Abstract
This study was conducted to characterize the gross and cytological characteristics of secretions from normal feline anal sacs. Thirty cats with no recent history of anal-sac disease were selected consecutively and their anal sacs manually expressed. The color, cell counts, or presence of solid portions of the secretions in the youngest cats (<1 year old) were not significantly different from the rest. Young cats (<1 year old) tended to more often have watery secretions. The secretions contained 13 (median) epithelial cells per 600× magnification microscopic field, basophilic background debris, and a mixture of Gram-positive cocci (83 per field; median), Gram-negative cocci (38 per field; median), and Gram-negative rods (1.8 per field; median). Most secretions had occasional neutrophils, and a minority of cats had occasional yeasts in their secretion. Erythrocytes were found only rarely. Extreme heterogeneity existed in gross color, consistency, and amount of solid material. Hence, these latter parameters would not be valid indicators of anal-sac disease.
Anal-sac disorders are uncommon in cats (Sherding 1994, Scott et al 2001, Scarff 2003, Zoran 2005, Norsworthy 2006, Sherding 2006). Feline anal-sac disorders are classified as impaction, inflammation, infection, abscess, and neoplasia (Sherding 1994, Scott et al 2001, Scarff 2003, Norsworthy 2006, Parry 2006).
Anal-sac secretion is composed of desquamated keratinocytes, material from sebaceous and apocrine glands, proteins, lipids, glycogen, and acid mucopolysaccharides. Melanin, bacteria, and yeasts are also present (Greer and Calhoun 1966, Scott et al 2001, Scarff 2003, Shabadash and Zelikina 2005). The bacteria and yeasts cultured from diseased anal sacs are the same as those cultured from normal sacs: staphylococci, streptococci, micrococci, Clostridium species, Bacillus species, Escherichia coli, Proteus species, and Malassezia (Scott et al 2001, Scarff 2003).
Cytological examination of stained specimens is of diagnostic value in dermatological and otic disorders of cats (Scott et al 2001). Cytological examination also has been recommended for the diagnosis of anal-sac diseases (Scarff 2003, Zoran 2005), but the cytological findings from anal sacs in either normal cats or cats with anal-sac diseases have not been reported.
The diagnosis of anal-sac disease via cytology requires knowledge of the cytology of the normal anal-sac secretion (Lake et al 2004). To the authors' knowledge, the cytological characteristics of normal feline anal-sac secretions have not been reported.
Our purposes were to (i) examine the cytology of clinically normal feline anal-sac secretions and to quantify the numbers of organisms and cells present, and (ii) determine whether there is an association between age or gross color/consistency of a secretion and the number of cells/organisms.
Materials and Methods
Thirty cats with no known history of anal-sac disease were selected from cats admitted to the Cornell University Hospital for Animals (CUHA) through the healthy-pet clinic or the spay-neuter program conducted with regional animal shelters. Fifteen of the selected animals were client-owned with no history of anal-sac disease: scooting, dragging, or rubbing their hind end on the ground; licking or chewing their anal or tail-base regions; tenesmus; or lesions of self-induced hypotrichosis or pyotraumatic dermatitis in the tail-base or perineal regions. Fifteen of the cats in the study were admitted by animal shelters in Cortland County and Tompkins County and had no recent history of the signs listed above, nor did they exhibit those signs during their stay at the CUHA. Client-owned cats ranged in age from 2 to 15 years. Although the exact age of the shelter cats was usually unknown, they were estimated to be between 5 months and 1 year old (based upon dentition, hair coat, and overall body condition). Nine cats were estimated to be <1 year old. Twenty-five of the cats were female and five were male. The gonads of 17 of the cats were intact at the time of sampling. Most cats were domestic shorthairs (n=27), but there were three purebreds: Siamese (two) and Persian (one).
All sampling occurred during the spring (March, April, and May) of 2007 as eligible cats were admitted to the CUHA; we sampled the first 30 cats to which we were allowed access. For each cat in the study, both anal sacs were simultaneously expressed by external compression between the thumb and the forefinger. This resulted in the secretions of both anal sacs being combined into one specimen. There was no visual difference in the secretions of the two anal sacs for any of the cats. Immediately after expression, each secretion was examined grossly and its color and consistency were recorded.
Following gross characterization, a portion of each cat's secretion was rolled onto glass slides using a sterile cotton swab. Two slides were made for each cat. One slide was stained with a modified Wright's stain (Diff-Quik, Baxter Healthcare Corporation, McGraw Park, IL, USA), and one slide was Gram-stained. After staining, the Diff-Quik (DQ) and Gram-stain (GS) slides were dipped in xylene and mounted with a cover slip using Permount (Fischer Scientific, Pittsburgh, PA, USA).
