Abstract
A 12-year-old cat was presented for investigation of weight loss and inappetence. Radiography and conventional grey-scale ultrasonography showed a large mid-body splenic mass. Contrast enhanced ultrasonography of the liver demonstrated a hypoechoic left lateral lobe nodular mass during the peak and late portal-phases of liver enhancement. Histopathology of the splenic mass and hepatic nodular mass confirmed haemangiosarcoma. The use of ultrasound microbubble contrast media in the diagnosis of hepatic metastasis in the cat has not been previously reported in the cat.
A 12-year-old male neutered domestic shorthair cat presented following a 2–4 week history of gradual weight loss and inappetence. At the time of initial clinical examination, the cat weighed 5.45 kg and was considered in good body condition. Clinical examination revealed an irregular, approximately 70 mm diameter, mid-abdominal mass. The remainder of the clinical examination was unremarkable. A mature neutrophilia (38.5×109/μl normal ranges 2.5–13.0/μl) and a mild elevation in cholesterol (4.2 mmol/l, normal ranges 1.9–3.9) were identified on routine haematology and serum biochemistry, respectively. Screening tests for feline immunodeficiency and feline leukaemia virus were negative.
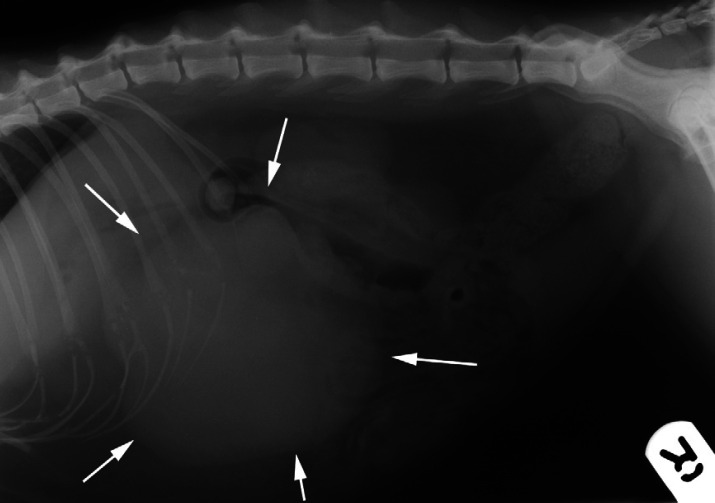
Abdominal radiography revealed a large, 90-mm diameter, irregular, circumscribed soft-tissue mass in the cranio-ventral abdomen (Fig 1). The mass displaced the pylorus cranially and the small intestines, transverse colon and kidneys caudally. A mild bronchial pattern and incidental ventral spondylosis were evident on thoracic radiographs. On abdominal ultrasonography (GE Vingmed Sound System Five, Horten, Norway) (Fig 2) the mass was localised to the spleen and its appearance was complex with numerous irregular internal cavitations. Doppler imaging revealed a highly vascularised mass. No other abnormalities were identified ultrasonographically within the remaining abdominal structures.
Fig 1.
Right lateral recumbent abdominal radiograph. There is a large irregularly marginated soft-tissue mass (white arrows) in the cranio-ventral abdomen displacing the small intestine and transverse colon caudally.
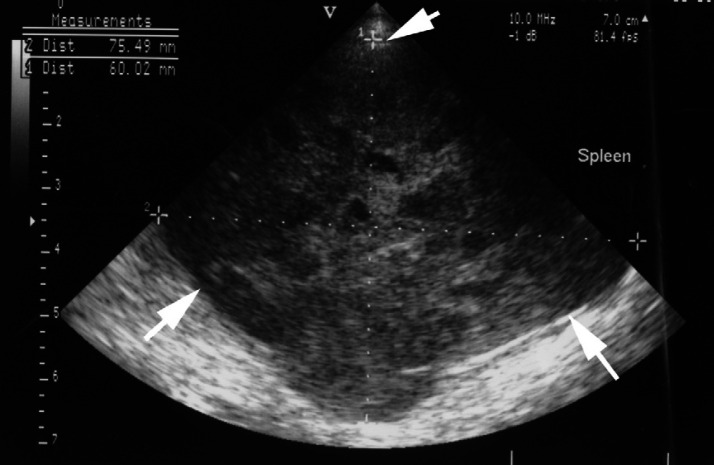
Fig 2.
