation, washed with phosphate-buffered saline (PBS), and resuspended to an optical density (A560) of 1.0 in PBS containing 0.1% bovine serum albumin and 0.1% Tween 20 (binding buffer). A 1.0-ml aliquot of the standardized cell suspension was placed in a 1.5-ml microcentrifuge tube that had been precoated overnight at 4°C with PBS containing 2% bovine serum. After addition of 105 dpm of 125I-labeled collagen and incubation at room temperature with constant end-over-end mixing for 1 h, cells were pelleted and the bulk of the supernatant was removed. The tube was then centrifuged a second time, and the remaining supernatant was removed. The radioactivity retained in the pellet was measured in a gamma counter (8). Binding assays were done in duplicate, with the results reported as the average ± standard deviation.
Competition assays were done as described above except that unlabeled collagen, fibronectin, or fibrinogen was mixed with 125I-labeled type I collagen immediately before addition to the standardized cell suspension. The amount of unlabeled protein was calculated to be in 10-fold excess with respect to labeled collagen, based on the assumption that 100% of the input collagen was labeled and recovered in the appropriate column fractions.
RESULTS
CBC as a function of cna.
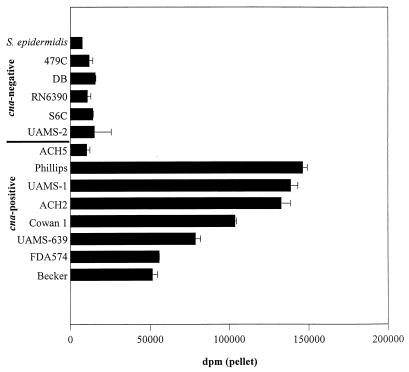
Collagen binding assays using 32 strains of S. aureus confirmed that strains that encode cna have a high CBC by comparison to cna-negative strains, all of which bound collagen at levels comparable to that of an S. epidermidis control (Fig. 1). In all cases, the results of our collagen binding assays were consistent with both type I (Fig. 1) and type II (Fig. 2) collagen. There were two exceptions to the correlation between cna and CBC, both of which involved cna-positive strains with a low CBC. The first was a single strain (ACH5) that had a CBC comparable to that of cna-negative strains (Fig. 1). Based on Northern blot analysis revealing the complete absence of cna mRNA (data not shown), the failure of ACH5 to bind collagen appears to arise from an as yet undefined transcriptional defect. The second exception involved the highly encapsulated strains Smith diffuse (SD) and M, both of which grow as mucoid colonies in vitro. As discussed below, the low CBC of SD and M appears to be due to masking of the collagen adhesin on the cell surface.
FIG. 1.
Type I collagen binding as a function of cna. The CBC of cna-negative and cna-positive strains (Table 1) was determined by using type I collagen. S. epidermidis was included as a negative control. CBC was determined in cells from cultures in mid-exponential growth (4 h). Results are reported as averages of two assays; error bars indicate standard deviations.
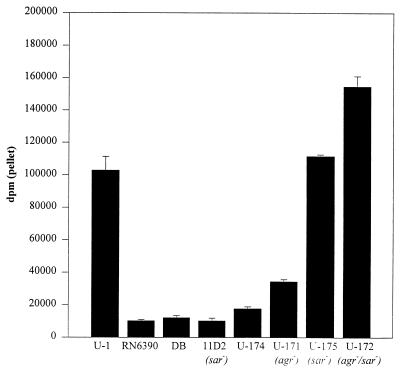
FIG. 2.
Type II collagen binding as a function of cna. CBC was determined by using type II collagen. Assays were done with cells from cultures in mid-exponential growth (4 h). Results are reported as averages of two assays; error bars indicate standard deviations. S. aureus strains and their relevant genotypes are indicated below the chart.
The CBC of cna-positive strains was competitively inhibited in the presence of unlabeled collagen but not in the presence of unlabeled fibrinogen or fibronectin (Fig. 3). In contrast, the CBC observed with cna-negative strains was not affected by the presence of any of the unlabeled proteins that we tested, including collagen (Fig. 3). These results indicate that the radioactivity observed in the pellet of cna-negative strains represents the background associated with our assay rather than the low-level binding of collagen by an adhesin present in strains that do not encode cna.
FIG. 3.
Competition assays. Collagen binding assays were done with type I collagen and cells from exponential-phase (4-h) cultures of UAMS-1 (cna positive) and RN6390 (cna negative). Assays were done with equal amounts of 125I-labeled collagen and an excess of unlabeled competitor protein. Results are reported as averages of two assays; error bars indicate standard deviations. None, no competitor protein; Cn, unlabeled collagen as competitor; Fb, unlabeled fibrinogen as competitor; Fn, unlabeled fibronectin as competitor.
