Abstract
A 10-year-old castrated male domestic cat domiciled in eastern Victoria (Australia) was presented for a subcutaneous mass on its nasal bridge in November 2006. Cytological examination of an aspirate demonstrated pyogranulomatous inflammation. At surgery, the lesion consisted of an encapsulated mass containing viscid fluid. Histological examination of the resected lesion revealed pyogranulomatous inflammation surrounding a central zone of necrosis. Sections stained with the Ziehl–Neelsen method revealed numerous acid-fast bacilli, intracellularly within macrophages and extracellularly. Molecular studies established the infection was caused by Mycobacterium ulcerans. As histology demonstrated that the infection extended to the margin of the excised tissues, the cat was treated subsequently with clarithromycin (62.5 mg orally once daily for 7 days, then twice daily for 3 months). The surgical wound healed unremarkably. The infection has not recurred at the time of writing, 1 year following discontinuation of treatment. Although M ulcerans infections have been recorded in variety of mammals, this is the first known case in a cat.
Mycobacterium ulcerans is the causative agent of Bairnsdale/Buruli ulcer, a chronic localised infection of the skin and subcutis of human patients typically associated with (often extensive) necrotising skin ulcers with undermined edges (MacCallum et al 1948). Pre-ulcerative lesions such as small red papules or painless subcutaneous nodules have also been described (Uganda Buruli Group 1970).
M ulcerans is closely related to M marinum, the ‘fish tank or swimming pool bacillus’ (Iredell et al 1992), which also causes lesions in the skin and subcutis; such infections develop when contact with contaminated water or soil occurs in association with penetrating injury or maceration of the skin. The extensive tissue destruction characteristic of ulcerative M ulcerans infections is caused by an unusual polyketide-derived macrolide, called mycolactone, which possesses cytotoxic and immunosuppressive properties (George et al 1999). The genes encoding the mycolactone biosynthetic pathway are located on a 174 kb plasmid (Stinear et al 2004) which is likely to have been acquired by horizontal gene transfer during the evolution of the M marinum/M ulcerans complex (Yip et al 2007).
Whereas infections caused by M marinum occur in almost all geographic regions around the world, disease due to M ulcerans is restricted to certain highly localised geographical regions, presumably because the causal organism has a restricted environmental presence. The precise niche has yet to be determined, but inferences have been made from the structure of the M ulcerans genome (Stinear et al 2007). The disease is most prevalent in West Africa where it is referred to as Buruli ulcer (http://www.who.int/buruli/en/ accessed September 1, 2007), whereas in Australia it occurs mainly in regions of coastal Victoria and Queensland, where it is known as Bairnsdale and Daintree ulcer, respectively. The initial Victorian cases described in the 1940s were from the Bairnsdale region of East Gippsland (MacCallum et al 1948). However, since then the main human disease foci have been Phillip Island and the Mornington Peninsula in the 1990s and more recently (since 2001), the Bellarine Peninsula (Johnson et al 2007 a,b).
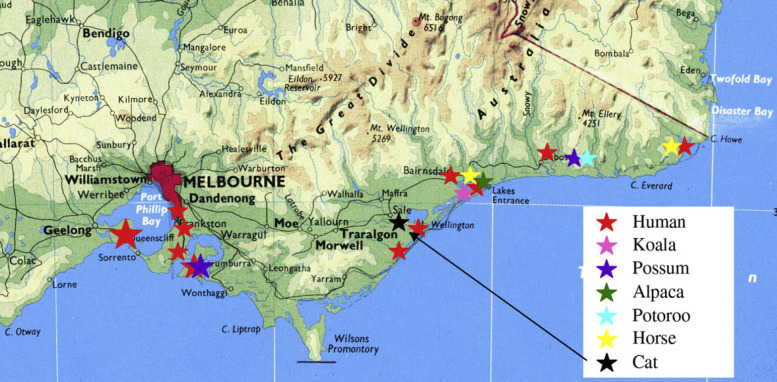
Non-human cases of M ulcerans infection have only been reported from Victoria, mainly in marsupial species such as koalas (Phascolarctos cinereus) (Mitchell et al 1987), common ringtail possums (Pseudocheirus peregrinus) (Portaels et al 2001) and a long-footed potoroo (Potorous longipes) (Fig 1). A single case has been described in an alpaca (Vicugna pacos) (Portaels et al 2001), and recently there have been two horses diagnosed with M ulcerans infections (van Zyl et al, personal communication). The geographical locations of confirmed M ulcerans infections in humans and animals in Victoria are shown in Fig 2. This report documents the first recorded feline M ulcerans infection.