Each DQ slide initially was scanned at low-power (10× magnification microscopic field) to select an appropriate region for the counting of epithelial cells, yeasts, and leukocytes. Although some heterogeneity did exist on each slide, efforts were made to select 10 oil-immersion fields (OIFs; 600× magnification) that were representative of the entire slide. The number of basal epithelial cells, parabasal squamous epithelial cells, corneocytes, yeasts, neutrophils, and mononuclear leukocytes were counted in each of the 10 OIFs. Basal epithelial cells were defined as round epithelial cells with basophilic cytoplasm and distinct nuclei. Parabasal squamous epithelial cells were angular and had pale cytoplasm and distinct nuclei. Corneocytes were angular and had pale cytoplasm and no nuclei.
The bacterial flora contained in the anal-sac samples was evaluated using the Gram-stained slides. Each slide was scanned at 10× magnification to check for quality of staining and homogeneity. Bacteria were counted at 600× magnification and placed into one of four categories: Gram-positive cocci, Gram-negative cocci, Gram-positive rods, and Gram-negative rods. A single bacterial counting unit was defined as follows: a single coccus, diploid cocci, a chain of cocci, a single rod or a chain of rods.
For each cell type and cat's secretion, the median count across 10 OIFs was calculated; the median then represented that cat's secretion for analyses. Counts for all the cytological variables were not Gaussian in distribution, making it necessary to use non-parametric statistical tests to evaluate relationships between them. Two-sided Wilcoxon's rank-sum tests were performed to determine the presence of age-associated differences in anal-sac cell and bacterial counts of adult cats versus cats <1 year old. Fisher's exact tests were used to compare cases <1 year old to all other cats regarding viscosity and the presence of solid portions (grossly visible as multiple ‘grains’ or ‘chunks’) of the secretion.
Two-sided Wilcoxon's rank-sum tests were used to evaluate the relationship between gross viscosity and the cell counts for each type of cell and microorganism. Spearman's rank correlation was used to test for association between (i) nucleated and non-nucleated epithelial cells; (ii) neutrophils and different types of epithelial cells; (iii) bacterial counts and epithelial cells, yeasts, and neutrophils; and (iv) the gross characteristic ‘solid portion’ (ordinal data) and epithelial cells, yeasts, neutrophils and bacteria.
The commercial software for all analyses was Statistix 8.0 (© 2003; Analytic Software, Tallahassee, FL, USA). Statistical significance for all tests was defined as P≤0.05 (two-sided); within logical sets of tests, Bonferroni adjustments were imposed (to account for multiple comparisons).
Results
Gross characteristics of the anal-sac secretions are presented in Table 1. The color and consistency of anal-sac secretion from normal cats were highly variable. Most (seven of nine) cats <1 year old had watery secretions, whereas only six of 21 adult cats had watery secretions (P=0.02).
Table 1.
Gross characteristics of normal feline anal-sac secretions (30 cats)
| Characteristic | Number of cats |
|---|---|
| Color | |
| Brown | 8 |
| Yellow-brown | 5 |
| Tan | 5 |
| Gray | 4 |
| White | 4 |
| Light yellow | 2 |
| Orange | 2 |
| Consistency | |
| Thick | 9 |
| Creamy | 8 |
| Watery | 13 |
| Solid portion (‘chunks’) | 19 |
Microscopic examination revealed a large amount of amorphous, basophilic material in all specimens. There was no evidence of cats <1 year old having different cell counts than adult cats; all P-values were ≥0.08 in Fisher's exact tests (data not shown).
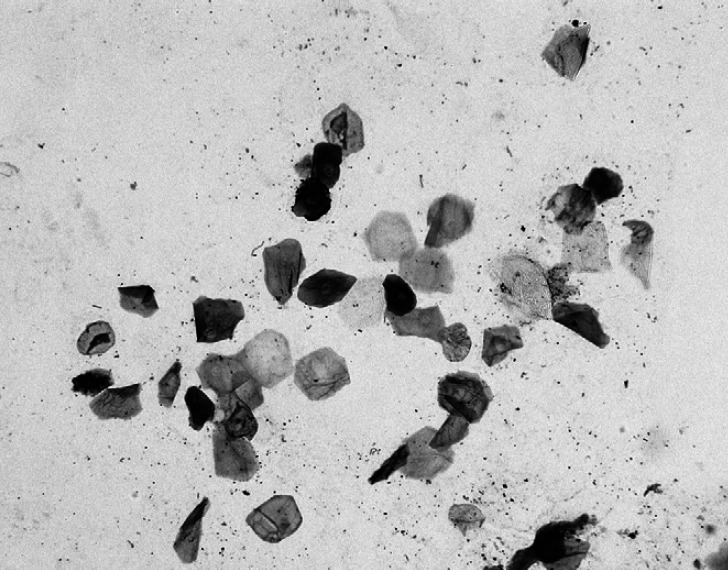
The cellularity data are presented in Table 2. Epithelial cells were the most commonly observed non-microbial cells (Fig 1). Basal epithelial cells, parabasal epithelial cells, and corneocytes were present at median counts per OIF of 0.8, 2.5, and 4.0, respectively. Yeasts were seen in only seven (23%) of the specimens. Small numbers (≤11 per OIF) of neutrophils and mononuclear leukocytes were seen in 19 (63%) and 15 (50%), respectively, of the specimens. The nuclei were degenerate, often pyknotic, but no intracytoplasmic microorganisms were seen. Erythrocytes were very rare and identified in only one (3%) of the specimens.