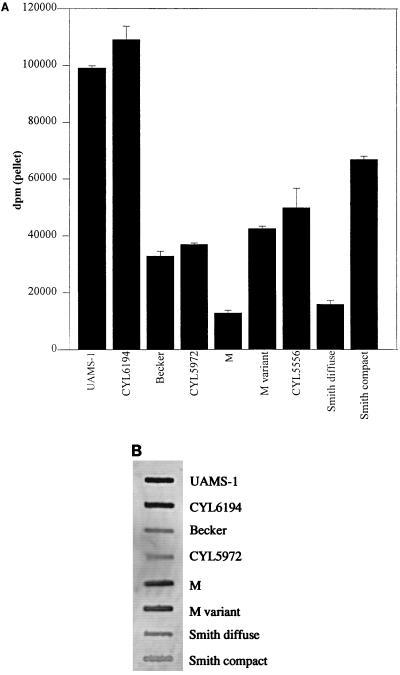
CBC as a function of capsule production.
The CBC of the SD and M strains was lower than that of other cna-positive strains (Fig. 4A) despite the fact that both strains encoded and expressed cna (Fig. 4B). To determine whether the low CBC was associated with the fact that these strains are heavily encapsulated, we compared the CBC of SD and M with that of capsule-deficient derivatives of each strain (Smith compact [SC] and M variant [MV], respectively). In both cases, the capsule-deficient strains had a CBC significantly higher than that of their parent strains (Fig. 4A). Although both SC and MV are spontaneous mutants that have not been characterized at the genetic level, the fact that the parent and mutant strains expressed similar levels of cna mRNA (Fig. 4B) indicates that the mutation responsible for the capsule-deficient phenotype of SC and MV did not affect cna transcription. Moreover, a subsequent experiment comparing the CBC of strain M and a derivative of M carrying a specific cap gene deletion (CYL5556) (24) confirmed that elimination of the capsule restored the ability to bind collagen (Fig. 4A). We conclude that the reduced CBC of SD and M is due to the presence of an extensive capsule that conceals the collagen adhesin and prevents its interaction with collagen. However, when we compared the CBC of UAMS-1 and Becker with that of cap mutants of each strain (CYL6194 and CYL5972, respectively), we observed only a slight increase in CBC with the capsule mutants (Fig. 4A). The relative amount of collagen binding in UAMS-1 and Becker and their capsule mutants did not change as a function of growth phase (i.e., the capsule mutants bound slightly more collagen when both exponential- and post-exponential-phase samples were examined) (data not shown). Because UAMS-1 and Becker are microencapsulated strains, these results suggest that any masking effect associated with the S. aureus capsule is limited to those strains that produce an abundance of capsular exopolysaccharide.
FIG. 4.
CBC as a function of capsule production. (A) CBC of two microencapsulated strains (UAMS-1 and Becker), two mucoid strains (SD and M), and their respective capsule-deficient mutants. CBC was determined by using type I collagen. (B) Northern slot blotting with a cna probe and total cellular RNA from exponentially growing cultures of the indicated strains.
CBC variation in cna-positive strains.
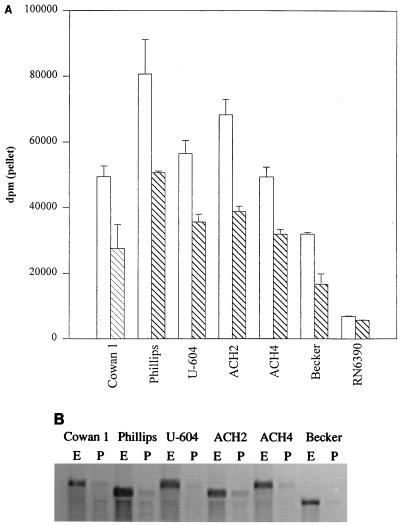
With the exceptions of ACH5, SD and M, all cna-positive strains had a CBC at least fivefold higher than that of any cna-negative strain (Fig. 1). However, we also observed a threefold difference in the CBC of cna-positive strains (Fig. 1). These differences were not correlated to structural variations in the cna gene itself. Specifically, our analysis included strains encoding cna variants with one (UAMS-1 and Becker), two (ACH2 and Phillips), three (Cowan, FDA574, ACH4, and UAMS-604), and four (UAMS-639) copies of the 561-bp B domain (26), and there was no correlation between the number of B domains and CBC (Fig. 1). Rather, the differences that we observed among cna-positive strains were correlated to differences in the level of cna transcription during exponential growth. For example, S. aureus Phillips had the highest CBC (Fig. 5A) and also appeared to have the highest level of cna mRNA (Fig. 5B). Conversely, Becker had the lowest CBC (Fig. 5A) and the lowest amount of cna mRNA (Fig. 5B). Strains that had a CBC between these extremes also appeared to express intermediate amounts of cna mRNA. These results suggest that with the exception of highly encapsulated strains of S. aureus, variations in CBC are a function of variations in cna transcription rather than structural differences within the collagen adhesin itself.
FIG. 5.