Fig 1.

Ulcerative lesion on the tail of a long-footed potoroo (Potorous longipes), caused by M ulcerans.
Fig 2.
Location of this case in relation to other human and animal cases of M ulcerans infections in Victoria since the 1940s.
A 10-year-old castrated male, medium haired, domestic crossbred cat was presented for a swelling on the bridge of the nose. The patient was an indoor/outdoor cat domiciled in Longford, a small town near Sale in the Gippsland region of Victoria (Fig 2). The only significant physical finding was a firm non-pruritic subcutaneous swelling at the junction of the nasal planum and the bridge of the nose. The lesion was neither painful nor ulcerated. Fluid was obtained from the lesion by fine needle aspiration. A smear of this material stained with a rapid Romanowsky stain (DiffQuik; Lab Aids, Australia) demonstrated neutrophils, macrophages, giant cells and cellular debris. It was considered that the lesion was most likely inflammatory in nature, and possibly associated with saprophytic micro-organisms such as fungi, Mycobacterium or Nocardia species (Malik et al 2004).
In order to obtain further representative material for laboratory investigations, the lesion was explored surgically under general anaesthesia. The lesion took the form of an encapsulated mass and was marginally resected using sharp and blunt dissection. The incision was closed routinely using a single layer of interrupted synthetic polyamide sutures. The mass was later bisected; it comprised a thick capsule containing viscid fluid within a necrotic central portion. The tissue specimen was placed in buffered formol saline and immediately sent to the laboratory where all tissue was processed and embedded in paraffin, cut into 4 μm sections and stained with haematoxylin and eosin (H&E), periodic acid–Schiff (PAS) and subsequently using the Ziehl–Neelsen (ZN) and modified ZN methods.
Microscopically, there was an intradermal multi-nodular infiltrate consisting of epithelioid macrophages, plasma cells and neutrophils adjacent to a large central zone of necrosis. The inflammatory infiltrate extended to the margins of the submitted tissues. ZN and modified ZN stained sections revealed numerous acid-fast bacilli; most acid-fast bacilli were located within the area of central necrosis. However, occasional organisms were seen also within macrophages at the periphery of the lesion (Fig 3).
Fig 3.
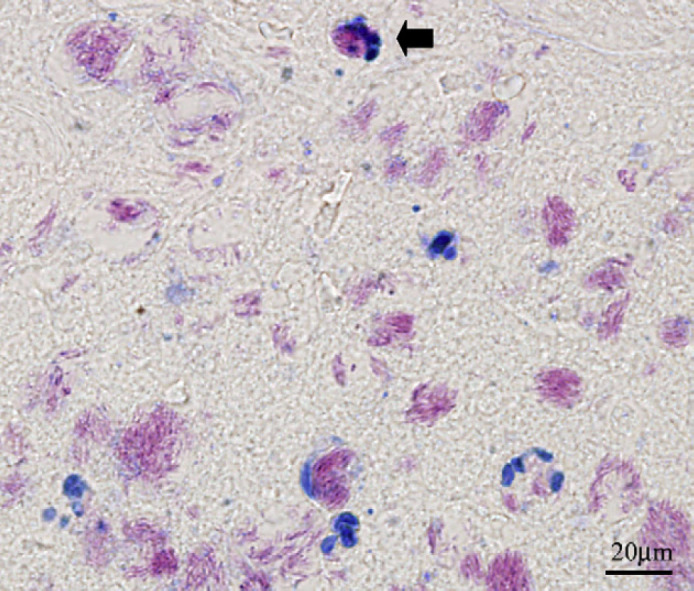
ZN stained section of the lesion from a cat with a localised M ulcerans infection. Note the acid-fast bacilli (which stain pink) intracellularly within macrophages (one is labelled with an arrow). Many of the extracellular collections are of a size and shape that indicate they are derived from phagosomes originally present in cells that have undergone lysis.