Table 2.
Cells per oil-immersion field of normal cat anal-sac secretions
| Cell type | Minimum | Q1 | Q2 | Q3 | Maximum |
|---|---|---|---|---|---|
| Basal epithelial cells | 0 | 0 | 0.8 | 3.5 | 93 |
| Parabasal epithelial cells | 0 | 1 | 2.5 | 8.1 | 15.5 |
| Corneocytes | 0 | 2.5 | 4.0 | 12 | 45 |
| Yeasts | 0 | 0 | 0 | 0 | 7.0 |
| Neutrophils | 0 | 0 | 0 | 0 | 6.5 |
| Mononuclear leukocytes | 0 | 0 | 0 | 0 | 1.5 |
| Gram-positive cocci | 2.5 | 23 | 83 | 168 | 200 |
| Gram-positive rods | 0 | 0 | 0.2 | 6.1 | 172 |
| Gram-negative cocci | 0 | 5.2 | 38 | 116 | 176 |
| Gram-negative rods | 0 | 0 | 1.8 | 13 | 76 |
Each specimen had 10 fields examined, and the median of the 10 fields was ‘the measurement’ for the specimen.
Q1, Q2, Q3, the three quartiles (Q2=median).
Fig 1.
Cytology of the normal feline anal-sac secretion illustrating clumped and single epithelial cells (DQ; 400× original).
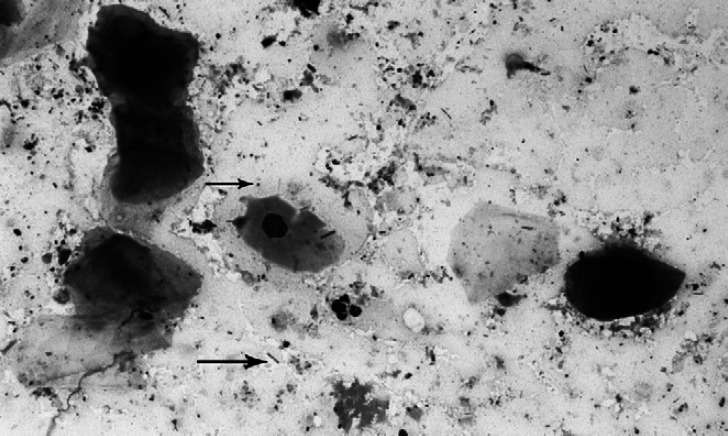
A comparison of the consistency (watery versus creamy/thick) of anal-sac secretions and their cellular composition revealed that parabasal epithelial cells were in significantly lower numbers in watery secretions (P=0.015) than in thick or creamy secretions. Bacterial analyses revealed: (i) a negative correlation between median Gram-negative cocci and Gram-positive cocci counts (r=0.69, P<0.0001); (ii) a positive rank correlation between median Gram-negative rod and Gram-negative cocci counts (r=0.50, P=0.006). The sum of each cat's median bacteria per OIF across all four categories of bacteria ranged from 50 to 203 bacteria per OIF; the median was 198. Sixty-three percent of the samples were dominated by Gram-positive cocci (Fig 2), and 30% by Gram-negative cocci. The remaining 7% of the samples contained predominantly rods (Gram-negative or Gram-positive) or mixed populations with no dominant microorganism.
Fig 2.
Cytology of the normal feline anal-sac secretion illustrating Gram-positive cocci (top arrow) and Gram-negative rods (bottom arrow) (Gram-stain; 600× original).
Discussion
The diagnosis of feline anal-sac diseases has been based upon assumptions made about the physical characteristics of normal anal-sac secretions (Sherding 1994, Scott et al 2001, Scarff 2003, Norsworthy 2006). Literature describes the pathophysiology, diagnosis, and therapy of anal-sac disease without defining the normal character of feline anal-sac secretion (Sherding 1994, Scott et al 2001, Scarff 2003, Zoran 2005, Norsworthy 2006, Sherding 2006). This study is the first numerical quantification of the gross and cytological characteristics of the clinically normal feline anal-sac secretion.