Ultrasonographic image of the splenic mass. The mass (white arrows) has a complex appearance with numerous small cavities distributed throughout the abnormal parenchyma.
The differential diagnoses for the splenic mass included neoplasia (lymphoma, mast cell tumour, visceral haemangiosarcoma, haemangioma), atypical nodular hyperplasia, abscess, granuloma and haematoma.
Because of the potential for metastatic disease associated with the presence of a large cavitated splenic mass, tissue contrast enhanced ultrasonography was performed before surgery using an ultrasound system and 5 MHz curvilinear transducer capable of contrast enhanced imaging (Philips HDI 5000, ATL, Bothell, USA) to identify potential hepatic metastases not identified with conventional grey-scale ultrasonography.
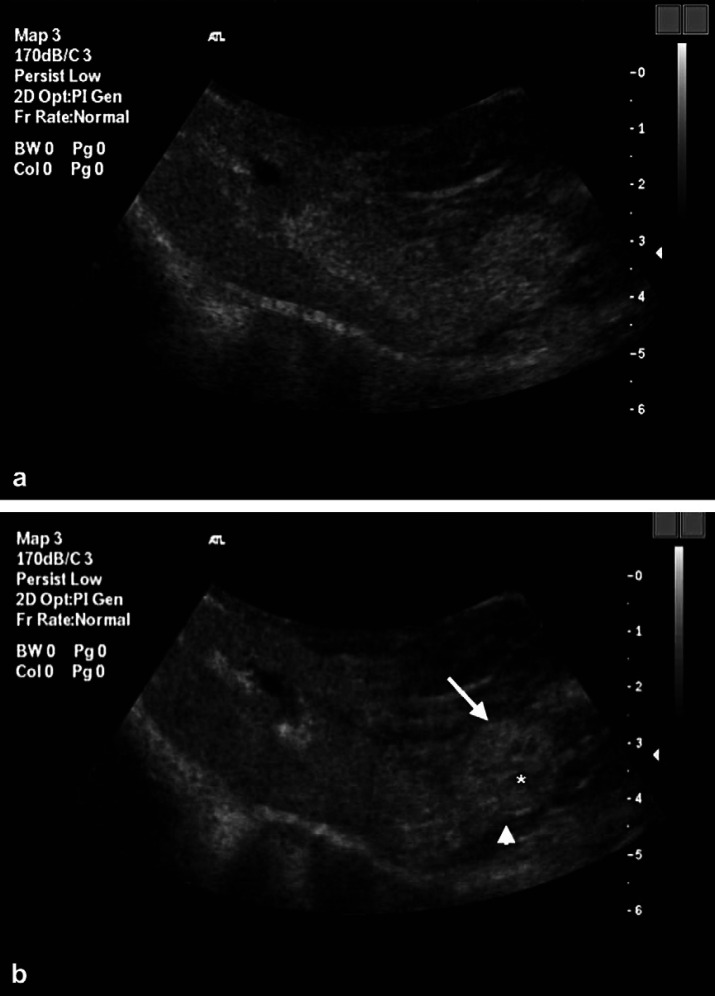
Under general anaesthesia contrast enhanced ultrasonography was performed using a continuous low acoustic power (mechanical index [MI]=0.1) pulsed inversion imaging technique. Two studies, each using 1.0 ml of a reconstituted microbubble contrast agent (Sonovue; Bracco International, The Netherlands) were performed. The contrast agent was injected intravenously through a 22 gauge cephalic catheter followed by 1.0 ml of saline flush. The liver was evaluated in the transverse plane from 10 s prior to, and until 3-min post-contrast administration on both studies. The period between the two studies was greater than 10 min. During the initial contrast examination the ultrasound beam was fanned through the liver to ensure that all areas were examined. At peak enhancement (26 s) the liver demonstrated homogeneous contrast uptake except for a single irregular hypoechoic nodular mass of 10 mm diameter extending from the caudodorsal aspect of one of the left liver lobes (Fig 3a). A second injection of contrast was performed to evaluate the nodule during all phases of the contrast study as the arrival of contrast during the early, arterial phase of contrast arrival was not evaluated during the initial injection. During this early period of liver enhancement the nodule demonstrated little or poor central enhancement, a rim of peripheral enhancing tissue and a prominent marginal feeder vessel (Fig 3b). The splenic mass itself was not imaged during the initial 3 min following contrast administration, hence only delayed images were obtained and these demonstrated extensive irregular unenhanced areas partially divided by enhanced splenic parenchyma. Re-evaluation of the liver with conventional grey-scale ultrasonography after the contrast study revealed a subtle, pedunculated isoechoic to hypoechoic nodular mass on the caudodorsal margin of one of the left liver lobes, in the location consistent with the lesion identified during contrast enhanced ultrasonography (Fig 4).