Ten, 10 μm sections were cut from the paraffin block using a fresh microtome blade and submitted to the Victorian Mycobacterium Reference Laboratory for polymerase chain reaction (PCR) studies. Unfortunately, no fresh material had been preserved for attempted culture and it was not possible to obtain further fresh material from the cat because the lesion had been completely excised.
A generic mycobacterium PCR targeting the 16s-23s internal transcribed spacer (ITS) region amplified a product with identical sequence to the ITS regions of both M marinum and M ulcerans. Subsequent testing in a real-time PCR assay (Fyfe et al 2007) targeting the M ulcerans-specific sequence, IS2404, indicated that M ulcerans (and not M marinum) DNA was present. Furthermore, molecular typing based on the amplification and size estimation of nine loci containing variable numbers of tandem repeats (VNTRs) (Ablordey et al 2005) confirmed that the strain shared the same profile as M ulcerans isolates causing disease in humans and animals in Victoria. A diagnosis of localised M ulcerans infection was, therefore, made.
To ensure that the infection did not recur at the site of excision, the cat was treated subsequently with a prolonged course of clarithromycin (Klacid; Abbotts, Australia, 62.5 mg once a day for 7 days, then twice daily). The incision healed by primary intention. Sutures were removed 10 days following surgery, and the cat completed a 3-month course of therapy. The cat tolerated the clarithromycin without adverse effects. The lesion went on to heal completely without scarring or disfigurement (Fig 4). At the time of writing (February 2008), there has been no recurrence of the lesion.
Fig 4.

The cat after successful surgical and medical therapy. It is just possible to discern the scar on the bridge of the cat's nose (small arrow).
This is the first report of M ulcerans causing disease in a cat. The lesion was located on a part of the head commonly subjected to cat scratch injuries (Malik et al 2004, 2005), and accordingly the initial assessment was that the cat had a localised infection with a saprophytic micro-organism associated with pyogranulomatous inflammation. The presence of acid-fast bacilli associated with caseous necrosis was initially most suggestive of an M lepraemurium infection (Pattyn and Portaels 1980, Malik et al 2002). However, PCR studies demonstrated that the infection was in fact referable to M ulcerans. Although few human M ulcerans infections have been known to occur in Longford or the nearby town of Sale several cases have occurred in coastal settlements 30 km to the east and to the south (Fig 2).
The rapid aetiological diagnosis was helpful in formulating the therapeutic regimen, for the antibiogram of this mycobacterium is relatively predictable, with susceptibility to clarithromycin and rifampicin. As the lesion had been largely removed surgically, it was decided to use clarithromycin monotherapy to prevent disease recurrence as this agent is generally well tolerated in companion animals (Gunn-Moore et al 1996, Malik et al 2001, 2002) and has less potential to produce adverse side effects (such as cutaneous erythema, hepatotoxicity and inappetence) than rifampicin. As in other mycobacterial infections of cats, including feline leprosy due to M lepraemurium (Malik et al 2002) and panniculitis due to rapidly growing mycobacteria (Malik et al 2000) ‘debulking’ lesions surgically is a very effective adjunct to medical therapy, especially in cases such as this cat in which it is straightforward to effectively remove virtually all of the infected tissues without recourse to reconstructive therapy. High levels of therapeutic agents are achieved at the well vascularised margins of the healing surgical incision, where residual organisms are likely to reside. In M ulcerans infections, en bloc resection of the lesion has the further advantage of removing the central area of necrotic debris harbouring the largest numbers of organism (Johnson et al 2007a).