CBC as a function of cna transcription. (A) CBC was determined by using type I collagen and cells taken from cultures in exponential (open bars) and post-exponential (hatched bars) growth. RN6390 was included as a cna-negative control. Error bars indicate standard deviations based on two assays. (B) Northern blotting done with a cna probe and total cellular RNA from early-exponential (E)- and post-exponential (P)-phase cultures of the six cna-positive strains shown in panel A.
Temporal expression of cna.
We recently demonstrated that cna transcription in S. aureus was temporally regulated, with expression being highest in exponentially growing cultures and falling to almost undetectable levels as cultures entered the post-exponential growth phase (8). Although two of the three agr mutants examined in the previous study appeared to express slightly elevated levels of cna mRNA during the exponential growth phase, mutation of agr did not alter the temporal pattern of cna transcription (8).
The temporal regulation of cna transcription was confirmed in this study. Specifically, Northern blot analysis of RNA samples taken from each of six cna-positive strains revealed that the level of cna mRNA was highest during the exponential growth phase and then fell to almost undetectable levels (Fig. 5B). The CBC of all six cna-positive strains was also highest when cells taken from exponential-phase cultures were assayed (Fig. 5A). However, despite the drastic drop in the level of cna mRNA present in stationary-phase samples, the CBC of all cna-positive strains remained higher than that of cna-negative strains (Fig. 5A). These results are consistent with our previous conclusion that the S. aureus collagen adhesin is relatively stable (8). The observation that the CBC of cna-negative strains did not change as a function of growth phase (data not shown) provides further support for our conclusion that the radioactivity detected in the pellet of cna-negative strains represents the background associated with our assay rather than collagen binding associated with an alternative collagen-binding adhesin.
Regulation of cna transcription.
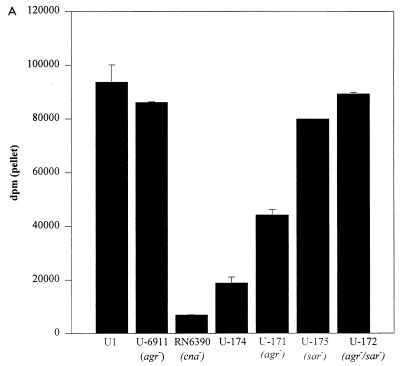
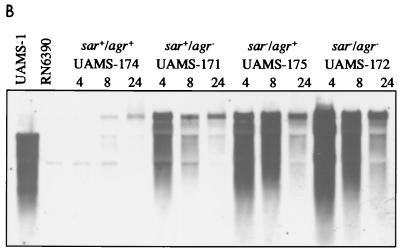
The previous suggestion that mutation of agr resulted in a slight increase in the CBC of some, but not all, S. aureus strains (8) was also confirmed during the course of this study. For instance, the CBC observed with a clinical osteomyelitis isolate (UAMS-1) was not significantly different from the CBC observed with an isogenic agr-null mutant (UAMS-6911) (Fig. 6A). However, we did observe an increase in the CBC of an agr-null mutant (UAMS-171) derived from UAMS-174 (Fig. 6A). Northern blot analysis confirmed that the increased CBC observed in UAMS-171 was a function of increased cna transcription (Fig. 6B). These results suggest that agr negatively regulates cna transcription during the exponential growth phase but that the regulatory influence of agr is strain dependent. Additionally, mutation of agr did not affect the temporal pattern of cna transcription (Fig. 6B).
FIG. 6.
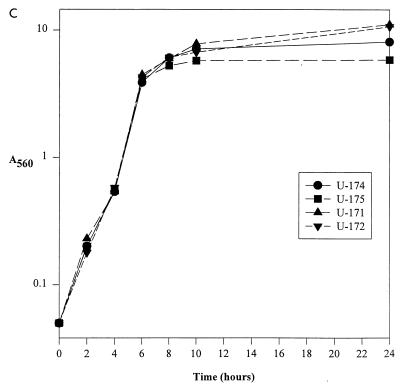
Effect of sar and agr mutation on cna transcription and CBC. (A) CBC of sar and agr mutants was determined by using type I collagen and cells taken from exponential-phase cultures. S. aureus strains and their relevant genotypes are indicated below the chart. RN6390 was included as a negative control. (B) Northern blotting done with a cna probe and 5 μg of total cellular RNA taken from cultures during exponential (4 h) and post-exponential (8 and 24 h) growth phases. S. aureus strains and their relevant genotypes are indicated above the appropriate lanes. Exponential-phase (4-h) samples from UAMS-1 and RN6390 were included as positive and negative controls, respectively. It should be noted that UAMS-1 encodes a cna gene with a single B domain, while the cna variant introduced into the RN6390 strains contains three B domains. (C) Growth curve for RN6390 agr and sar mutants in panels A and B.