Fig 3.
(a) Transverse ultrasonographic image of the liver at peak enhancement (26 s post-contrast injection) demonstrating diffuse enhancement of the hepatic parenchyma including a 15-mm diameter pedunculated nodule at the caudal aspect of one of the left liver lobes. The periphery of the nodule has a similar echogenicity to adjacent enhanced parenchyma but centrally there is a hypoechoic poorly enhancing region. (b) Second injection of contrast media. Transverse ultrasonographic image of the liver 12 s post-injection showing arterial and early portal venous phases of contrast enhancement. The hepatic nodule shows a prominent peripheral feeder vessel (arrowhead) and peripheral contrast uptake within the nodule (arrow) and a central non-enhancing area (asterisk).
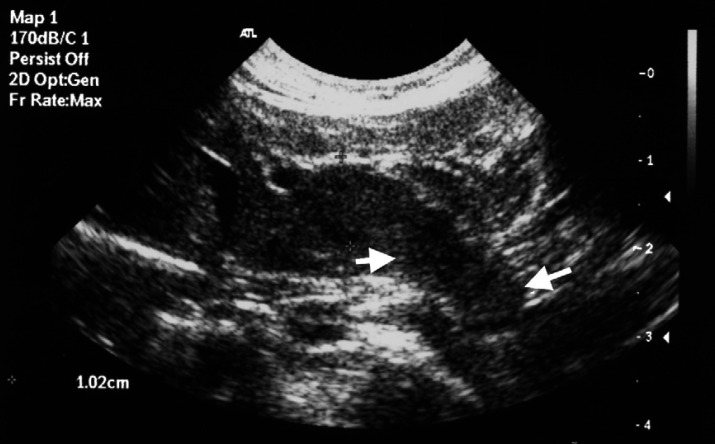
Fig 4.
Oblique conventional grey-scale ultrasonographic image of the liver obtained after the contrast examination demonstrating a poorly defined pedunculated nodular lesion (arrows) extending from the caudal aspect of one of the left liver lobes.
Although the features of poor early enhancement and rapid contrast wash out of the nodular liver mass identified on ultrasonography were considered suggestive of metastatic disease, and a poor prognostic indicator, the owner elected surgical exploration to establish a histopathological diagnosis. A pre-surgical coagulation profile was within normal limits. A mid-line laparotomy was performed 10 days after the initial examination. A large nodular, mid-body, splenic mass was identified. A small 10-mm diameter pedunculated nodule attached to the left lateral lobe of the liver was visualised and removed. The cat recovered uneventfully.
Histopathology of the splenic mass and hepatic nodule were typical of a haemangiosarcoma with extensive areas of spindle cell proliferation, haemorrhage and necrosis as well as extensive areas of irregular blood space formation. Histopathology of the hepatic nodule showed a zone of transition from normal liver to solid neoplastic tissue to irregular areas of neoplastic tissue with foci of necrosis and blood spaces. In this case, primary splenic haemangiosarcoma with hepatic metastasis was considered more likely due to the size of the splenic lesion.
The owner elected not to pursue chemotherapy due to the poor prognosis associated with feline visceral haemangiosarcoma and the lack of clinical data to support the use of chemotherapeutic protocols in cats. The cat re-presented 3 months after surgery with anorexia and marked weight loss. Clinical examination revealed very thin body condition and multiple, firm, non-mobile masses ranging from 30 to 50 mm diameter within the cranial abdomen. Abdominal ultrasonography was repeated revealing multiple hypoechoic to isoechoic nodules within the liver parenchyma causing bulging and deformation of the capsule. The cat underwent euthanasia. A post-mortem examination was not permitted. Metastatic hepatic haemangiosarcoma was considered the most likely cause of the nodular hepatic masses.