The presence of a small focal lesion in the oculonasal region is most consistent with inoculation of M ulcerans organisms via a scratch injury (Malik et al 2004, 2005), with constraint of the lesion to the primary site of infection by a well-directed immune response (Bartralot et al 2000, Peduzzi et al 2007). The observation of intracellular M ulcerans organisms within macrophages at the periphery of the lesion is consistent with recent studies (Torrado et al 2007) suggesting that like M marinum, M ulcerans is readily phagocytosed by mammalian macrophages, and after an initial phase of intracellular growth, the mycobacteria induce host cell lysis through the production of mycolactone. The presence of M ulcerans within macrophages is not generally a feature of ulcerated lesions in humans, although in the initial paper (MacCallum et al 1948), intracellular organisms were described and well illustrated in rat macrophages.
It is conceivable that cats, as a species, may be inherently resistant to M ulcerans, despite being susceptible to infection by numerous other mycobacterial species (de Lisle et al 1990, Gunn-Moore et al 1996, Hughes et al 1997, Appleyard and Clark 2002). There have been no recorded feline infections referable to M marinum, the species most closely related to M ulcerans, despite its ubiquitous presence in watery environments.
Although we favour a cat scratch as the most likely portal of entry, a scratch injury from a native animal, such as a brush tailed possum, cannot be discounted. Alternatively, the cat may have been bitten by mosquitoes, which may act as passive mechanical vectors of M ulcerans in regions where the organisms are prevalent in the environment (Johnson et al 2007b). The location of the cat's lesion is consistent also with a mosquito bite.
An increase in human and animal infections with M ulcerans cases has been perceived since 2005, with new cases being observed in marsupial species, as well as species not previously known to be infected such as cats and horses (Fyfe J, unpublished observations). Over the same time period we have diagnosed over 10 new cases of a new fastidious mycobacterial infection in cats in rural Victoria, attributable to a novel species closely related to M simiae and the canine leproid granuloma organism (Malik et al 1998, Hughes et al 2000, Fyfe et al 2008). Our speculation is that conditions associated with the drought then affecting rural Victoria have somehow increased the likelihood of animals being infected by mycobacteria normally residing in certain environmental niches.
The value of PCR investigations such as this cannot be overemphasised. Without molecular diagnostics, this infection could easily have been attributed to M lepraemurium, considering the location of the infection, rural domicile and central necrosis within the lesion (Pattyn and Portaels 1980, Malik et al 2002). Knowing the specific microbial aetiology was helpful in formulating an antibiotic regimen and it also enabled us to appreciate that the presence of M ulcerans in the local environment may pose a public health risk to humans living nearby.
Acknowledgement
Richard Malik is supported by the Valentine Charlton Bequest of the Post Graduate Foundation of Veterinary Science of the University of Sydney.