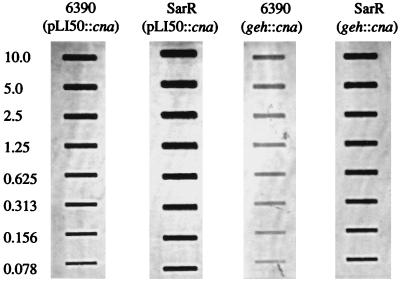
To determine whether mutation of the staphylococcal accessory regulator (sar) had any effect on the temporal pattern of cna transcription, we introduced cna into RN6390 and an isogenic sar mutant (SarR) both as part of a multicopy plasmid (pLI50::cna) and as a single-copy chromosomal insertion (geh::cna). In both the plasmid-borne and chromosomal variants, the cna-positive derivatives of SarR (UAMS-177 and UAMS-175, respectively) had an elevated CBC by comparison to the corresponding cna-positive derivatives of RN6390 (UAMS-176 and UAMS-174, respectively) (Fig. 7A). Importantly, the increased CBC observed in the UAMS-175 sar mutant (Fig. 7A) exceeded the increase observed in the UAMS-171 agr mutant (Fig. 6A). Northern blot analysis confirmed that the increased CBC observed in the sar mutants was due to an increase in the level of cna transcription (Fig. 7B). To quantitate this increase, we repeated the Northern blot analysis using twofold dilutions of total cellular RNA taken from exponential-phase cultures of the cna-positive derivatives of RN6390 and SarR (Fig. 8). By comparison to UAMS-176 (RN6390, pLI50::cna), UAMS-177 (SarR, pLI50::cna) appeared to express at least eightfold more cna mRNA (Fig. 8). The increase was even more apparent when UAMS-174 (RN6390, geh::cna) and UAMS-175 (SarR, geh::cna) were compared. Specifically, UAMS-175 appeared to express cna at a level at least 32-fold higher than that for UAMS-174 (Fig. 8). Although less apparent, the increase in cna transcription seen in UAMS-175 was also reflected in the CBC. Specifically, UAMS-177 had a CBC approximately twice as high as that of UAMS-176, while UAMS-175 had a CBC approximately fourfold higher than that of UAMS-174 (Fig. 7A).
FIG. 7.
Role of sar in cna transcription and CBC. (A) CBC of cna-positive variants of RN6390 and SarR. Plasmid (pLI50::cna) and chromosomal cna variants (geh::cna) are indicated below the chart. (B) Northern slot blotting using a cna probe and total cellular RNA from exponential-phase (4-h) cultures of the strains in panel A.
FIG. 8.
Quantitative analysis of cna transcription in sar mutants, using twofold serial dilutions of total cellular RNA from exponential-phase (4-h) cultures of cna-positive derivatives of RN6390 and SarR. RNA was analyzed by slot blotting using a probe specific for cna. Approximate amounts of RNA are indicated in micrograms at the left.
By comparison to UAMS-174, cna transcription in UAMS-175 not only was elevated during the exponential growth phase but also persisted at a relatively high level as cultures entered the post-exponential growth phase (Fig. 6B). These results suggest that the sar regulatory locus also contributes either directly or indirectly to the temporal regulation of cna transcription. The same temporal pattern of cna transcription was observed when we examined a sar agr double mutant (Fig. 6B). Additionally, the effect of the double mutation on cna transcription appeared to be additive, with the amount of cna mRNA in the sar agr double mutant being slightly higher than the amount observed in either the sar or the agr mutant strains (Fig. 6B). These results were also confirmed by our collagen binding assays. Specifically, the CBC observed during the exponential growth phase with the sar agr double mutant consistently exceeded that observed in the sar mutant in assays using both type I (Fig. 6A) and type II (Fig. 2) collagen. Interestingly, although the level of cna mRNA was increased during the post-exponential growth phase in both the sar mutant and the sar agr double mutant, the temporal pattern of collagen binding was unchanged compared to the wild-type strain. Specifically, the CBC of the sar mutants still peaked during the exponential growth phase and fell as cultures entered the post-exponential growth phase (Fig. 6A).
DISCUSSION
There is mounting evidence to suggest that S. aureus MSCRAMMs (adhesins) are important in the pathogenesis of various forms of staphylococcal disease. For example, the ability to bind fibronectin has been associated with the colonization of heart valves and endocarditis (17), while the production of a 19-kDa extracellular fibrinogen-binding protein has been associated with the colonization of wounds (25). The ability to bind fibronectin, fibrinogen, laminin, or thrombospondin has also been shown to promote the adherence of S. aureus to various biopolymers, including those used in the construction of indwelling medical devices (10, 11). Similarly, inactivation of the collagen adhesin gene (cna) has been correlated to reduced virulence in animal models of endocarditis and septic arthritis (12, 28). The latter is consistent with the observation that S. aureus isolates obtained from patients with septic arthritis and osteomyelitis generally exhibit an enhanced capacity to bind collagen (26). Moreover, we recently demonstrated that the collagen adhesin encoded by cna is present on the surface of S. aureus cells growing in bone (8). Taken together, these observations clearly suggest an important role for S. aureus adhesins in various forms of staphylococcal disease.