The ability to detect metastatic disease by ultrasonography is based on lesion size, vascularity, echogenicity, border definition, operator skill, and the contrast resolution of the ultrasound machine (Feeney et al 1984, Nyman et al 2004). In particular, the accurate detection and characterisation of lesions with acoustic properties similar to those of the surrounding normal parenchyma (ie, isoechoic lesions) is a significant limitation of grey-scale B-mode ultrasonography (Nyman et al 2004). Conventional ultrasonography is also relatively insensitive at discriminating between benign and malignant lesions in the liver (Nyland et al 2002, Ziegler et al 2003). The use of ultrasonographic contrast media to assist in the characterisation of benign and malignant hepatic lesions in man and the dog has been reported (Blomley et al 2001, Bryant et al 2004, Quaia et al 2004, O'Brien 2007). Ultrasound contrast media have also been employed to increase the detection rate of primary and metastatic focal hepatic lesions (Harvey et al 2000, Albrecht et al 2001). To the authors' knowledge, there are no reports of the use of contrast ultrasonography in the detection of hepatic metastases in the cat.
Ultrasonographic contrast media are blood pool and/or tissue-specific agents and consist of membrane-stabilised microbubbles containing gas or air that scatter the ultrasound energy in all directions, increasing the intensity of the backscattered ultrasound waves (Szatmari et al 2003, Nyman et al 2004). The marked non-linear increase in acoustic backscatter produced by ultrasound contrast agents at their harmonic frequency compared to tissue permits increased tissue contrast and can improve the visualisation of lesions (Nyman et al 2004). Tumour identification is improved with contrast media because of increased differences in echogenicity between normal and diseased areas of the hepatic parenchyma (Nyman et al 2004, 2005). Contrast specific protocols and transducers are required to detect the harmonic frequencies generated by ultrasound contrast media and produce images. Sonovue, the ultrasound contrast agent employed in this case, is a second-generation ultrasound contrast agent, available in Europe, composed of sulphur hexafluoride-filled microbubbles in a phospholipid sphere. The microbubble stability and size uniformity of this agent at low acoustic pressures limit destruction of the microbubbles preserving the agent in circulation for several minutes and allowing real-time assessment of hepatic perfusion. Although primarily a blood pool agent, Sonovue is also reported to be slowed or delayed within the hepatic sinusoids producing a late hepatic parenchymal phase in humans and the dog (Ohlerth and O'Brien 2007). As a result early (arterial), portal venous (peak phase of enhancement) and late phases of contrast enhancement are recognised. Malignant lesions are usually best visualised at peak enhancement (O'Brien et al 2004, Ohlerth and O'Brien 2007).
In the dog, contrast ultrasonography has been shown to assist in the differentiation of malignant hepatic nodules from benign lesions (O'Brien et al 2004, O'Brien 2007). In one study of 32 dogs (O'Brien et al 2004) the use of contrast ultrasound resulted in increased conspicuity of malignant hepatic nodules. Canine malignant hepatic masses appeared hypoechoic compared with the surrounding liver at peak normal liver enhancement and, with the exception of a hepatoma, all benign nodules appeared isoechoic to the surrounding liver at peak enhancement (O'Brien et al 2004). The hypoechoic appearance of malignant nodules during peak enhancement is assumed to be due to a relative lack of portal vessels and sinusoids (O'Brien 2007). Hence, metastatic liver nodules have been described as filling defects in the otherwise uniformly enhanced normal liver at peak enhancement. During the initial arterial phase the appearance of malignant lesions is variable and both hyperintense (hypervascular) and hypointense (hypo- or avascular) malignancies have been reported (O'Brien 2007). An early hyperintense (hypervascular) appearance in malignant lesions is presumed to result from induction of primarily hepatic arterial neovascularisation. In two previous studies, however (O'Brien et al 2004, O'Brien 2007), the appearance of metastatic haemangiosarcoma following ultrasound contrast media in the dog has been reported to be avascular (non-enhancing) or poorly enhancing (hypovascular) during both the arterial and peak phases of enhancement. The cause of this hypovascularity during all phases of contrast perfusion is not known. Although characteristic of haemangiosarcoma, this pattern has also been reported in metastatic spindle cell sarcoma, metastatic carcinoma and metastatic neuroendocrine neoplasia in the dog (O'Brien et al 2004). In this report although the centre of the metastatic lesion appeared to enhance poorly throughout perfusion there was conspicuous enhancement of the periphery of the nodule during both arterial and peak enhancement phases of the study. The solid neoplastic tissue surrounding the central necrosis identified histopathologically was presumed to be responsible for the peripheral enhancement. An additional finding of the nodular mass in this cat was the presence of a conspicuous peripheral feeder vessel. This feature of small but distinct vessels at the periphery of non-enhancing metastatic haemangiosarcoma nodules has also been reported in the dog (O'Brien 2007).