References
- Ablordey A., Swings J., Hubans C., Chemlal K., Locht C., Portaels F., Supply P. Multilocus variable-number tandem repeat typing of Mycobacterium ulcerans, Journal of Clinical Microbiology 43, 2005, 1546–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard G.D., Clark E.G. Histologic and genotypic characterization of a novel Mycobacterium species found in three cats, Journal of Clinical Microbiology 40, 2002, 2425–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartralot R., Pujol R.M., Patos V. Garcia, Sitjas D., Martin-Casabona N., Coll P., Alomar A., Castells A. Cutaneous infections due to nontuberculous mycobacteria: histopathological review of 28 cases. Comparative study between lesions observed in immunosupressed patients and normal hosts, Journal of Cutaneous Pathology 27, 2000, 124–129. [DOI] [PubMed] [Google Scholar]
- Fyfe J.A.M., Lavender C.J., Johnson P.D.R., Globan M., Sievers A., Azuolas J., Stinear T.P. Development and application of two multiplex real-time PCR assays for the detection of Mycobacterium ulcerans in clinical and environmental samples, Applied and Environmental Microbiology 73, 2007, 4733–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J.A., McCowan C., O'Brien C.R., Globan M., Birch C., Revill P., Barrs V.R.D., Wayne J., Hughes M.S., Holloway S., Malik R. Molecular characterization of a novel fastidious mycobacterium causing lepromatous lesions of the skin, subcutis, cornea, and conjunctiva of cats living in Victoria, Australia, Journal of Clinical Microbiology 46, 2008, 618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George K.M., Chatterjee D., Gunawardana G., Welty D., Hayman J., Lee R., Small P.L. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence, Science 283, 1999, 854–857. [DOI] [PubMed] [Google Scholar]
- Gunn-Moore D.A., Jenkins P.A., Lucke V.M. Feline tuberculosis: a literature review and discussion of 19 cases caused by an unusual mycobacterial variant, Veterinary Record 138, 1996, 53–58. [DOI] [PubMed] [Google Scholar]
- Hughes M.S., Ball N.W., Beck L.-A., de Lisle G.W., Skuce R.A., Neill S.D. Determination of the etiology of presumptive feline leprosy by 16S rRNA gene analysis, Journal of Clinical Microbiology 35, 1997, 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M.S., James J.G., Ball N., Scally M., Malik R., Wigney D., Martin P., Chen S., Mitchell D., Love D.N. Identification by 16S rRNA gene analyses of a potential novel mycobacterial species as an etiological agent of canine leproid granuloma syndrome, Journal of Clinical Microbiology 38, 2000, 953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iredell J., Whitby M., Blacklock Z. Mycobacterium marinum infection: epidemiology and presentation in Queensland 1971–1990, Medical Journal of Australia 157, 1992, 596–598. [DOI] [PubMed] [Google Scholar]
- Johnson P.D.R., Hayman J.A., Quek T.Y., Fyfe J., Jenkin G.A., Buntine J.A., Athan E., Birrell M., Johnstone B., Graham J., Lavender C.J. Consensus recommendations for the diagnosis, treatment and control of Mycobacterium ulcerans infection (Bairnsdale or Buruli ulcer) in Victoria, Australia, Medical Journal of Australia 186, 2007a, 64–68. [DOI] [PubMed] [Google Scholar]
- Johnson P.D.R., Azuolas J., Lavender C.J., Wishart E., Stinear T.P., Hayman J.A., Brown L., Jenkin G.A., Fyfe J.A.M. Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia, Emerging Infectious Diseases 13, 2007, 1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lisle G.W., Collins D.M., Loveday A.S., Young W.A., Julian A.F. A report of tuberculosis in cats in New Zealand, and the examination of strains of Mycobacterium bovis by DNA restriction endonuclease analysis, New Zealand Veterinary Journal 38, 1990, 10–13. [DOI] [PubMed] [Google Scholar]
- MacCallum P., Tolhurst J.C., Buckle G., Sissons H.A. A new mycobacterial infection in man, Journal of Pathology and Bacteriology 60, 1948, 93–122. [PubMed] [Google Scholar]