As part of our efforts to assess the role of adhesins in the pathobiology of S. aureus, we recently mapped each of the recognized adhesin genes within the S. aureus chromosome and assessed the prevalence of each gene among clinical isolates (33). The genes encoding the elastin-binding protein (ebpS), two fibrinogen-binding proteins (fib and clfA), and at least one of the two fibronectin-binding proteins (fnbA and/or fnbB) were present in every strain that we examined (n = 25). The map gene, which has been reported to encode an adhesin capable of mediating the low-level binding of a number of host proteins, was also present in all strains. In contrast, the cna collagen adhesin gene was present in only 10 of 25 strains (33).
The observation that cna is not present in most S. aureus strains is important because (i) cna is known to encode a high-affinity, collagen-specific adhesin (29, 30) and (ii) S. aureus strains isolated from patients with septic arthritis or osteomyelitis typically exhibit an enhanced capacity to bind collagen (2, 13). The fact that a relatively rare characteristic of the population is conserved among isolates that cause a specific form of staphylococcal disease suggests that the phenotype is associated with the pathogenesis of the disease. However, to date there has not been a comprehensive analysis establishing a direct correlation between the collagen adhesin encoded by cna and the ability to bind collagen. It therefore remains possible that cna-negative strains have some capacity to bind collagen as a function of either a broad-specificity adhesin (e.g., Map) or some as yet unrecognized adhesin. Indeed, Nilsson et al. (22) reported that mutation of sar in the cna-negative strain DB resulted in an enhanced capacity to bind type II collagen. Additionally, no binding was observed with type I collagen (22). Because DB does not encode cna, and because the collagen adhesin encoded by cna is known to bind both type I and type II collagen (26), these results suggest the existence of a second collagen adhesin that exhibits specificity for certain types of collagen.
We believe that the results presented here rule out the possibility that S. aureus encodes a second adhesin that makes a significant contribution to collagen binding by comparison to the adhesin encoded by cna. Specifically, our results for a number of cna-positive and cna-negative strains, using both type I and type II collagen, indicate that the high level of collagen binding in S. aureus is a direct function of the presence and expression of cna. We base that conclusion on the observations that (i) with only two exceptions, every cna-positive strain examined had a CBC at least fivefold higher than that of any cna-negative strain, (ii) the only exceptions to the correlation between cna and CBC can be explained either by the failure to transcribe cna or by the presence of an extensive capsule that masks the collagen adhesin itself (see below), (iii) in strains that were not heavily encapsulated and expressed cna, variations in CBC corresponded to the level of cna transcription, (iv) the alterations observed in the CBC of our sar and agr regulatory mutants can be explained by the effect of each mutation on cna transcription, and (v) none of the cna-negative strains examined bound collagen above the background level of our assay. The last is based on the observation that the amounts of radioactivity observed in pellets from all cna-negative strains were essentially identical, did not change as a function of growth phase, and were not reduced in the presence of other host proteins, including unlabeled collagen. In contrast, the binding of 125I-labeled collagen in cna-positive strains was specifically inhibited by the presence of unlabeled collagen. It should be emphasized that our studies included the DB and 11D2 strains examined by Nilsson et al. (22) and that we did not observe collagen binding above background levels in either strain, using either type I or type II collagen. Indeed, type I and type II collagen worked equally well in our binding assays, and we did not observe any strain-dependent distinctions in the ability to bind different collagen types. Although we cannot explain the difference between our results and those reported by Nilsson et al. (22), we believe that our results demonstrate that the high-level binding of collagen by S. aureus is not restricted to certain types of collagen. However, we cannot rule out the possibility that S. aureus encodes a second adhesin that mediates collagen binding in a type-specific manner at a level below the sensitivity of our assay.
The observation that the highly encapsulated strains SD and M had a CBC lower than that of their corresponding capsule-deficient derivatives suggests that the presence of an extensive capsule may mask the adhesin on the surface of the bacterial cell. A similar masking effect helps the bacterial cell avoid phagocytosis by preventing recognition of C3b deposited on the cell surface (3). It is possible that masking of the collagen adhesin also helps the bacterial cell avoid recognition by host immune system. However, the S. aureus collagen adhesin appears to contribute to the colonization of musculoskeletal tissues (28), which suggests that the inhibition of collagen binding associated with capsule production might inhibit the colonization of at least some tissues. Although not conclusive, that observation is consistent with our demonstration that S. aureus SD is not virulent in a rabbit osteomyelitis model despite the fact that it encodes and expresses cna and is highly virulent in a murine peritonitis model of staphylococcal disease (35). However, given the fact that capsule-deficient mutants of UAMS-1 and Becker did not have a CBC significantly higher than that their microencapsulated parent strains, the inhibitory effect of the capsule is apparently restricted to those strains that produce an abundance of capsular exopolysaccharide.