In addition to the characterisation of hepatic lesions, contrast ultrasound has been shown to increase the detection rate of focal liver lesions. In a study of three dogs the use of contrast ultrasound enabled the detection of metastatic haemangiosarcoma nodules in the liver not visualised with grey-scale ultrasonography (O'Brien 2007). Similarly in the cat in this report, metastatic disease within the abdomen, including the liver was not recognised on conventional grey-scale ultrasonography. Contrast ultrasonography was performed allowing identification of a previously undetected hepatic metastatic lesion. The failure to detect this lesion on grey-scale ultrasonography was due to its peripheral location, pedunculated structure and isoechoic appearance to normal liver.
Although contrast enhanced ultrasonography established the presence of a second, probable metastatic mass lesion, the owners chose to pursue exploratory surgery as histopathology was required in order to obtain a definitive diagnosis. While needle aspiration cytology of suspicious masses is simple and cost effective, it is of low diagnostic value where haemangiosarcoma is suspected, thus exploratory surgery remains the primary method of diagnosis and treatment for almost all dogs and cats with haemangiosarcoma (O'Keefe and Couto 1987, Thamm 2007). The prognosis for visceral haemangiosarcoma in the cat is poor (Chun 1999, Thamm 2007). The surgical planning was altered by the identification of the hepatic nodule as the surgeon had more information regarding the potential extent of the disease in this patient.
This case demonstrates that ultrasonography combined with contrast media may have a role in the diagnosis and staging of feline cancer patients with suspected hepatic metastatic disease. The evaluation of contrast media in the microcirculation of a focal liver lesion has the potential to improve the ability of ultrasonography to identify and characterise liver lesions whilst retaining the non-invasive properties of this imaging modality (Nyman et al 2005).
Although historically contrast enhanced imaging capabilities require dedicated transducers and software generally available only on high-end ultrasound machines, these machines are becoming more widely available and affordable. In clinical practice, ultrasonography is often more accessible than magnetic resonance imaging or computed tomography. Thus, contrast ultrasonography may lead to improved sensitivity and diagnosis of liver metastasis in cats in clinical practice. In the United Kingdom, Sonovue is available commercially at £39.90 per vial direct from the manufacturer, making it a relatively cost effective and rapid additional diagnostic test that may be performed in conscious, sedated or anaesthetised cats. Dose rates for the use of Sonovue in dogs have been described ranging from 0.5 to 1.2 ml (Ziegler et al 2003, O'Brien et al 2004, Nyman et al 2005). When reconstituted, the 5-ml microbubble solution is stable for up to 6 h, allowing multiple examinations per vial. Sonovue is not licensed for veterinary use in Europe or USA at this time. Although this report is limited to a single patient, the findings suggest that a prospective study examining the use of ultrasound contrast media in hepatic metastases is needed to establish the validity of the technique in the cat.
Acknowledgements
The authors would like to thank Robert C. Gumbrell BVSc, Dip Microbiol, MRCVS, MV, Dr Martin Havlicek MACVSc and Jonathan D. Wray BVSc, DSAM, MRCVS for their assistance.