- Malik R., Love D.N., Wigney D.I., Martin P. Mycobacterial nodular granulomas affecting the subcutis and skin of dogs (canine leproid granuloma syndrome), Australian Veterinary Journal 76, 1998, 403–407. [DOI] [PubMed] [Google Scholar]
- Malik R., Wigney D.I., Dawson D., Martin P., Hunt G.B., Love D.N. Infection of the subcutis and skin of cats with rapidly growing mycobacteria: a review of microbiological and clinical findings, Journal of Feline Medicine and Surgery 2, 2000, 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R., Martin P., Wigney D.I., Swan D., Slatter P.S., Cibilic D., Allen J., Mitchell D.H., Chen S.C., Hughes M.S., Love D.N. Treatment of canine leproid granuloma syndrome: preliminary findings in seven dogs, Australian Veterinary Journal 79, 2001, 30–36. [DOI] [PubMed] [Google Scholar]
- Malik R., Hughes M.S., James G., Martin P., Wigney D.I., Canfield P.J., Chen S.C.A., Mitchell D.H., Love D.N. Feline leprosy: two different clinical syndromes, Journal of Feline Medicine and Surgery 4, 2002, 43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R., Vogelnest L., O'Brien C.R., White J., Hawke C., Wigney D.I., Martin P., Norris J.M. Infections and some other conditions affecting the skin and subcutis of the naso-ocular region of cats-clinical experience 1987–2003, Journal of Feline Medicine and Surgery 6, 2004, 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R., Norris J., White J., Jantulik B. ‘Wound cat’, Journal of Feline Medicine and Surgery 8, 2005, 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P.J., McOrist S., Bilney R. Epidemiology of Mycobacterium ulcerans infection in koalas (Phascolarctos cinereus) on Raymond Island, southeastern Australia, Journal of Wildlife Diseases 23, 1987, 386–390. [DOI] [PubMed] [Google Scholar]
- Pattyn S.R., Portaels F. In vitro cultivation and characterization of Mycobacterium lepraemurium, International Journal of Leprosy 48, 1980, 7–14. [PubMed] [Google Scholar]
- Peduzzi E., Groeper E., Schutte D., Zajac P., Rondini S., Mensch-Ouainoo E., Spagnoli G.C., Pluschke G., Daubenberger C.A. Local activation of the innate immune system in Buruli ulcer lesions, Journal of Investigative Dermatology 127, 2007, 638–645. [DOI] [PubMed] [Google Scholar]
- Portaels F., Chemlal K., Elsen P., Johnson P.D., Hayman J.A., Hibble J., Kirkwood R., Meyers W.M. Mycobacterium ulcerans in wild animals, Revue Scientifique et Technique 20, 2001, 252–264. [DOI] [PubMed] [Google Scholar]
- Stinear T.P., Mve-Obiang A., Small P.L., Frigui W., Pryor M.J., Brosch R., Jenkinn G.A., Johnson P.D., Davies J.K., Lee R.E., Adusumilli S., Garnier T., Haydock S.F., Leadlay P.F., Cole S.T. Giant plasmid-encoded polyketide synthases produce the macrolide toxin of Mycobacterium ulcerans, Proceedings of the National Academy of Sciences of the United States of America 101, 2004, 1345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear T.P., Seemann T., Pidot S., Frigui W., Reysset G., Garnier T., Meurice G., Simon D., Bouchier C., Ma L., Tichit M., Porter J.L., Ryan J., Johnson P.D., Davies J.K., Jenkin G.A., Small P.L., Jones L.M., Tekaia F., Laval F., Daffé M., Parkhill J., Cole S.T. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer, Genome Research 17, 2007, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrado E., Fraga A.G., Castro A.G., Stragier P., Meyers W.M., Portaels F., Silva M.T., Pedrosa J. Evidence for an intramacrophage growth phase of Mycobacterium ulcerans, Infection and Immunity 75, 2007, 977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uganda Buruli Group. Clinical features and treatment of pre-ulcerative Buruli lesions (Mycobacterium ulcerans infection). Report II of the Uganda Buruli Group, British Medical Journal 2, 1970, 390–393. [PMC free article] [PubMed] [Google Scholar]
- Yip M.J., Porter J.L., Fyfe J.A., Lavender C.J., Portaels F., Rhodes M., Kator H., Colorni A., Jenkin G.A., Stinear T. Evolution of Mycobacterium ulcerans and other mycolactone-producing mycobacteria from a common Mycobacterium marinum progenitor, Journal of Bacteriology 189, 2007, 2021–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]