Our results also demonstrate that variations in CBC among cna-positive strains is associated with the level cna transcription and is independent of any functional difference associated with structural variations in the collagen adhesin. Specifically, our studies included strains that encode each of four cna variants that differ in the number (one, two, three, or four) of B domains (30). The B domain is a 561-bp region that is directly repeated in strains that have multiple domains. Other than the fact that it is not responsible for binding collagen (27), the function of the B domain is unknown. One intriguing possibility is that multiple B domains extend the ligand-binding A domain outward such that it is more exposed even in encapsulated strains. It should be noted that all of our capsule-deficient mutants were generated in strains that encode a cna gene with a single B domain. We are currently investigating the possibility that cna variants that have multiple B domains are less subject to the inhibitory effect of the capsule.
Finally, our results establish that the temporal pattern of cna transcription observed in our earlier experiments (8) is a consistent feature of all cna-positive strains. The temporal pattern observed with cna is characteristic of S. aureus surface proteins, most of which are regulated by the accessory gene regulator (agr) and/or the staphylococcal accessory regulator (sar). In a previous report, we demonstrated that mutation of agr had no effect on the temporal pattern of cna transcription (8). That finding was confirmed in this study. Our earlier study also suggested that mutation of agr resulted in an increase in cna transcription during the exponential growth phase in some, but not all, strains (8). The strain-dependent increase in the level of cna transcription during the exponential growth phase was also confirmed during the course of this study. Taken together, our results suggest that agr encodes or induces some factor that represses cna transcription during exponential growth but that the more complete repression observed as cultures enter the post-exponential growth phase requires an additional, agr-independent regulatory signal.
One possibility is the temporal signal described by Vandenesch et al. (37), who demonstrated that the induction of hla transcription requires induction of the agr-encoded RNA III effector molecule and an additional agr-independent regulatory signal that is induced during the transition between exponential and post-exponential growth. At present, the nature of this additional regulatory signal and its contribution to the regulation of S. aureus virulence factors other than hla are unknown. A second possibility is the staphylococcal accessory regulator (sar). To examine that possibility, we introduced cna into RN6390 and its otherwise isogenic SarR mutant. Our results indicate that mutation of sar results in a significant increase in the exponential-phase expression of cna and a delay in the post-exponential-phase repression of cna transcription. These results suggest that sar is the primary regulatory element controlling expression of cna. At present, we do not know whether the sar-mediated regulation of cna transcription is a function of a direct interaction between SarA and cis elements upstream of cna or involves an additional regulatory element that is not encoded within the sar locus.
Interestingly, mutation of both sar and agr had an additive effect on CBC by comparison to the corresponding sar and agr single mutants. One intriguing possibility that would explain these results is that the sensory component of the agr system somehow modulates a response regulator that is either encoded within sar or induced in response to some sar-encoded regulatory signal. Because mutation of the sensory component of two-component regulatory systems typically has a more moderate effect on phenotype than mutation of the response regulator (20), such as scenario would explain the moderate increase in CBC observed with an agr mutant and the more drastic increase in CBC observed with the sar mutant. However, mutation of agr has no effect on the production of any sar transcript (1). Moreover, the additive effect observed with sar agr double mutants would not be expected based on the scenario described above. In fact, an additive effect would not be expected in any scenario in which the sar and agr regulatory signals were interdependent. For that reason, we conclude that the regulatory effects exerted by the sar and agr loci on cna transcription are independent of each other.
Finally, although mutation of sar resulted in a delay in the post-exponential-phase repression of cna transcription, the delay did not have a significant impact on the post-exponential-phase CBC. Specifically, while collagen binding and cna transcription were highest during the exponential growth phase, the post-exponential-phase decline in CBC was apparent even in sar mutants. These results suggest that sar may have an impact on collagen binding at some posttranscriptional level. One possibility for the decrease in CBC despite the continued production of cna mRNA is that mutation of sar results in overproduction of proteases that degrade the collagen-binding adhesin. Indeed, mutation of sar is known to result in enhanced proteolytic activity (5). A second possibility is that the overproduction of the cna-encoded adhesin or some other surface protein affects the surface architecture of S. aureus in such a way that the adhesin cannot be appropriately localized to the cell surface. Indeed, the observation that the cna-encoded adhesin is anchored in the cell envelope in a manner that is distinct by comparison to other S. aureus surface proteins (32) suggests that the collagen adhesin may be differentially affected in strains carrying mutations that alter expression of multiple target genes. Although we cannot yet explain the discrepancy between cna transcription and CBC in post-exponential-phase cultures of sar mutants, it is important to note that the discrepancy involves the failure to bind collagen despite expression of cna rather than the ability to bind collagen in the absence of cna transcription. These results therefore do not detract from our conclusion that the collagen adhesin encoded by cna is the primary adhesin responsible for the ability of S. aureus to bind collagen.