References
- Albrecht T., Hoffmann C.W., Schmitz S.A., Schettler S., Overberg A., Germer C.T., Wolf K.J. Phase-inversion sonography during the liver-specific late phase of contrast enhancement: improved detection of liver metastases, American Journal of Roentgenology 176 (5), 2001, 1191–1198. [DOI] [PubMed] [Google Scholar]
- Blomley M.J., Sidhu P.S., Cosgrove D.O., Albrecht T., Harvey C.J., Heckemann R.A., Butler-Barnes J., Eckersley R.J., Basilico R. Do different types of liver lesions differ in their uptake of the microbubble contrast agent SH U 508A in the late liver phase? Early experience, Radiology 220 (3), 2001, 661–667. [DOI] [PubMed] [Google Scholar]
- Bryant T.H., Blomley M.J., Albrecht T., Sidhu P.S., Leen E.L., Basilico R., Pilcher J.M., Bushby L.H., Hoffmann C.W., Harvey C.J., Lynch M., MacQuarrie J., Paul D., Cosgrove D.O. Improved characterization of liver lesions with liver-phase uptake of liver-specific microbubbles: prospective multicenter study, Radiology 232 (3), 2004, 799–809. [DOI] [PubMed] [Google Scholar]
- Chun R. Feline and canine hemangiosarcoma, Compendium on Continuing Education for the Practicing Veterinarian 21 (7), 1999, 622–629. [Google Scholar]
- Feeney D.A., Johnston G.R., Hardy R.M. Two-dimensional, gray-scale ultrasonography for assessment of hepatic and splenic neoplasia in the dog and cat, Journal of the American Veterinary Medical Association 184 (1), 1984, 68–81. [PubMed] [Google Scholar]
- Harvey C.J., Blomley M.J., Eckersley R.J., Cosgrove D.O., Patel N., Heckemann R.A., Butler-Barnes J. Hepatic malignancies: improved detection with pulse-inversion US in late phase of enhancement with SH U 508A – early experience, Radiology 216 (3), 2000, 903–908. [DOI] [PubMed] [Google Scholar]
- Nyland T.G., Mattoon J.S., Herrgesell E.R., Wisner E.R. Liver. Nyland T.G., Mattoon J.S. Small Animal Diagnostic Ultrasound, 2002, Saunders: Philidelphia. [Google Scholar]
- Nyman H.T., Kristensen A.T., Flagstad A., McEvoy F.J. A review of the sonographic assessment of tumor metastases in liver and superficial lymph nodes, Veterinary Radiology and Ultrasound 45 (5), 2004, 438–448. [DOI] [PubMed] [Google Scholar]
- Nyman H.T., Kristensen A.T., Kjelgaard-Hansen M., McEvoy F.J. Contrast-enhanced ultrasonography in normal canine liver. Evaluation of imaging and safety parameters, Veterinary Radiology and Ultrasound 46 (3), 2005, 243–250. [DOI] [PubMed] [Google Scholar]
- O'Brien R.T. Improved detection of metastatic hepatic hemangiosarcoma nodules with contrast ultrasound in three dogs, Veterinary Radiology and Ultrasound 48 (2), 2007, 146–148. [DOI] [PubMed] [Google Scholar]
- O'Brien R.T., Iani M., Matheson J., Delaney F., Young K. Contrast harmonic ultrasound of spontaneous liver nodules in 32 dogs, Veterinary Radiology and Ultrasound 45 (6), 2004, 547–553. [DOI] [PubMed] [Google Scholar]
- Ohlerth S., O'Brien Contrast ultrasound: general principles and veterinary clinical applications, Veterinary Journal 174 (3), 2007, 501–512. [DOI] [PubMed] [Google Scholar]
- O'Keefe D.A., Couto C.G. Fine-needle aspiration of the spleen as an aid in the diagnosis of splenomegaly, Journal of Veterinary Internal Medicine 1 (3), 1987, 102–109. [DOI] [PubMed] [Google Scholar]
- Quaia E., Calliada F., Bertolotto M., Rossi S., Garioni L., Rosa L., Pozzi-Mucelli R. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence, Radiology 232 (2), 2004, 420–430. [DOI] [PubMed] [Google Scholar]
- Szatmari V., Harkanyi Z., Voros K. A review of nonconventional ultrasound techniques and contrast-enhanced ultrasonography of noncardiac canine disorders, Veterinary Radiology and Ultrasound 44 (4), 2003, 380–391. [DOI] [PubMed] [Google Scholar]
- Thamm D.H. Miscellaneous tumours. Withrow S.J., Vail D.M. Withrow & MacEwen's Small Animal Clinical Oncology, 2007, Saunders Elsevier: St. Louis, Missouri. [Google Scholar]
- Ziegler L.E., O'Brien R.T., Waller K.R., Zagzebski J.A. Quantitative contrast harmonic ultrasound imaging of normal canine liver, Veterinary Radiology and Ultrasound 44 (4), 2003, 451–454. [DOI] [PubMed] [Google Scholar]