There is great variability in the gross characteristics of the normal feline anal-sac secretion. At least seven anal-sac secretion color varieties were identified in this study: brown, yellow-brown, tan, gray, white, light yellow, and orange. There is also variability in the consistency (thick, creamy, and watery) and in the presence or absence of solid material (‘chunks’) among the secretions from normal feline anal sacs. Hence, the color, consistency, and presence or absence of solid material are not reliable indicators of feline anal-sac disease. This is identical to what has been reported for dogs (Lake et al 2004).
Microscopic examination of the normal feline anal-sac secretion reveals a large amount of amorphous, basophilic background debris. This has also been reported for dogs (Lake et al 2004). Epithelial cells are commonly found in normal feline anal-sac secretions, with a median count per OIF of 0.8, 2.5, and 4.0, respectively, for basal epithelial cells, parabasal cells, and corneocytes. Erythrocytes are rarely seen in normal feline anal-sac secretion and their presence may indicate a disease state.
Sixty-three percent of the cats had small numbers (1–11 per OIF) of neutrophils. Although these neutrophils had degenerate-to-pyknotic nuclei, intracytoplasmic bacteria were not identified. As in other secretions, neutrophils containing phagocytosed microorganisms would represent compelling evidence of infection. Small numbers of mononuclear leukocytes (1–4 per OIF) were found in 50% of the specimens.
Yeasts were rare in any single OIF. However, we found at least one yeast in at least one OIF in 23% of our cats. Therefore, the presence of yeasts by itself does not determine the presence or absence of anal-sac disease.
Bacteria, mainly Gram-positive cocci (63%) or Gram-negative cocci (30%) were the most common bacteria identified. Gram-negative or Gram-positive rods were also seen, but were rarely the dominant bacterial population. None of the samples had a median>203 bacteria per OIF. Cultures were not performed on the anal-sac secretions in our study, so it is not possible to confirm or deny the presence of specific bacterial species in our samples. In general, significant relationships were not identified between color, consistency, and any of the cellular variables. An exception was the negative association between numbers of parabasal epithelial cells and watery secretions.
In summary, the normal feline anal-sac secretion is highly variable in gross appearance (color and consistency) and its cytological composition between different cats. The ‘typical’ normal feline anal-sac secretion contains mostly nucleated and non-nucleated epithelial cells and a mixed-bacterial population dominated by Gram-positive cocci in a sea of amorphous basophilic debris. Some healthy cats have a small number of neutrophils. Yeasts as well as bacteria and small numbers of mononuclear leukocytes were also identified. Intracellular bacteria or erythrocytes are not routinely identified in these secretions.
References
- Greer W.B., Calhoun M.L. Anal sacs of the cat (Felis domesticus), American Journal of Veterinary Research 27, 1966, 733–781. [PubMed] [Google Scholar]
- Lake A.M., Scott D.W., Miller W.H., Jr., Erb H.N. Gross and cytological characteristics of normal canine anal-sac secretion, Journal of Veterinary Medicine A 51, 2004, 249–253. [DOI] [PubMed] [Google Scholar]
- Norsworthy G.D. Anal sac impaction and infection. Norsworthy G.D. The Feline Patient, 3rd edn, 2006, Blackwell Publishing: Ames, 11–12. [Google Scholar]
- Parry N.M.A. Anal sac gland carcinoma in a cat, Veterinary Pathology 43, 2006, 1008–1010. [DOI] [PubMed] [Google Scholar]
- Scarff D.H. An approach to anal sac diseases. Foster A.P., Foil C.S. British Small Animal Veterinary Association Manual of Small Animal Dermatology, 2nd edn, 2003, British Small Animal Veterinary Association: Gloucester, 121–124. [Google Scholar]
- Scott D.W., Miller W.H., Jr., Griffin C.E. Muller & Kirk's Small Animal Dermatology, 6th edn, 2001, WB Saunders: Philadelphia, pp. 1200–1203 [Google Scholar]
- Shabadash S.A., Zelikina T.I. Comparative morphological analysis of different hepatoid glands: histological variants and the morphofunctional unit, Biological Bulletin 32, 2005, 354–359. [PubMed] [Google Scholar]
- Sherding R.G. Diseases of the intestine. Sherding R.G. 2nd edn, The Cat: Diseases and Clinical Management Vol. 2, 1994, Churchill Livingstone: New York, 275–276. [Google Scholar]
- Sherding R.G. Anal sac disease. Birchard S.J., Sherding R.G. Saunders Manual of Small Animal Practice, 3rd edn, 2006, Saunders Elsevier: St Louis, 840–841. [Google Scholar]
- Zoran D.L. Rectoanal disease. Ettinger S.J., Feldman E.C. 6th edn, Textbook of Veterinary Internal Medicine. Diseases of the Dog and Cat Vol. II, 2005, Saunders Elsevier: St Louis, 408–420. [Google Scholar]