ACKNOWLEDGMENTS
This work was supported by grants AI37729 (M.S.S.) and AI37027 (C.Y.L.) from the National Institute of Allergy and Infectious Disease and by grant 96-046 from the Orthopaedic Research and Education Foundation (M.S.S.). A.F.G. was supported in part by funds from the UAMS Graduate Student Research Fund.
We thank Joe Patti for his generous gift of CYL574.
REFERENCES
- 1.Bayer M G, Heinrichs J H, Cheung A L. The molecular architecture of the sar locus in Staphylococcus aureus. J Bacteriol. 1996;178:4563–4570. doi: 10.1128/jb.178.15.4563-4570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buxton T B, Rissing J P, Horner J A, Plowman K M, Scott D F, Sprinkle T J, Best G K. Binding of a Staphylococcus aureus bone pathogen to type I collagen. Microb Pathog. 1990;8:441–448. doi: 10.1016/0882-4010(90)90031-k. [DOI] [PubMed] [Google Scholar]
- 3.Cheung A L, Eberhardt K, Heinrichs J H. Regulation of protein A synthesis by the sar and agr loci of Staphylococcus aureus. Infect Immun. 1997;65:2243–2249. doi: 10.1128/iai.65.6.2243-2249.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung A L, Yeaman M R, Sullam P M, Witt M D, Bayer A S. Role of the sar locus of Staphylococcus aureus in induction of endocarditis in rabbits. Infect Immun. 1994;62:1719–1725. doi: 10.1128/iai.62.5.1719-1725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung A L, Ying P. Regulation of α- and β-hemolysins by the sar locus of Staphylococcus aureus. J Bacteriol. 1994;176:580–585. doi: 10.1128/jb.176.3.580-585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen G D, Baddour L M, Madison B M, Parisi J T, Abraham S N, Hasty D L, Lowrance J H, Josephs J A, Simpson A. Colonial morphology of staphylococci on Memphis agar: phase variation of slime production, resistance to β-lactam antibiotics, and virulence. J Infect Dis. 1990;161:1153–1169. doi: 10.1093/infdis/161.6.1153. [DOI] [PubMed] [Google Scholar]
- 7.Gillaspy A F, Hickmon S G, Skinner R A, Thomas J R, Nelson C L, Smeltzer M S. Role of the accessory gene regulator (agr) in the pathogenesis of staphylococcal osteomyelitis. Infect Immun. 1995;63:3373–3380. doi: 10.1128/iai.63.9.3373-3380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillaspy A F, Patti J M, Smeltzer M S. Transcriptional regulation of the Staphylococcus aureus collagen adhesin gene, cna. Infect Immun. 1997;65:1536–1540. doi: 10.1128/iai.65.4.1536-1540.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart M E, Smeltzer M S, Iandolo J J. The extracellular protein regulator (xpr) affects exoprotein and agr mRNA levels in Staphylococcus aureus. J Bacteriol. 1993;175:7875–7879. doi: 10.1128/jb.175.24.7875-7879.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrmann M, Suchard S J, Boxer L A, Waldvogel F A, Lew P D. Thrombospondin binds to Staphylococcus aureus and promotes staphylococcal adherence to surfaces. Infect Immun. 1991;59:279–288. doi: 10.1128/iai.59.1.279-288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrmann M, Vaudaux P E, Pittet D, Auckenthaler R A, Lew P D, Schmacher-Perdreau F, Peters G, Waldvogel F A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988;158:693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- 12.Hienz S A, Schennings T, Heimdahl A, Flock J I. Collagen binding of Staphylococcus aureus is a virulence factor in experimental endocarditis. J Infect Dis. 1996;174:83–88. doi: 10.1093/infdis/174.1.83. [DOI] [PubMed] [Google Scholar]
- 13.Holderbaum D, Spech T, Ehrhart L A, Keys T, Hall G S. Collagen binding in clinical isolates of Staphylococcus aureus. J Clin Microbiol. 1987;25:2258–2261. doi: 10.1128/jcm.25.12.2258-2261.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horinouchi S, Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982;150:815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonsson K, McDevitt D, McGavin M H, Patti J M, Höök M. Staphylococcus aureus expresses a major histocompatibility complex class II analog. J Biol Chem. 1995;270:21457–21460. doi: 10.1074/jbc.270.37.21457. [DOI] [PubMed] [Google Scholar]
- 16.Karakawa W W, Fournier J M, Vann W F, Arbeit R, Schneerson R S, Robbins J B. Method for the serological typing of the capsular polysaccharides of Staphylococcus aureus. J Clin Microbiol. 1985;22:445–447. doi: 10.1128/jcm.22.3.445-447.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuypers J M, Proctor R A. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect Immun. 1989;57:2306–2312. doi: 10.1128/iai.57.8.2306-2312.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee C Y. Cloning of genes affecting capsule expression in Staphylococcus aureus strain M. Mol Microbiol. 1992;6:1515–1522. doi: 10.1111/j.1365-2958.1992.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 19.Lin W S, Cunneen T, Lee C Y. Sequence analysis and molecular characterization of genes required for the biosynthesis of type I capsular polysaccharide in Staphylococcus aureus. J Bacteriol. 1994;176:7005–7016. doi: 10.1128/jb.176.22.7005-7016.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magasanik B. Historical perspective. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: ASM Press; 1995. pp. 1–5. [Google Scholar]
- 21.McGavin M H, Krajewska-Pietrasik D, Rydén C, Höök M. Identification of a Staphylococcus aureus extracellular matrix-binding protein with broad specificity. Infect Immun. 1993;61:2479–2485. doi: 10.1128/iai.61.6.2479-2485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson I, Bremell T, Rydén C, Cheung A L, Tarkowski A. Role of the staphylococcal accessory gene regulator (sar) in septic arthritis. Infect Immun. 1996;64:4438–4443. doi: 10.1128/iai.64.11.4438-4443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novick R P, Ross H F, Projan S J, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouyang S, Lee C Y. Transcriptional analysis of type 1 capsule genes in Staphylococcus aureus. Mol Microbiol. 1997;23:473–482. doi: 10.1046/j.1365-2958.1997.d01-1865.x. [DOI] [PubMed] [Google Scholar]
- 25.Palma M, Nozohoor S, Schennings T, Heimdahl A, Flock J-I. Lack of the extracellular 19-kilodalton fibrinogen-binding protein from Staphylococcus aureus decreases virulence in experimental wound infection. Infect Immun. 1996;64:5284–5289. doi: 10.1128/iai.64.12.5284-5289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patti J M, Allen B L, McGavin M J, Höök M. MSCRAMM-mediated adherence of microorganisms to host tissue. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 27.Patti J M, Boles J O, Höök M. Identification and biochemical characterization of the ligand binding domain of the collagen adhesin from Staphylococcus aureus. Biochemistry. 1993;32:11428–11435. doi: 10.1021/bi00093a021. [DOI] [PubMed] [Google Scholar]
- 28.Patti J M, Bremell T, Krajewska-Pietrasik D, Abdelnour A, Tarkowski A, Rydén C, Höök M. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect Immun. 1994;62:152–161. doi: 10.1128/iai.62.1.152-161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patti J M, House-Pompeo K, Boles J O, Garza N, Gurusiddappa S, Höök M. Critical residues in the ligand-binding site of the Staphylococcus aureus collagen-binding adhesin (MSCRAMM) J Biol Chem. 1995;270:12005–12011. doi: 10.1074/jbc.270.20.12005. [DOI] [PubMed] [Google Scholar]
- 30.Patti J M, Jonsson H, Guss B, Switalski L M, Wigberg K, Lindgerg M, Höök M. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J Biol Chem. 1992;267:4766–4772. [PubMed] [Google Scholar]
- 31.Sau S, Sun J W, Lee C Y. Molecular characterization and transcriptional analysis of type 8 capsule genes in Staphylococcus aureus. J Bacteriol. 1997;179:1614–1621. doi: 10.1128/jb.179.5.1614-1621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneewind O, Mihaylora-Petkov D, Model P. Cell wall sorting signals in surface proteins of Gram-positive bacteria. EMBO J. 1993;12:4803–4811. doi: 10.1002/j.1460-2075.1993.tb06169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smeltzer M S, Gillaspy A F, Pratt F L, Jr, Thames M D, Iandolo J J. Prevalence and chromosomal map location of Staphylococcus aureus adhesin genes. Gene. 1997;196:249–259. doi: 10.1016/s0378-1119(97)00237-0. [DOI] [PubMed] [Google Scholar]
- 34.Smeltzer M S, Pratt F L, Jr, Gillaspy A F, Young L A. Genomic fingerprinting for the epidemiological differentiation of Staphylococcus aureus clinical isolates. J Clin Microbiol. 1996;34:1364–1372. doi: 10.1128/jcm.34.6.1364-1372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smeltzer M S, Thomas J R, Hickmon S G, Skinner R A, Nelson C L, Griffith D L, Parr T R, Jr, Evans R P. Characterization of a rabbit model of staphylococcal osteomyelitis. J Ortho Res. 1997;15:414–421. doi: 10.1002/jor.1100150314. [DOI] [PubMed] [Google Scholar]
- 36.Switalski L M, Butcher W G, Patti J M, Speziale P, Gristina A G, Höök M. Collagen receptor of Staphylococcus aureus. In: Hook M, Switalski L, editors. Microbial adhesion and invasion. New York, N.Y: Springer-Verlag; 1992. pp. 101–114. [Google Scholar]
- 37.Vandenesch F, Kornblum J, Novick R P. A temporal signal, independent of agr, is required for hla but not spa transcription in Staphylococcus aureus. J Bacteriol. 1991;173:6313–6320. doi: 10.1128/jb.173.20.6313-6320.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]